INTRODUCTION
Apart from listening to the cry of a healthy newborn, it is the declaration by the attending paediatrician in the labour room that the child is normal, which brings utmost joy to parents. Congenital anomalies are being increasingly recognized as an important global cause of paediatric disease[1]. The global incidence of children born with severe congenital anomalies has been reported to be 3%-6% with more than 90% of them occurring in low- and middle-income group (LMIG) countries[2]. These countries also report 90% of deaths in these children. A significant number of survivors also suffer life-long disabilities, with birth defects accounting for a staggering 25.3 to 35.8 million disability-adjusted life years (DALYs) worldwide[1]. DALYs are a well-established metric for measuring the burden of disease in terms of both mortality and morbidity. One DALY is 1 healthy year of life lost due to disability or premature death[1]. World Health Organization’s global burden of disease study reports that these anomalies rank 17th in causes of disease burden. Despite being impressive figures, these are definitely underestimates because of several reasons, viz, non-inclusion of all the anomalies in the study, absence of national congenital anomaly surveillance systems/registry in many LMIG countries and the difficulties in evaluating incidence, morbidity and mortality. The cultural stigma associated with congenital anomalies prevents many parents from going to and availing medical services or even leads to infanticide. There is also an inherent bias of hospital-based data. The last cause by its nature, excludes from estimations those infants with immediately life-threatening conditions who die prior to reaching treatment[3]. Deficient diagnostic capacity and poor awareness are contributing factors. Some anomalies may not be obvious at the time of birth, e.g., submucous cleft palate (Figure 1), congenital muscular torticollis (sternomastoid tumour) etc. A great number of children in LMIG countries are born in homes or in places where no records are maintained. Sarkar et al reporting data from eastern India found the prevalence of congenital malformations in the newborns as 2.22% which was comparable with data of 2.72% and 1.9% from earlier studies from India[4].
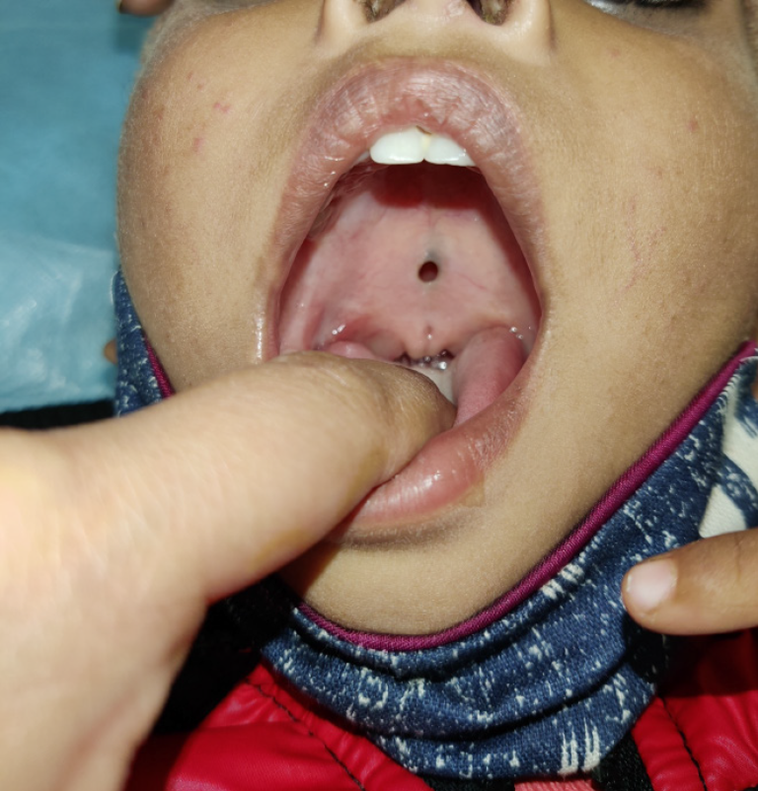
Figure 1 A submucous cleft palate is often missed until the child is much older and has poor speech development.
Note the zona pellucida and bifid uvula in this 3-year-old child.
The children with congenital anomalies are operated under several surgical disciplines, e.g., paediatric-, plastic reconstructive-, neuro-, cardiothoracic-, orthopaedic surgery etc. These conditions may be categorised as follows: (1) Life-threatening: e.g., oesophageal atresia with or without trachea-oesophageal fistula, critical pulmonary stenosis, etc. and require immediate surgical intervention. Even in these conditions, the patient should be optimally stabilised hemodynamically and reasonably well investigated in the shortest possible time after transfer to an appropriate centre with available technical expertise and then taken for surgery; (2) need very early intervention: e.g., hydrocephalus, may need intervention as soon as the patient is fit for surgery; (3) need the so-called ‘wait and watch’ policy: Natural history of the disease may be such that it is advantageous to follow this policy, also sometimes known as ‘masterly inactivity’. However, it is really not so. Rather, one has to be more careful that intervention is not unduly delayed and the disease does not worsen or lead to some secondary problems. For example, while one waits for oculomotor ptosis surgery, it should not cause deleterious effects on vision. A patent ductus arteriosus is kept under observation and followed upto a certain age in the hope of spontaneous recovery. The natural history of haemangiomas is one of spontaneous resolution with better outcome than if surgical intervention would have been rushed, usually on the demand of parents to do something at the earliest. Additionally, infantile haemangiomas are now being treated with drugs (e.g., propranolol) which cause resolution of the lesion earlier. Umbilical hernias and many children with sternomastoid tumour may resolve of their own, the latter with some non-operative measures; and (4) need elective surgery based on several ‘common to all’ and some ‘specific to deformity’ related considerations: A category of patients exists where the operative intervention is done based on several considerations which serves the child best by delivering an optimal outcome, e.g., the age and weight of the child, size of the part to be operated etc. There are advantages and disadvantages of intervention at different ages which may need to be balanced. The structures may become more robust with growth of child. The greater risks of anaesthesia to smaller children is always a cause of concern although it has become less important than in the past.
Most of the emergency surgical interventions done by paediatric surgeons at the time of birth (or within a few days) are life-threatening and their successful treatment leads to normal survival. However, almost all the congenital anomalies or birth defects coming under care of a plastic surgeon are operated as elective surgery, with several as multiple stages of correction, at appropriate ages. It is well known that children are anatomically and functionally different from adults and are not to be considered as mini-adults. Some plastic surgical procedures just cannot be done as soon as the child is fit for anaesthesia/surgery. For example, pinna reconstruction requires harvesting of sufficient autologous costal cartilage which is not available till 6-7 years of age or so. Moreover, the reconstructed pinna does not grow to adult size with age. Similarly, micropenis cannot be treated till late adolescence period. Breast reconstruction in a female child afflicted with Poland syndrome can be considered only in late teens.
In this article, we present a review of optimal timings, along with basis/reasoning behind them for surgery of many of the common congenital anomalies treated by plastic surgeons. Obstetricians, paediatricians and general practitioners/family physicians, who most often are the first ones to come across such children, must be able to guide and convince the parents appropriately. It must also be emphasized that although parents must consult a plastic surgeon as soon as possible, they should not expect that the child will be operated immediately. It is a common observation that many patients reach late to a plastic surgeon because they never consulted anyone. There is another category who were not guided appropriately by the medical personnel as to when they should consult a plastic surgeon. All the parents wish that they take a healthy newborn baby from labour room to home with no aesthetic defects because that is what is visible to relatives and friends visiting to see and bless the new born as is the custom in many societies. It will be ideal if every parent is directed to consult a plastic surgeon at the earliest who tells them about the surgical procedure and its timing and keeps the child under his/her follow up so that no undue delay occurs. On follow up visits, the plastic surgeon not only replies to the queries of the parents arisen since last visit and reassure them that everything best possible is being done for their child but also checks if the child is thriving normally. Another advantage of these visits is that the child’s parents get an opportunity to meet parents of other children, waiting in the out-patient department, who are in different stages of their post-operative follow up. This reassures them and also provides them with a real picture of what to expect.
Several of the most common congenital birth defects can be treated by a plastic surgeon operating as an individual (e.g., hypospadias, torticollis etc.) while others need a multi-disciplinary team (e.g., cleft lip and palate, craniosynostosis etc.). Great strides have been made in the treatment of numerous congenital conditions because of the intensified efforts toward achieving better functional and aesthetic results. Advances in microsurgery, craniofacial surgery, tissue expansion and anaesthesia etc. have significantly impacted the outcome of these unfortunate children. It goes without saying that all children with congenital anomalies requiring surgical intervention must remain under the constant follow up of a paediatrician as well.
