Published online Jun 9, 2024. doi: 10.5409/wjcp.v13.i2.92263
Revised: February 13, 2024
Accepted: March 14, 2024
Published online: June 9, 2024
Processing time: 138 Days and 24 Hours
Acute fulminant liver failure rarely occurs in the neonatal period. The etiologies include viral infection (15%), metabolic/genetic disease (10%), hematologic disorders (15%), and ischemic injury (5%). Gestational alloimmune liver disease usually manifests as severe neonatal liver failure, with extensive hepatic and extrahepatic iron overload, sparing the reticuloendothelial system. Empty liver failure is a rare cause of liver failure where a patient presents with liver failure in the neonatal period with no hepatocytes in liver biopsy.
A 5-week-old male presented with jaundice. Physical examination revealed an alert but deeply icteric infant. Laboratory data demonstrated direct hyperbilirubinemia, a severely deranged coagulation profile, normal transaminase, and normal ammonia. Magnetic resonance imaging of the abdomen was suggestive of perinatal hemochromatosis. Liver biopsy showed histiocytic infiltration with an absence of hepatocytes. No hemosiderin deposition was identified in a buccal mucosa biopsy.
Neonatal liver failure in the absence of hepatocellular regeneration potentially reflects an acquired or inborn defect in the regulation of hepatic regeneration.
Core Tip: We report a rare case of liver failure in which a term infant, with no history of perinatal complication, presented at age of 4-wk with an insidious onset of liver failure. We speculated that a severe liver insult may have occurred sub-clinically in the first week of life which was not detected. Regardless of the etiology of the marked hepatocyte destruction, there appears to be a. complete absence of hepatocellular regeneration, indicating a possible acquired or inborn defect in the regulation of hepatic regeneration.
- Citation: Al Atrash E, Azaz A, Said S, Miqdady M. Unique presentation of neonatal liver failure: A case report. World J Clin Pediatr 2024; 13(2): 92263
- URL: https://www.wjgnet.com/2219-2808/full/v13/i2/92263.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v13.i2.92263
Neonatal liver failure presents a unique clinical challenge as it is usually part of broader systemic disorder. Identifying of liver disease in newborns is difficult since biochemical findings such as hyperbilirubinemia and coagulopathy may be due to various physiological and pathophysiological processes. The leading cause of acute liver failure in the neonatal period is gestational alloimmune liver disease (GALD), previously known as perinatal hemochromatosis (PH). Other causes are viral infections, metabolic diseases, hemophagocytic lymphohistiocytosis, and other rare disorders[1]. Here, we describe a term infant who developed hepatic failure at the age of 28 d. Liver pathology showed a hepatic parenchyma lacking hepatocytes, as well as iron accumulation distributed in a hemochromatotic pattern.
Worsening jaundice at 28 d old.
The infant was doing well at birth but was noticed to be jaundiced at the age of 14 d. However, he was gaining weight, and the parents did not have any concerns until 28 d when the jaundice worsened.
Pregnancy was spontaneous and was the first pregnancy for the 28-year-old mother (no history of previous pregnancy losses or stillbirth). The mother has taken a prenatal multivitamin.
A full-term male newborn had been delivered by elective cesarean section owing to malpresentation after an uneventful pregnancy and had a birth weight of 2400 g and no perinatal complications.
There was no family history of liver failure, metabolic disease, or any chronic illness.
The patient was well nourished and not dysmorphic, and there was no hepatosplenomegaly or features of encephalopathy.