The most common congenital defects requiring surgical correction by a plastic surgeon can be divided into three groups: (1) Aesthetic defect alone, e.g., cleft lip alone, unilateral microtia/anotia have mainly cosmetic concerns with far less of functional issues; (2) functional defect alone, e.g., submucous cleft palate alone; and (3) combined functional and aesthetic defect, e.g., bilateral microtia with atresia of external auditory canals, congenital muscular torticollis, congenital blepharoptosis, congenital constriction ring syndrome, syndactyly/polydactyly/absent thumb, haemangiomas and vascular malformations, craniosynostosis, pectus excavatum, etc.
GENERAL CONSIDERATIONS FOR TIMING OF SURGICAL INTERVENTION
Paediatric plastic surgery is a surgical subspecialty focused on the reconstructive and aesthetic improvement of a child’s appearance with the goal of restoring functionality and improving quality of life for those with anomalies, be they congenital or acquired from an illness or traumatic event.
The question being discussed in this article is, what is the optimal time a child should be operated for a particular congenital condition and why he/she should be operated at that age, why not earlier or later. There are a number of general considerations, common to all congenital conditions which call for a delay in operation and some specific considerations for each condition which determine the actual timing for that condition. The specific considerations are discussed separately for each condition later.
The general considerations ‘common to all’ are: (1) Age and weight are very important. It allows the surgeon to work with larger tissues and give more symmetry to reconstructed structures like in cleft lip, pinna (microtia) etc. A number of anomalies require accurate measurements. Thus, in cleft lip or ptosis surgery, even 0.5 to 1.0 mm over/under measurement may ultimately spoil the result as the child grows and need revision later in adult age. ‘If one makes a small mistake in a neonate, it becomes a "big" mistake in an adult! It also avoids inadvertent injuries to very small structures (like fingers, thumb etc.) and gives more working space to surgeon, e.g., during palatoplasty. Amount of blood loss may also be important (e.g., craniosynostosis); (2) in all aesthetic defects, the timing is usually also dependent on whether the reconstruction requires tissues like skin grafts, skin flaps, cartilage, bone etc. and their availability in sufficient amount, extent of operation, etc; (3) condition of patient. As a rule, every patient who undergoes an elective surgical intervention, is expected to be hemodynamically stable. It is very important to evaluate every child, especially one who is syndromic or has multiple, associated congenital anomalies, especially of cardio-respiratory system. The child should be healthy, thriving well and be able to withstand anaesthesia and surgery. Over a period of last few decades, with advances in paediatric anaesthetic gadgets and techniques, availability of trained paediatric anaesthesiologists, greatly improved pre and post operative care in neonatal and paediatric Intensive Care Units, at least in bigger centres of metropolitan cities, postponement of operation for this reason at least has diminished. Aesthetic and elective surgical procedures demand safety with nil mortality. Most minimal morbidity/complications are the prime objectives; (4) other associated, obvious or hidden, congenital conditions may delay the surgery, if life-threatening. For example, isolated cleft lip repair may have to be delayed because of ventricular septal defect which needs an earlier management; (5) long term psychological disturbances are also very important for most congenital defects requiring surgical management. An improved understanding of psychological implications of genital surgery have changed the timing of surgery in hypospadias, epispadias etc. over a period of last several decades. An undue delay in reconstruction of cleft lip or pinna may force a child to avoid school to safeguard himself/herself from the taunts of his/her classmates. While a few children may be impervious to ridicule and may get along without correction, others may need a change of school even after correction for full psychological benefit. Thus, the personality of the child also matters a lot. As a rule, all congenital anomalies especially with aesthetic defects not only affect the psychology of the child but also of the parents of the affected child; (6) technique of operation also matters. Thus, one-staged or multi-staged operations cannot have same time guidelines, e.g., in cleft lip and palate cases. Similarly, different techniques of pinna reconstruction demand different age group of the child; (7) a very important factor, which has not been given due importance by the novice surgeons, is that all ideal timings are advocated by highly experienced surgeons working in high volume centres with teams of experienced anaesthetists, nursing and other paramedical staff and resident doctors for usual postoperative care and any untoward rare problem. Thus, cleft lip/palate and craniosynostosis management requires a team of several specialities and paramedical personnel available in developed countries and advanced centres in metropolitan cities of LMIG countries. If these are not available, it may not be wise to mechanically follow the time guidelines. In these countries, many children have lower body weight and body frame and one may need to delay the operation for some more time. For example, unless the body size is big enough to harvest the required size and volume of costochondral cartilage, microtia reconstruction should not be undertaken; and (8) in LMIG countries, every child is not fortunate enough to get the best of institutional treatment at optimal timings. Many reach very late to the appropriate clinician and centre and one may have to accept less than best outcome. Thus, ‘late comers’ in congenital muscular torticollis may not get perfect facial symmetry but do improve tremendously and are offered treatment even in adulthood. The number of trained plastic surgeons falls severely short of required to deal the massive numbers of these children.
SPECIFIC CONSIDERATIONS FOR TIMING OF SURGICAL INTERVENTION
Cleft lip and palate
Children affected with cleft lip and/or palate (CLP) form a large spectrum of deformities from unilateral microform cleft lip alone on one end and bilateral cleft lip and palate with severely protruding premaxilla on the other end (Figure 2A and B). In addition, the nose is also deformed to varying extent and needs correction[5]. These clefts are quite common and may be unilateral or bilateral. About 1 in 700 children are born with a cleft lip and/or cleft palate. They are also sometimes associated with other congenital anomalies, especially of the heart. They may be syndromic or non-syndromic. Almost 500 syndromes have been associated with them[6]. The importance of presence of other congenital anomalies and being part of a syndrome is that very often this delays the usual timings of operation as they may have to be given priority[7]. Apart from obvious problems of feeding and aesthetics at the time of birth, a CLP has significant effect on the child’s hearing and speech with psychosocial difficulties at school due to bullying and teasing by peers because of appearance of their face and consequently attaining lower educational standards. Frequent visits to doctors and operative interventions are stressful in themselves[8]. The objectives of cleft lip and palate surgery are to achieve symmetry of lip, nose, maxillary arches and their normal growth upto adulthood when the facial growth stops and no further alterations are expected. The separation of nasal and oral cavities should allow development of normal speech (without any velopharyngeal incompetence) and normal hearing.
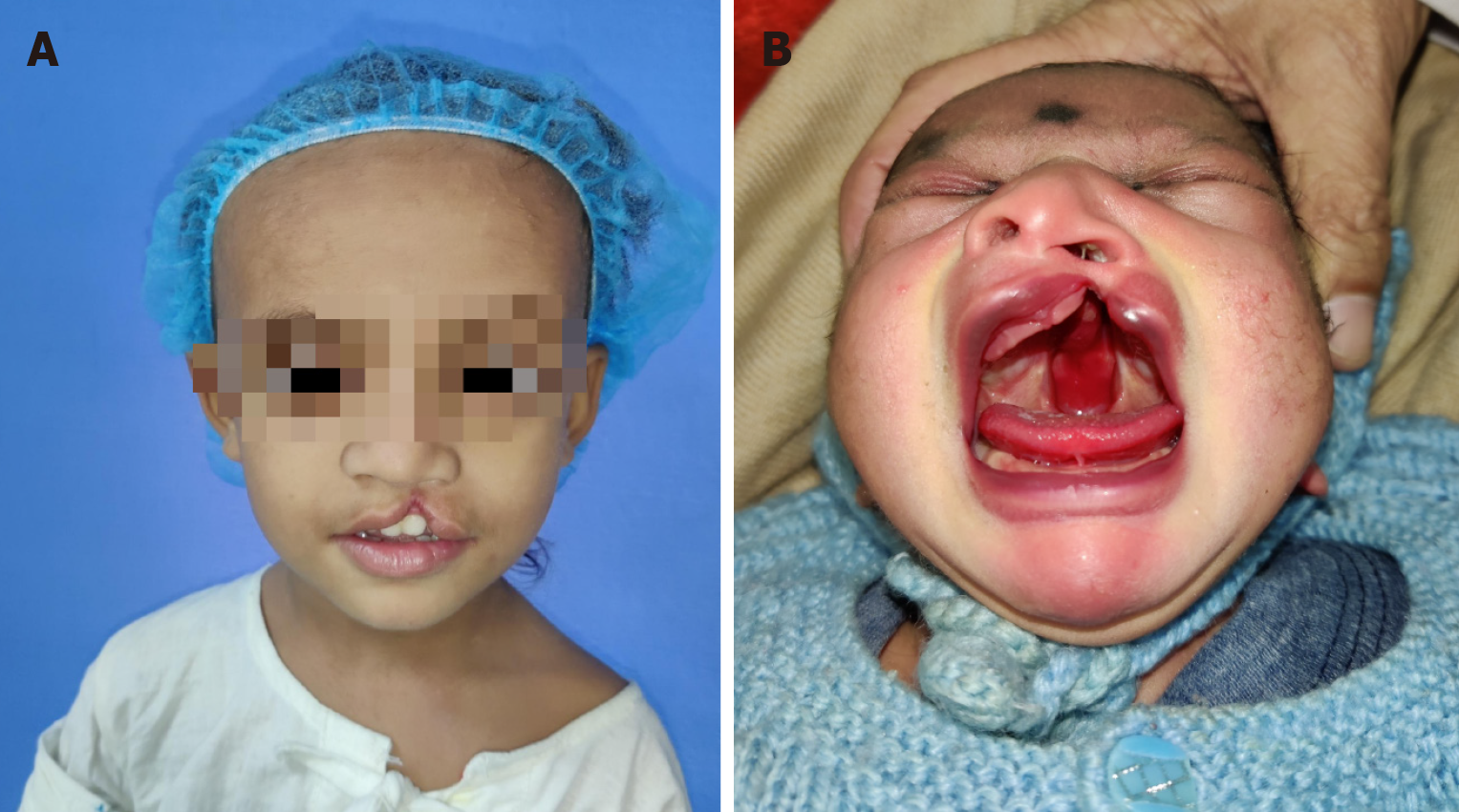
Figure 2 Cleft lip and palate.
A: 2-years-old boy with left unilateral incomplete cleft lip; B: Neonate with complete left cleft lip, alveolus and palate with Simonart band.
A newborn with cleft lip and palate does not constitute a surgical emergency. Many paediatricians and obstetricians who see the child in labour room have a mistaken notion that this child needs immediate closure of the defect lest the child starves to death or else needs a nasogastric tube feeding (Figure 3). Thus, cleft lip and palate constitutes one condition where a plastic surgeon needs to see the child immediately after birth for appropriate feeding and other advice. The problem of feeding is for initial few months only when the mother cannot breast feed the child directly because cleft in the palate does not allow the child to develop intraoral negative pressure required. The mother is instructed to express the milk manually from her breast in a bowl and then feed the child using a small spoon with child’s head being elevated at 45 degrees and milk dropped over the posterior part of the tongue. The child gulps the milk down with each breath/cry. The child swallows a lot of air along with milk, regurgitates milk into nose and may stop more intake of feed. The child is made to burp by holding vertically against the chest of mother and patting on the back of child repeatedly so that more milk is accepted. A successful feeding by the mother is judged by the satisfactory gain in weight measured every week initially. Some workers also advocate feeding with bottle using a bigger opening in the nipple so that the milk drops of its own without the child sucking it. A number of feeding devices made by dental colleagues to feed the child are probably not required as the mother and child soon adjust to bowl-spoon routine.
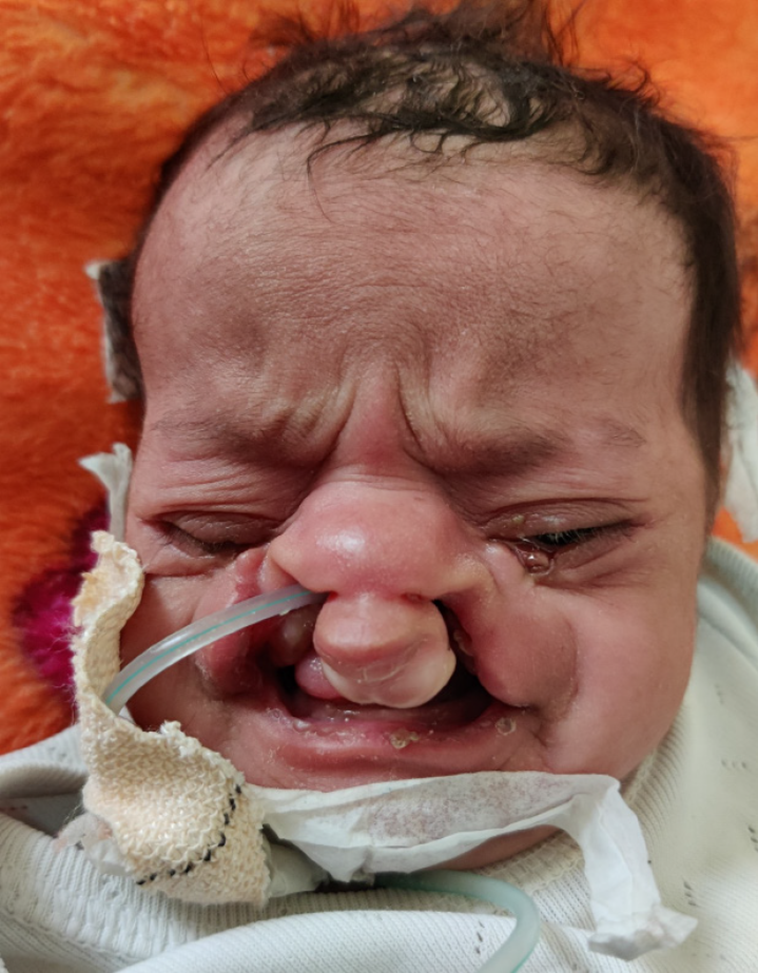
Figure 3
Neonate with a nasogastric tube inserted by the paediatrician for feeding.
Many children are given presurgical infant orthopaedic dentofacial treatment before the cleft lip and palate are operated. It is started as soon as possible after birth. Here, the cleft maxillary and soft tissue segments are moved closer while waiting for surgical reconstruction. Although it can be used in any patient, it is typically reserved for cases with a very wide cleft. The objective is to reposition the alar base and restoring the skeletal and soft tissue anatomy. Repositioning of the maxillary alveolar segments provides symmetric maxilla and nasal floor and a narrower alveolar cleft. In unilateral cases of complete cleft lip and palate, segments of the maxillary arch are brought in an anatomically neutral position without collapse or constriction (Nasoalveolar moulding)[9-12].
Millard gave his ‘rule of 10’ for cleft lip repair. The child should be over 10 wk of age, over 10 pounds of weight with a hemoglobin of at least 10 gm/dL and a leucocyte count of less than 10000/mm3. The bottom line is that the child should be thriving well and without any other congenital anomalies which can increase the risk of anaesthesia[13]. Because of free communication of oral and nasal passages in cases of cleft lip and palate, these children are prone to upper respiratory infections which may need postponement of operation and appropriate management. The timing is also dictated to some extent by the technique. Thus, some surgeons do correction of nasal deformity (primary rhinoplasty) with cleft lip repair and thus, it may be better to operate at a slightly higher age than if only lip repair is done. Similarly, in patients with complete cleft of lip, alveolus and palate, most surgeons perform lip and anterior repair in first stage and palatal (hard and soft palate) repair in second stage 3-4 months later. However, some like to repair all in one-stage operation and obviously, child should be older and weigh more for this technique in view of prolonged surgery. One-stage cleft lip and palatal repair before the age of 6 months is rarely applied in clinical practice due to the technical difficulty of the surgical procedure and potential risks such as increased blood loss, airway obstruction and anaesthetic problems. However, there are surgeons who routinely do it and find no such shortcomings[14]. One of the very important considerations in these patients is that the surgeon and anaesthesiologist share the upper airway and have to take care throughout the operation that one does not encroach upon each other’s interests.
The main concern in lip repair is of scar. It is known that scarless healing occurs in fetus and hence, experimental attempts have been made to diagnose and perform surgery in utero. This is still not done clinically. Surgery on the lip can be performed even at the time of birth sparing the psychological trauma to parents who wish to take a normal child to their home. However, this is not followed as it is very difficult to measure and accurately suture the cleft edges in a newly born (with oedematous facial tissues) and any minor discrepancy gets enlarged with facial growth, spoiling all the result with need to revise on non-virgin tissues later. Moreover, as the parents have not spent even a few hours with the neonate, even the best result may not be acceptable to them. Spending a few months allows greater development of the bond between child and parents and sudden tremendous improvement in the child’s look after operation makes them far happier. Thus, there is absolutely no need to take the child from labour room to operation theatre. A big advantage of repeated visits to the plastic surgeon during the course of treatment is that it gives the parents an opportunity to meet and interact with parents of other affected children to gain insight into the course of management.