The patient’s total bilirubin was 553 µmol/L (normal value < 17 µmol/L), out of which, the direct bilirubin was 250 µmol/L (normal range < 17 µmol/L). The infant was anemic with hemoglobin of 84 g/L, platelets were low at 74 × 103 µL (normal range 140–400 × 103/µL) while white blood cell counts were normal at 9 × 109/L. Work up for hemolysis including Coombs test, reticulocyte count, and haptoglobin were normal. However, the patient’s coagulation profile was significantly deranged with a prothrombin time of > 120 s (normal range 12–15 s), partial thromboplastin time at > 102 s (normal range 27–42 s), and international normalized ratio > 10 (normal range 0.7–1.1). Initial serum glucose was normal at 4 mmol/L (normal range 2.5–7.00 mmol/L). Liver enzymes remained normal with aspartate aminotransferase (AST) at 35 IU/L (normal range < 63 IU/L) and alanine aminotransferase (ALT) at 19 IU/L (normal range < 46 IU/L). Serum ammonia was normal at 45 mmol/L and serum albumin was low at 27 g/L (normal range 34–54 g/L). Work up for infectious etiology was negative for rubella polymerase chain reaction (PCR), cytomegalovirus PCR, herpes simplex PCR, enteroviruses PCR, Epstein-Barr virus (EBV) PCR, hepatitis B surface antigen, hepatitis B virus PCR, human immunodeficiency virus PCR, and toxoplasmosis serum serology. Blood and stool cultures were negative, but urine culture grew Klebsiella pneumonia. Metabolic evaluation demonstrated that urine was negative for reducing substance and succinylacetone, red blood cells had normal galactose-1-phosphate uridylyl-transferase activity, and serum amino profile and lactate were normal. In addition, alpha fetoprotein, thyroid function test, serum cortisol level, serum ferritin, and serum triglycerides were all normal. Urine testing for organic acid and amino acid were normal.
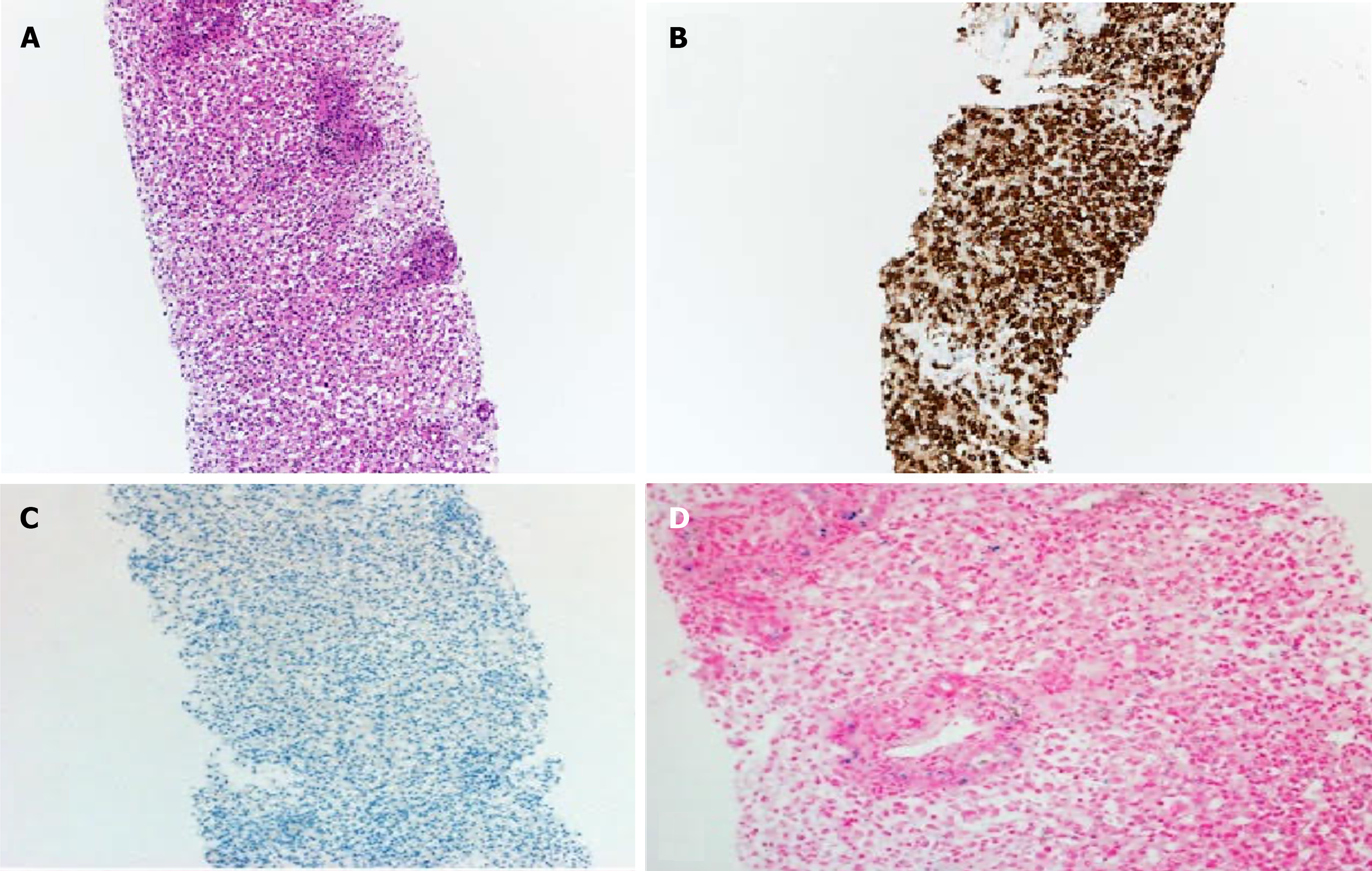
The patient underwent trans-jugular liver biopsy because of severe coagulopathy, and histopathology of the liver biopsy demonstrated histiocytic infiltration between the portal areas (Figure 1A), diffusely positive staining for CD68 (a histiocytic marker) (Figure 1B), and negative staining for HbPar (a hepatocyte marker; Figure 1C). These HbPar results were indicative of an absence of hepatocytes. Iron staining showed mild iron deposition, predominantly in the proliferating ducts and histiocytes (Figure 1D).