While cleft lip repair is almost totally aesthetic, the palatal repair has lot of functional consequences, the most important being speech. The goals of palatoplasty operation are to have normal speech, hearing and maxillofacial growth The palate forms the floor of the nose and the roof of the oral cavity. Thus, palatal cleft leads to a free communication between these two cavities. The palatoplasty has to be done before the child starts speaking. Over a period of last several decades the age at which the child starts speaking has been decreased by the paediatricians again and again as they have included babbling and cooing of infants as an important prelude to normal speech development[15]. Consequently, the plastic surgeons who used to perform palatoplasty at 18-24 months of age are now advocating 6-9 months or even earlier[16].
Apart from speech, the timing of cleft palatal repair has to consider several other issues concurrently. Prevention of otitis media in the first year of life are of prime importance. Otitis media with effusion in middle ear is almost universal in unoperated cleft palate patients and this leads to diminished hearing, affecting the speech development in return. Eustachian tube dysfunction is a primary cause of middle ear disease. The Eustachian tube in a child is not fully developed and is shorter than that of adults. Also, it is positioned more horizontally and its opening into the nasopharynx is narrower. An episode of upper respiratory tract infection leads to inflammatory swelling of mucosa with narrowing/blockade of the Eustachian tube meatus resulting in a negative pressure in the middle ear. Microbes in the nasopharynx enter the middle ear resulting in effusion and infection. Abnormal tensor veli palatini and levator veli palatini muscles also cause maladjustment in the regular opening of the Eustachian tube. It needs placement of tube (ventilating grommet) in the tympanic membrane and may be done as part of palatoplasty surgery or independently, if required earlier[17,18].
In unoperated CLP patients, the midfacial growth is similar to non-cleft children without apparent restriction of growth (Figure 4A)[19,20]. However, along with varying degrees of inherent maxillary deficiency[15], surgical intervention further retards the maxillary growth due to periosteal stripping and scarring following raw areas left on either side of mucoperiosteal flaps raised to close the palatal cleft[21]. These raw areas heal by secondary intention (contraction and epithelialization from the margins). Thus, timing in palatoplasty is a compromise where speech demands as soon as possible after birth and facial growth demands only after adulthood, i.e., after complete facial growth. Midfacial growth restriction after the palatoplasty operation becomes apparent much later in adolescence and may require corrective jaw surgery. Although this revision surgery is taken to be part of an overall treatment plan, it can definitely not be viewed as inconsequential. It is quite complex and burdensome and can further affect facial and palatal growth due to scar and wound contraction. In situations where limited interdisciplinary facilities are available, the child may be left with excellent speech but severe mid face hypoplasia. After the repair of lip, alveolus and palate, the child needs to be sent to a speech therapist who assesses the speech and gives therapy for its normal development[22]. The speech abnormalities are multifactorial. There may be hypoplasia and hypomobility of the levator and tensor veli palatini muscles and their abnormal course and insertion into the palate. Unless properly repositioned, it may not allow for perfect speech development. Assessment for any velopharyngeal incompetence is done and, in some children, pharyngoplasty is required at around 5 years of age. Velopharyngeal incompetence is the inability to completely close the velopharyngeal sphincter which is required for the normal production of all but the nasal consonants. It results in nasal air escape and hypernasality leading to decreased intelligibility of speech[23].
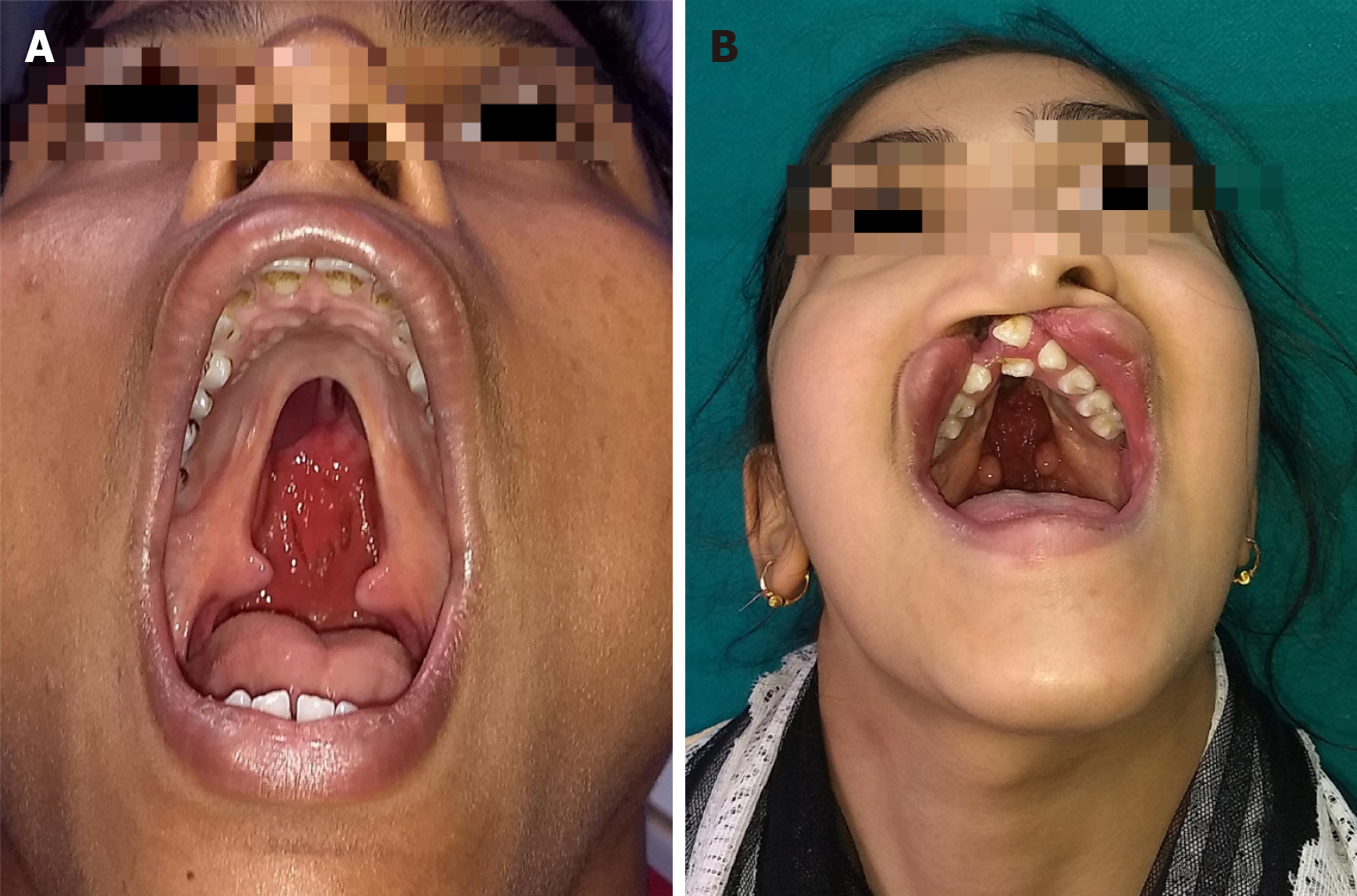
Figure 4 Cleft lip and palate in adults.
A: Normal maxillary growth in an adult un-operated cleft palate patient; B: Adult un-operated patient of cleft lip and palate with multiple dental abnormalities and nasal deformity.
As the child grows, one finds that the dentition is disturbed with malocclusion. Some teeth are absent while others are mal-positioned or mal-aligned or even supernumerary or smaller than normal (Figure 4B). Thus, a pedodontist and an orthodontist need to see the child as the teeth erupt. These specialists have to work with primary, mixed and permanent dentitions[8]. Orthodontic treatment in children with CLP consists of maxillary arch expansion, correction of upper incisor misalignments, gross rotations of incisors, crossbites and correction of class III skeletal growth pattern. Some patients require secondary alveolar bone grafting. When the bone grafting is done before or along with palatoplasty, it is called primary while secondary bone grafting implies that the procedure is performed after the repair of cleft palate[24-26]. Primary bone grafting prevents collapse of the maxillary arch and stabilizes it creating more uniform growth of maxilla and improvement of articulation. The disadvantage is that it can provoke attenuation of the maxillary growth especially in the vertical dimension. The optimal timing of secondary alveolar bone grafting balances the ability to provide bone for eruption and periodontal support of the teeth adjacent to cleft, establish continuity of dental arch in the alveolar cleft, allowing orthodontic space closure and future placement of an implant or bridgework, achieve closure of any oronasal fistulae, establish nasal skeletal base and improve speech on one side and the minimization of inhibition of maxillofacial growth on the other side[24]. Secondary bone grafting is usually performed just before permanent canine eruption, seen in radiograph to be about half or more of its normal size, using autogenous cancellous bone from iliac crest (8-11 years of age.) It minimizes the growth disturbances of the maxillary arch, giving it integrity with periodontal support for the teeth proximal to the cleft. It is now widely used and considered to be a standard procedure for alveolar repair[27]. Some children may need orthognathic surgery (osteotomies, distraction osteogenesis and bone grafting). These interventions are usually done only in late teenage, a little earlier in females (14-16 years) as compared to males (16-18 years). Some patients may require dental implants and prosthetics in adult age. Correction of the underlying skeletal base by alveolar bone grafting, Le Forte I advancement or prosthetic reconstruction of the anterior maxilla in bilateral clefts is done prior to undertaking secondary rhinoplasty.
The repair of cleft in the lip and palate and other measures by the plastic and dental surgeons restores the nasal floor maxillary symmetry but several problems remain which are due to misplaced nasal cartilages. Their correction is done either as part of lip repair (primary rhinoplasty) or later (secondary rhinoplasty)[28]. Primary rhinoplasty can be limited or extensive. By giving reasonable shape to nose, primary rhinoplasty allows most children to defer definitive rhinoplasty. A secondary or definitive rhinoplasty operation is carried out only after the facial growth is complete and maxillary arch is symmetrical and hypoplasia taken care of. Definitive rhinoplasty is usually undertaken at 14 to 16 years of age in girls and 16 to 18 years of age in boys[6]. During definitive rhinoplasty, aggressive osteotomies, septal correction and nasal cartilage repositioning/carving and fixations are done as there is neither a risk of their alterations by growth nor the growth itself will be altered by them. The goal is to create a permanent symmetry, desired nasal shape, dorsum as well as base and relief from various deformities, not only congenital but also due to effects of previous surgical interventions[6,29]. In addition to restoration of nasal shape, secondary rhinoplasty also requires to correct functional component as majority of these patients have functional airway obstruction which is due to external nasal deformity, septal deviation, vomerine spurs, inferior turbinate hypertrophy or maxillary hypoplasia. Secondary cleft rhinoplasty has been considered one of the more challenging subcategories of rhinoplasty because each of previous procedures add to scarred nasal skin, cartilage and mucosa and absent virgin tissue planes and often needs autogenous costal cartilage graft as well[30].
Some surgical interventions may also be required for secondary deformities which need correction only after the facial growth is over and the patient himself/herself puts forth the demands to the surgeon (e.g., improper direction of hair or alopecia in the area of moustache region). The fact is that the treatment of these children by a plastic surgeon is longitudinal in nature starting right at the time of birth and stopping only after adulthood. Throughout this period, the child is under care of not only a plastic surgeon but also of paediatrician, otorhinolaryngologist, speech therapist, dental surgeons (pedodontist, orthodontist etc.).
In several LMIG countries, a large percentage of the CLP children reach a plastic surgeon far later than the above optimal timelines because of shortage of trained medical personnel as well as remote areas and financial constraints. These children are still offered operative intervention and satisfactory, if not perfect results obtained. In these patients palatoplasty may be done first so that speech problems are minimised. Aesthetic outcome of isolated clefts of lip are usually dependent on the skills of the surgeon and even late middle-aged have also been offered treatment with excellent results! Palatoplasty is generally not offered to adults because of poor speech outcome.
Microtia and other deformities
Congenital absence of pinna or external ear is a very common anomaly (Figure 5). The deformity can range from mild malformation to complete absence of the pinna (anotia). Congenital aural atresia, a common association with microtia, is failure of development of the external auditory canal. Microtia may be part of a syndrome (e.g., hemifacial microsomia). Microtia is of great aesthetic concern to parents of newborn and to the child once he/she starts going to school and wishes to avoid the torment meted out by the classmates. Pinna is also required to wear glasses, may not be now but later on when an individual needs correction of refractory errors or at least in old age for presbyopia. It is also needed for wearing sunglasses and hence needs restoration. So, theoretically, as for several other conditions with aesthetic concerns, it should be reconstructed before the child starts going to school (around 3 years). However, there are a number of important considerations as to why this is just not possible. The two most important one’s are: (1) The reconstructed pinna does not grow. The normal pinna continues to grow after birth and attains 85% of adult size by the age of 5 years or so[31]; and (2) pinna reconstruction for microtia is done using autologous costochondral cartilage[32]. The amount of cartilage required depends upon the technique of reconstruction while the amount available depends on the age and body build of the child. While Brent[33] operated on children more than 6 years of age (preferred 7-8 years of age) with costal cartilage from opposite side, Nagata advocated use of ipsilateral cartilage at 10 years of age with a chest circumference of 60 cm at the level of xiphisternum[31,34-36]. Many other pioneers of pinna reconstruction like Bauer, Firmin, Park also prefer 10 years of age because of insufficient amount of costal cartilage at lesser ages and better results at 10 years[37]. In general, 6th, 7th, 8th and 9th costal cartilages are harvested for pinna framework fabrication. Pinna reconstruction is a multi-staged operation. A very early operation may make the pinna very small (as compared to the opposite normal side) and the amount of cartilage may be highly deficient in length and volume. The reconstructed pinna may also prove to be rotated and abnormally positioned once the adult size of normal pinna is reached. There may be a chest deformity also. An undue delay may affect the psychology of the child while still greater delay may even land the surgeon in harvesting a cartilage which is ossified and difficult to carve. Thus, the best time for reconstruction has to be balanced and comes to around 10 years for most children.
Figure 5 Bilateral microtia in a 12-year old boy.
A: Front view; B: Right ear; C: Left ear.
The time to attend the school and the development of self-image of a child are much before the time-lines of a good pinna reconstruction. They do have an impact on the emotional state of the child and the parents which needs to be looked after by the plastic surgeon and the paediatrician till the pinna is reconstructed. It is important to note that although the operation is to be done at a particular age, the parents and child must visit the surgeon regularly, may be yearly. As the child grows and understands, he/she is taken into confidence for the reasons of delay and may be shown pictures of pinna reconstructed by the surgeon on every visit. All must understand that once the cartilage is harvested, there is no way to harvest more for a second operation, if it proved inadequate earlier. The ear being reconstructed is for rest of life. First attempt is the best attempt, be it pinna reconstruction or academic exams!
In addition to aesthetic reasons, ability to hear normally is another big concern. Thus, the other consideration of concern is whether microtia is unilateral or bilateral and whether the external auditory canal is absent. All children need to be seen by the otologist at 3-4 months of age and kept under follow up for assessment of hearing capabilities. Even in unilateral cases, one must know that the other side is normal, the protection of which is very essential for development of speech. Identification of extent of hearing loss allows appropriate corrective measures to be instituted. In patients with unilateral microtia, absent canal is usually not reconstructed as the child adjusts to monoaural hearing. Patients with bilateral microtia with bilateral absence of canal are usually functionally deaf bilaterally. Autologous ear reconstruction takes care of cosmetic outcomes only. It does not address the functional hearing issues in patients of microtia with associated atresia of external auditory canal. It precedes canal restoration as the plastic surgeon needs virgin tissues for cartilaginous pinna framework placement at the appropriate site while delay in canal restoration in bilateral cases is bound to affect development of speech. To achieve best results from auricular reconstruction for cosmetic purpose and canal restoration for hearing rehabilitation, the plastic surgeon and the otologist colleague must look at all aspects of the procedure and plan in advance. Thus, the site of surgical incision, position of bone conducting hearing implant etc. must be discussed. Treatment of functional deficits must not compromise aesthetic reconstructive options and vice versa. These children need non-operative management for hearing in the form of bone conducting hearing device on soft band or osseointegrated implants like bone anchored hearing aid (BAHA) during this period[31,36]. Bilateral cases have to be staggered and one side should have settled before undertaking the opposite side lest the manipulations of child’s head spoil the reconstructed pinna. Canal restoration has to be done at least after 3 months of framework placement and lobule transposition and is generally combined with the last stage of pinna reconstruction. Even after canal restoration the child may need BAHA.