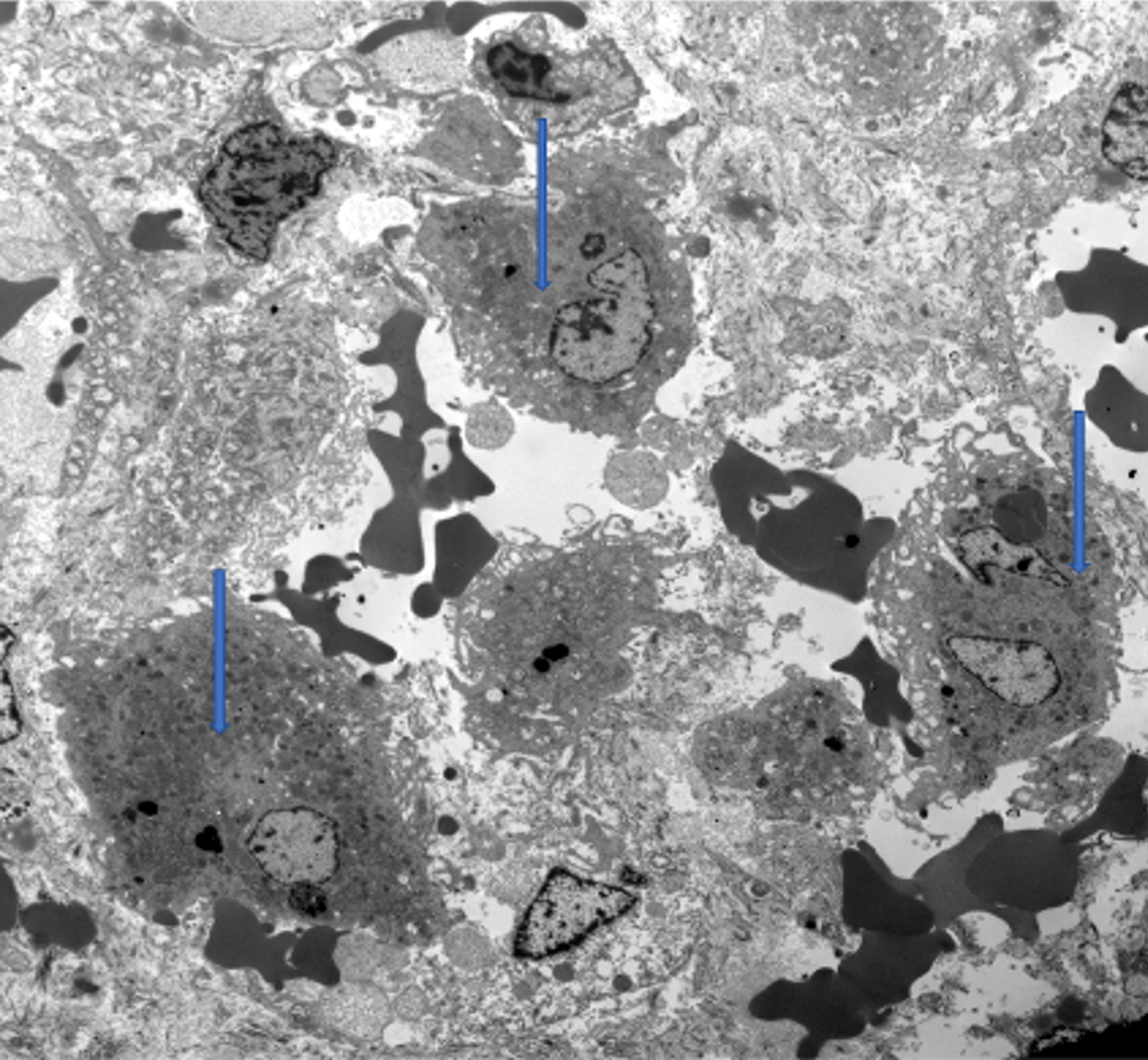
Similar to light microscopy, electron microscopy showed histiocytic infiltration (Figure 2). Adenovirus and EBV stains were negative. A buccal biopsy was obtained to look for extrahepatic iron deposition, and no hemosiderin-laden macrophages were observed. Bone marrow aspirate was also normal. Whole-exome sequencing and mitochondrial genome analysis were normal.