Similar to many other congenital conditions, microtia may also be associated with other congenital malformations like facial clefts and cardiac defects. The latter may have a bearing on the timing of pinna reconstruction.
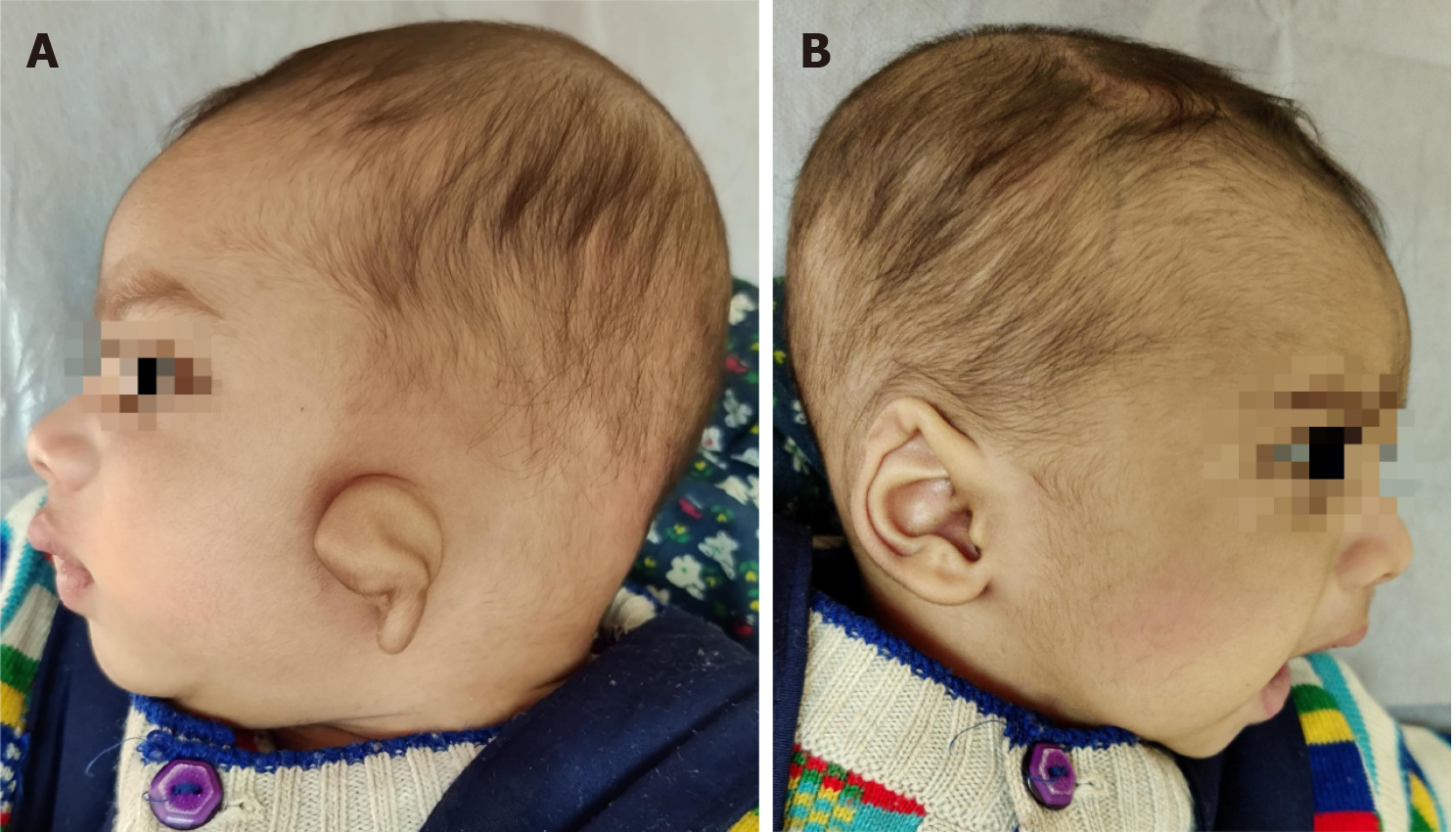
Other deformities of pinna such as prominent ear or lop ear (Figure 6) also have aesthetic concerns and affect the psychology of the child because of bullying by children at school and calling them names like ‘bat ear’ or ‘Mickey Mouse ear’. Hence, child should be operated before admission to school. Some severe cases of lop ear need reconstruction as for microtia and hence they follow similar timelines for operation.
Figure 6 Neonate with unilateral lop ear.
A: Left affected ear; B: Right unaffected ear.
Craniosynostosis
Craniosynostosis is defined as premature fusion of one or more cranial sutures in the newborn. It leads to restrictions in the growth of calvarium, predictable compensatory deformations and also affects the brain morphology with resultant neurological sequelae[38]. The growth of the skull is typically restricted perpendicular to the fused suture and expanded in a plane parallel to it (Virchow’s law). This fusion can be either isolated (or non-syndromic, in 80%; e.g., sagittal synostosis) or part of a syndrome (or syndromic, in 20%; e.g., Apert syndrome, Crouzon syndrome etc.)[39]. The incidence is approximately 1 in 2500 births[40]. While surgical treatment is the mainstay of treatment, its timing is controversial[41-44]. The considerations for timing of operative intervention are aesthetic and functional. By restoring the normal shape of the cranium as soon as possible, cosmetic goals are achieved. Just like other aesthetic deficiencies, craniosynostosis also leads to psychosocial stress for the parents and the child. The functional goals of craniosynostosis surgery are to restore adequate intracranial volume enough for unimpeded cerebral growth and expansion, reducing the risk of raised intracranial pressure (ICP) and to minimize cognitive sequelae. An uncorrected deformity does not tend to worsen over a period of time. At the same time, it does not improve of its own as well. The calvarium of a newborn undergoes significant changes until one year of age. Maldevelopment of skull and brain go side by side. The weight of brain is 400 gm at birth, double at 6 months and triple at 2.5 years of age, reaching almost adult size of 1400-1600 gm by 5 years. Greater the number of fused sutures, greater is the potential for raised ICP[40]. Thus, operative intervention is important not only for cosmetic purposes but also to safeguard against brain dysfunction.
Being a highly complicated problem, even all plastic surgeons do not perform this surgery. It requires major centres with a team of plastic surgeon, paediatric neurosurgeon, paediatrician, ophthalmologist, anaesthetist and trained nursing staff etc. to operate on such patients[45]. Different units have their own protocols for operative intervention[41-44] and hence, the child should be under care of the team as soon after birth as possible[46]. Some unfortunate children reach the clinician late because the parents consider their abnormal shape to be due to child’s preferred posture while lying. Indications for emergency surgery include an immediate threat to the airway or eyes or the presence of raised ICP[39]. Surgery before the age of 1 year (most surgeons start at 3-6 months) reduces potential impacts on crucial brain development, risk of raised ICP and deformities of skull base[47]. Advantages also include increased malleability of the softer and younger bone amenable to bending and reshaping. The ongoing growth of brain encourages continued growth of the cranial vault and ossification of craniectomy defects due to surgery occurs satisfactorily. No bone grafting is required for these defects as the osteogenic potential of the dura enables spontaneous bone regeneration. However, softness and increased malleability of bone also means that its fixation by plates and screws is unsatisfactory. There are greater risks of anaesthesia and excessive blood loss in smaller children[39,48] (plus complications of massive blood transfusions) and more chances of re-do surgery required during youth because of ongoing craniocerebral disproportion resulting in craniostenosis again. After 1 year of age, the bones become more and more mineralised, thick and brittle and pose difficulty in remodelling. The deformity also increases and becomes too severe for improvement. The small defects left in calvarium do not ossify of their own and need bone grafting[39]. Despite surgery, some children do develop raised ICP which needs to be looked after to prevent complications. Thus, the timing of surgery needs a balance between prevention of undesirable effects of craniosynostosis and child’s ability to tolerate a prolonged and complicated major surgery.
Congenital blepharoptosis
Congenital blepharoptosis is characterized by an abnormal drooping of the upper eyelid resulting in narrowing of the palpebral fissure and may be: (1) Simple, seen as an isolated anomaly; or (2) complicated, which is associated with maldevelopment of the surrounding structures, ocular motor anomalies, blepharophimosis syndrome and Marcus Gunn ptosis[49]. Examination of the infant is difficult and the presence of congenital ptosis is generally confirmed from photographs taken during childhood by the parents. It may be uni- or bilateral and is mostly due to defective development of the muscles and is nonprogressive. Although the primary reason for correction of ptosis surgery is functional (vision), production of symmetry in lid height, contour and eyelid crease lead to better cosmesis taking care of any psychological consequences.
A number of operative techniques are available to correct the ptosis. The timing of surgical correction depends on the severity of the ptosis and the strength of the levator muscle. The eyelids are primarily elevated by the contraction of the levator palpebrae superioris muscle which is innervated by the superior branch of the third cranial nerve[49]. Usually, it is done at 4-5 years as it allows accurate measurements preoperatively which need reliable cooperation of the child during physical examination, improving the surgical outcome in return. Also, the risk of postoperative exposure keratopathy is less as these children cooperate better with forced closure exercises and the status of cornea can be better monitored[50]. An earlier surgical correction is indicated when the droopy upper eyelid interferes with the visual axis causing stimulus deprivation amblyopia[51,52] or associated strabismus induced amblyopia. Sometimes, a temporary operation may be needed to tide over the period in first year of life itself. Greater the delay in treatment of amblyopia, more difficult it becomes to achieve good final visual acuity and after 7-10 years, it may not be possible to reverse amblyopia. Thus, timely treatment of ptosis is very important. Refractive error is another criterion, especially anisometropia or increasing amblyogenic astigmatism. An earlier intervention is required in cases with torticollis of ocular origin as this may delay mobility in toddlers because of the balance problems from extreme chin-up head posture.
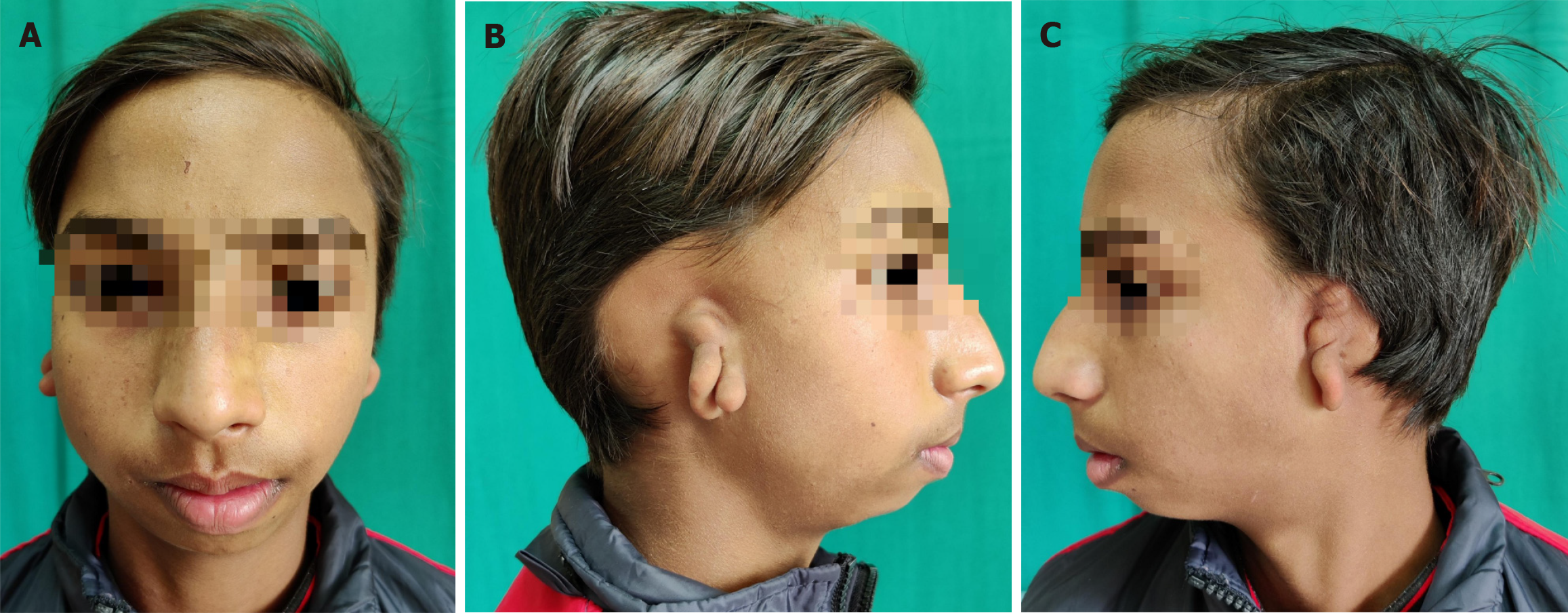
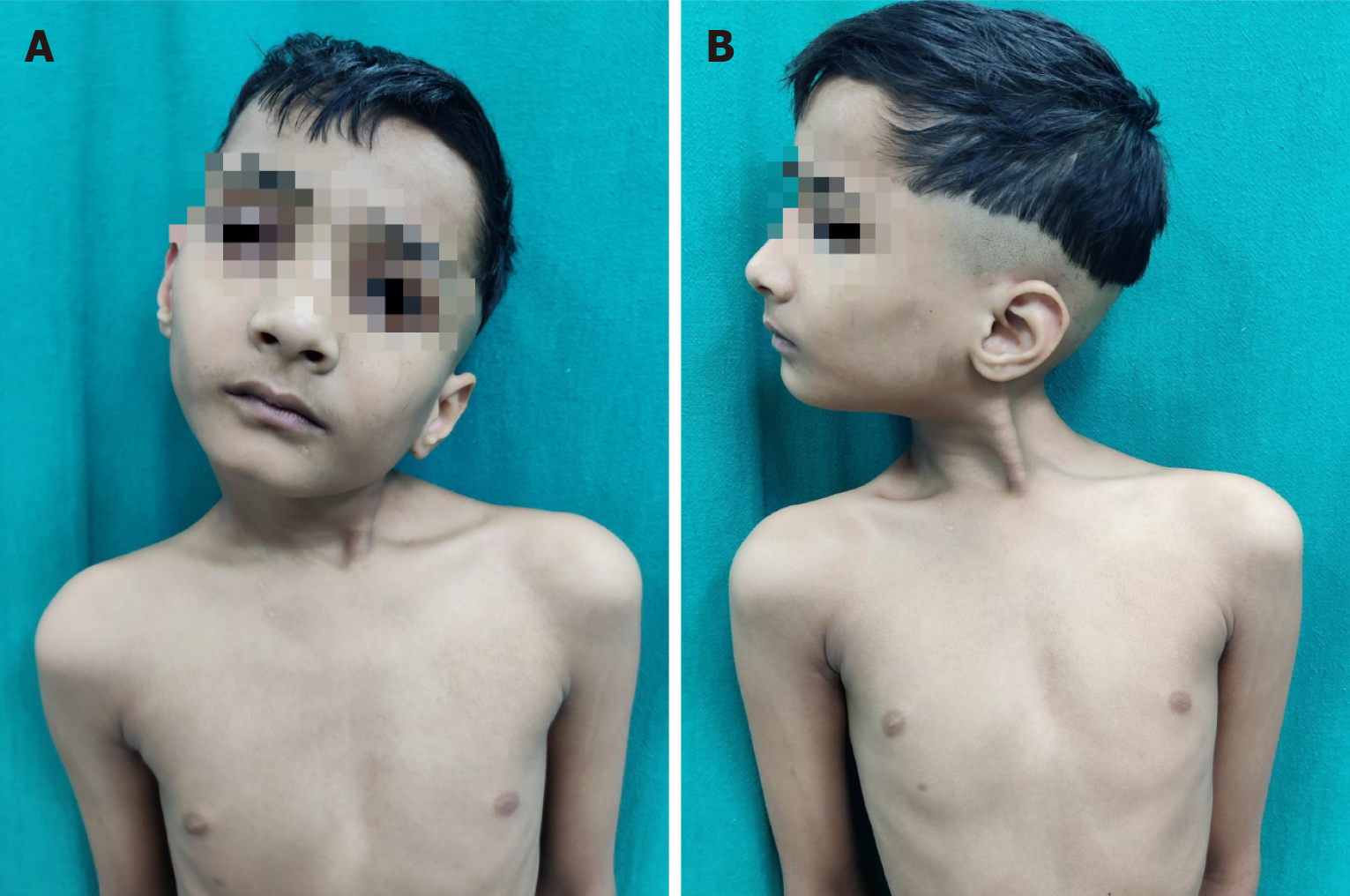
Congenital muscular torticollis
Congenital muscular torticollis is the third most common congenital musculoskeletal anomaly, after clubfoot and congenital hip dysplasia. There is shortening of the sternomastoid muscle in the neck and it presents as a unilateral deformity leading to inclination of the head towards the affected side (Figure 7). Torticollis is not a simple static, fixed deformity. Rather, it has far-reaching effects on craniofacial growth, development of spine and possibly ocular and vestibular development. In an attempt to compensate for torticollis, the child elevates the affected shoulder and laterally shifts the head. This involuntary compensation may lead to the development of craniofacial asymmetry, cervical scoliosis and compensatory lumbar scoliosis. It may result in rotation, lateral flexion of head, deviation of the face, limitation in neck movements and diplopia as well[53]. Thus, congenital muscular torticollis has aesthetic as well as functional consequences. It must be remembered that that torticollis is a sign also and hence, other causes must be ruled out.