Abdomen ultrasound showed an unremarkable liver with normal size, echogenicity, and vascularity. Magnetic resonance imaging (MRI) of the abdomen showed a markedly shrunken liver, moderate splenomegaly, mild prominence of the common bile duct, and marked reduction in pancreatic signal intensity in comparison to the spleen, suggestive of extensive pancreatic iron deposition with splenic sparing. A skeletal survey showed no lytic lesions.
Acute neonatal liver failure of unknown etiology.
The patient’s unstable clinical condition precluded liver transplant. Although PH was unlikely and because of the critical condition of the patient, intravenous immunoglobulin (IVIG), pulse steroid, and empirical anti-viral medication and maximal medical support in intensive care were initiated.
Over the following 4 wk, the patient’s hospital course was complicated by encephalopathy, high blood pressure, bradycardia, and seizure. The patient required invasive ventilation and died from pulmonary hemorrhage at the age of 66 d.
We report an unusual case of liver failure in which a term infant-with no history of perinatal complication-presented at the age of 4 wk with an insidious onset of liver failure. There was no history of acute illness or ischemic insult that could precipitate liver failure. Histology of the liver showed an absence of hepatocytes. Laboratory data revealed profound liver synthetic dysfunction, direct hyperbilirubinemia, and profound coagulopathy, while serum aminotransferases were constantly normal or near normal despite significant liver failure which is attributed to reduced liver parenchymal mass, rather than normal hepatocytes or liver cirrhosis.
The initial presumptive clinical diagnosis was GALD, where the mother produces IgG antibodies against fetal hepatocytes, resulting in complement-mediated hepatocytes injury leading to markedly decreased or absent hepatocytes[2]. GALD usually manifests as acute severe neonatal liver failure with extensive hepatic and extrahepatic iron overload, sparing the reticuloendothelial system. GALD is rare and typically presents early in the newborn period[2], often occurring in slightly preterm or low-birth-weight infants, whereas the reported patient was full term of normal birth weight.
GALD is characterized by severe liver disease in a newborn accompanied by extrahepatic siderosis[3]. A diagnosis of GALD is established by the presence of iron overload in the serum, liver, and other organs, with sparing of the reticuloendothelial system, along with the classic clinical picture. This situation was not found in the reported patient; his blood ferritin level was normal, and no iron deposits were found in buccal mucosa. The liver biopsy did not detect any identifiable hepatocytes but showed mild iron deposition, predominantly in the proliferating ductules. Typically, in GALD, surviving hepatocytes show coarsely granular siderosis, and severe pan-lobular parenchymal fibrosis is a dominant feature; however, all these features were absent in the reported case.
GALD has been reported to cause acute liver injury to the fetal liver, resulting in stillbirth or neonatal demise[4]. In affected infants, liver pathology revealed hepatocyte necrosis without evidence of collapse, fibrosis, or inflammation, indicating a hyperacute process, consistent with the reported case. There may not be any siderosis in the liver or other tissues. The reason why certain infants experience this hyperacute liver failure while others present with congenital cirrhosis remains unclear.