Figure 7 Eight-year-old child with left congenital muscular torticollis.
A: Note the shortened left sternocleidomastoid muscle as a fibrotic band, the facial asymmetry, restricted mobility of the neck; B: Lateral view of the same.
Because of short neck in children, many cases especially the mild ones may be missed even by clinicians. Affected children present with a painless swelling in the neck, called ‘sternomastoid tumour’ which resolves spontaneously, usually within a year or so. In patients where the tumour persists, it is replaced by fibrosis and contracture in the muscle which, if not treated, leads to progressive facial asymmetry. The cranial asymmetry is already determined by 6 months of age. Non-surgical treatment in the form of cervical collars, massage and physiotherapy with active and passive stretching exercises of sternomastoid muscle is started as soon as diagnosed. However, this non-operative treatment is not successful after 1 year of age[54]. The patient's age at operation, duration of the disease and severity of the deformity before the operation are the major determinants affecting both cosmetic as well as functional outcomes. The surgical intervention is required to correct the abnormality for aesthetic and functional reasons. Although optimal age has not yet been established but intervention from 1-4 years is said to be optimal, if diagnosed by that time[55-57]. Earlier the better. After that age, as the delay occurs, it may not be possible to achieve perfect symmetry. However, marked improvement in symmetry, aesthetics and psychology has been reported after operation in teenagers and young adults, the so called ‘late comers’ by several workers including authors[58-60].
Pectus excavatum
Pectus excavatum is the most common congenital chest wall deformity (constituting 95% of patients of all chest wall deformities) with males being affected several times more. The sternum is sunken and gives an appearance of a dent in the anterior chest. The sternum and lower costal cartilages are pushed posteriorly with severity ranging from mild depression to ones where the sternum almost abuts the vertebral bodies. In 90% of patients, the deformity can be detected in first year of life. It increases in severity gradually till adolescence when growth spurt makes it rapid until full skeletal growth is achieved. After adulthood the deformity remains constant for most patients throughout life[61]. Scoliosis may be present in 15% of cases[62]. This can be very disturbing to young teenagers who develop poor self-esteem and body image perception leading to psychological disturbances. In general, any deformity of the anterior chest is not acceptable to adolescent males as well as females leading to significant restrictions in their social activities. This is seen so commonly with the increasing number of teenaged males with gynecomastia and females with hypoplastic breasts thronging the aesthetic surgery clinics all over the world. These children try to camouflage the chest contours and avoid sports and swimming activities etc. Corrective surgery tremendously improves the quality of life[63].
Functional effects are on heart and lungs. In milder cases, the cardiorespiratory functions may be within normal limits. More severe cases may require surgical intervention for cardiac and/or respiratory impairment. Older children, teenagers and young adults may find exercise intolerance and endurance issues or even exercise-induced asthma[64]. Thus, there are aesthetic as well as functional considerations for deciding the optimal age. Earlier, mild to moderate cases of pectus excavatum were advised operation from 2-5 years even if they were asymptomatic because the surgery could be done more easily as compared to in bigger children. However, surgical techniques which involve resection of large amounts of rib cartilages performed too early may risk chest wall growth and result in pulmonary dysfunction[65,66]. Thus, most surgeons wish to wait till 8 years or later when there is adolescent growth spurt[61]. Symptomatic cases might need intervention earlier. The optimal age for repair appears to be in the range of 12 to 16 years as the rib cage is more malleable allowing for rapid recovery with a lower recurrence rate. Adults with persistent deformities extending into 4th-5th decades have achieved excellent results after corrective surgery[61]. Fonkalsrud in his retrospective study of 375 patients found the age at surgery ranged from 2.5 to 53 years with 47% from 2-11 years[67]. Horch et al[68] advocate that in children with no reduction of the respiratory volume and no compression of mediastinal structures, a sternal elevation procedure or rib transection may not be required. The aesthetic defect, without altering the rib cage pathology, can be treated using customized silicone implants or autologous tissues such as cartilage, fat etc. in teenagers and adults[69,70]. However, very few patients are suitable for augmentation procedures and maximum require sternal elevation only[68]. While obviously the former do not address the functional issues, their migration may produce additional deformity.
Poland’s syndrome
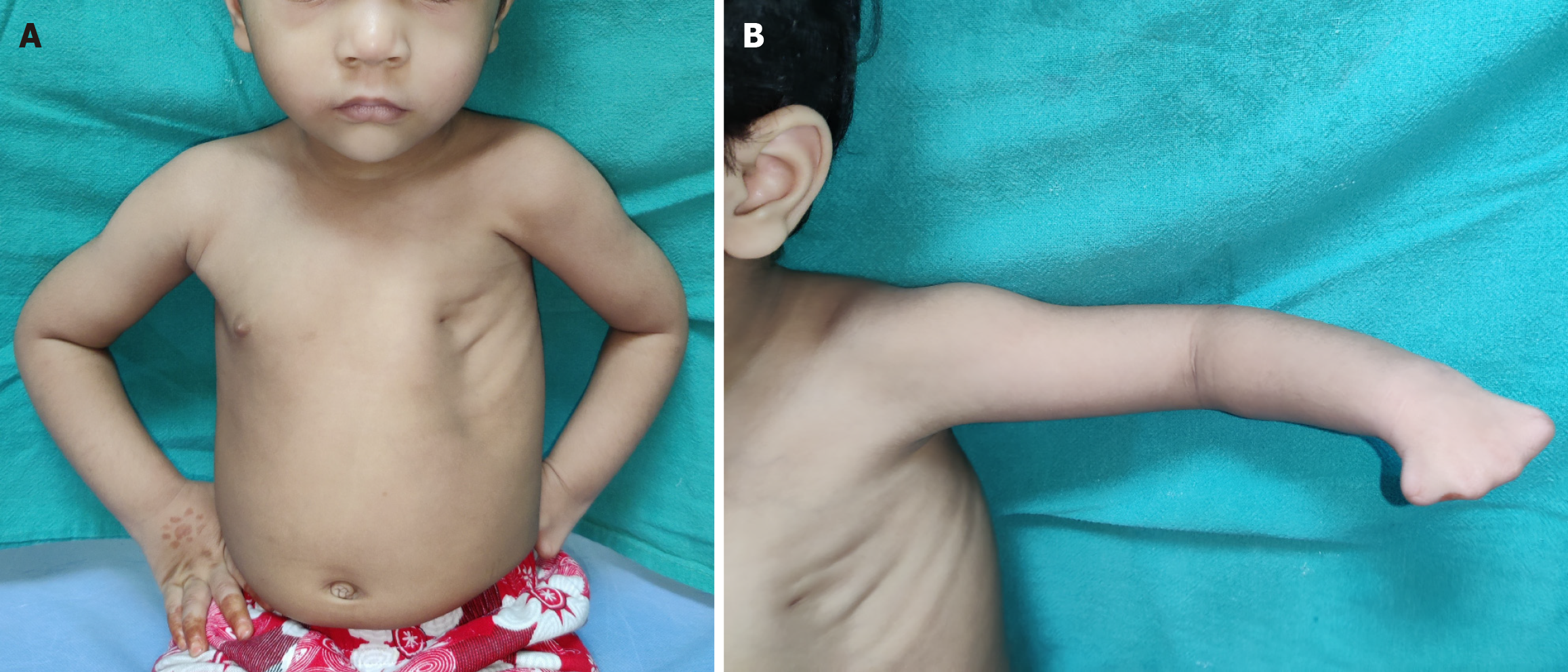