In addition, for patients with GALD, the T2-weighted MRI scan is often used as a noninvasive approach to show iron accumulation in the liver and other organs[4]. The MRI of the patient in this case report did not show any iron deposition in the liver; however, severe loss of hepatocytes can give a false impression of low iron levels on MRI scans. Pancreatic siderosis was detected by the patient’s MRI; however, this is a nonspecific finding and can be observed in other conditions including hemolytic disease, galactosemia, and sepsis, all of which were ruled out in the reported patient.
Following the delivery of an infant of GALD, the likelihood of recurrence in subsequent pregnancies exceeds 90%[5], which can be prevented by treatment with IVIG during gestation[5]. In this case, the mother of the reported patient subsequently gave birth to a healthy baby who outgrew the infancy period with no issues and the mother did not receive any preventive treatment[6].
Hemophagocytic lymphohistiocytosis was unlikely because serum ferritin and triglycerides were persistently normal and bone marrow aspirate did not detect hemophagocytosis.
Although neonatal hemochromatosis was unlikely, the critical condition of the patient meant that intravenous immunoglobin, pulse steroid, and empirical anti-viral medication and maximal medical support in intensive care were initiated. Over the following 4 wk, the patient’s hospital course was complicated by encephalopathy, high blood pressure, bradycardia, and seizure. The patient required invasive ventilation and died of pulmonary hemorrhage at the age of 66 d.
A rare liver disease, termed empty liver syndrome (Le Foi Vide), was reported by Gilmour et al[4] in 1996, and had similar findings to our case, with a 5-wk-old female patient who presented at the age of 2 d with liver failure and profound coagulopathy. The hospital course of Gilmour et al’s patient[4] was complicated by hypoglycemia and seizure, and the infant died at the age of 39 d after pulmonary hemorrhage. Histological examination of the liver showed a total absence of hepatocytes with minimal stromal proliferation. Gilmour et al[4] discussed theories that could lead to complete absence of hepatocytes, including infection, severe hypoxic-ischemic insult, hepatic tumors, or inborn error of metabolism (IEOM). In our reported patient, extensive studies for bacterial and viral infections were negative. IEOM leading to perinatal liver failure will usually show fatty changes in hepatocytes rather than absent hepatocytes. Gilmour et al[4] speculated that a severe hepatic insult occurred sub-clinically in the first week of life which was not detected. Regardless of the etiology of the marked hepatocyte destruction, there appears to be total lack of hepatocellular regeneration, which may reflect an acquired or inborn defect in the regulation of hepatic regeneration[4].
In conclusion, GALD presents as a severe liver disease in a newborn accompanied by extrahepatic siderosis. A diagnosis of GALD is established by detecting iron overload in the serum, liver, and other organs, while sparing of the reticuloendothelial system, along with the classic clinical picture[7]. Early diagnosis and prompt medical intervention can lead to an improved prognosis.
We thank Dr. Taofic Mounajjed for providing electron microscopy figure and figure legend.
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
| 1. | Jackson R, Roberts EA. Identification of neonatal liver failure and perinatal hemochromatosis in Canada. Paediatr Child Health. 2001;6:248-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Staicu A, Popa-Stanila R, Albu C, Chira A, Constantin R, Boitor-Borza D, Surcel M, Rotar IC, Cruciat G, Muresan D. Neonatal Hemochromatosis: Systematic Review of Prenatal Ultrasound Findings-Is There a Place for MRI in the Diagnostic Process? J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Debray FG, de Halleux V, Guidi O, Detrembleur N, Gaillez S, Rausin L, Goyens P, Pan X, Whitington PF. Neonatal liver cirrhosis without iron overload caused by gestational alloimmune liver disease. Pediatrics. 2012;129:e1076-e1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Gilmour SM, Hughes-Benzie R, Silver MM, Roberts EA. Le foie vide: a unique case of neonatal liver failure. J Pediatr Gastroenterol Nutr. 1996;23:618-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Whitington PF, Pan X, Kelly S, Melin-Aldana H, Malladi P. Gestational alloimmune liver disease in cases of fetal death. J Pediatr. 2011;159:612-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Whitington PF, Kelly S. Outcome of pregnancies at risk for neonatal hemochromatosis is improved by treatment with high-dose intravenous immunoglobulin. Pediatrics. 2008;121:e1615-e1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Whitington PF, Hibbard JU. High-dose immunoglobulin during pregnancy for recurrent neonatal haemochromatosis. Lancet. 2004;364:1690-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |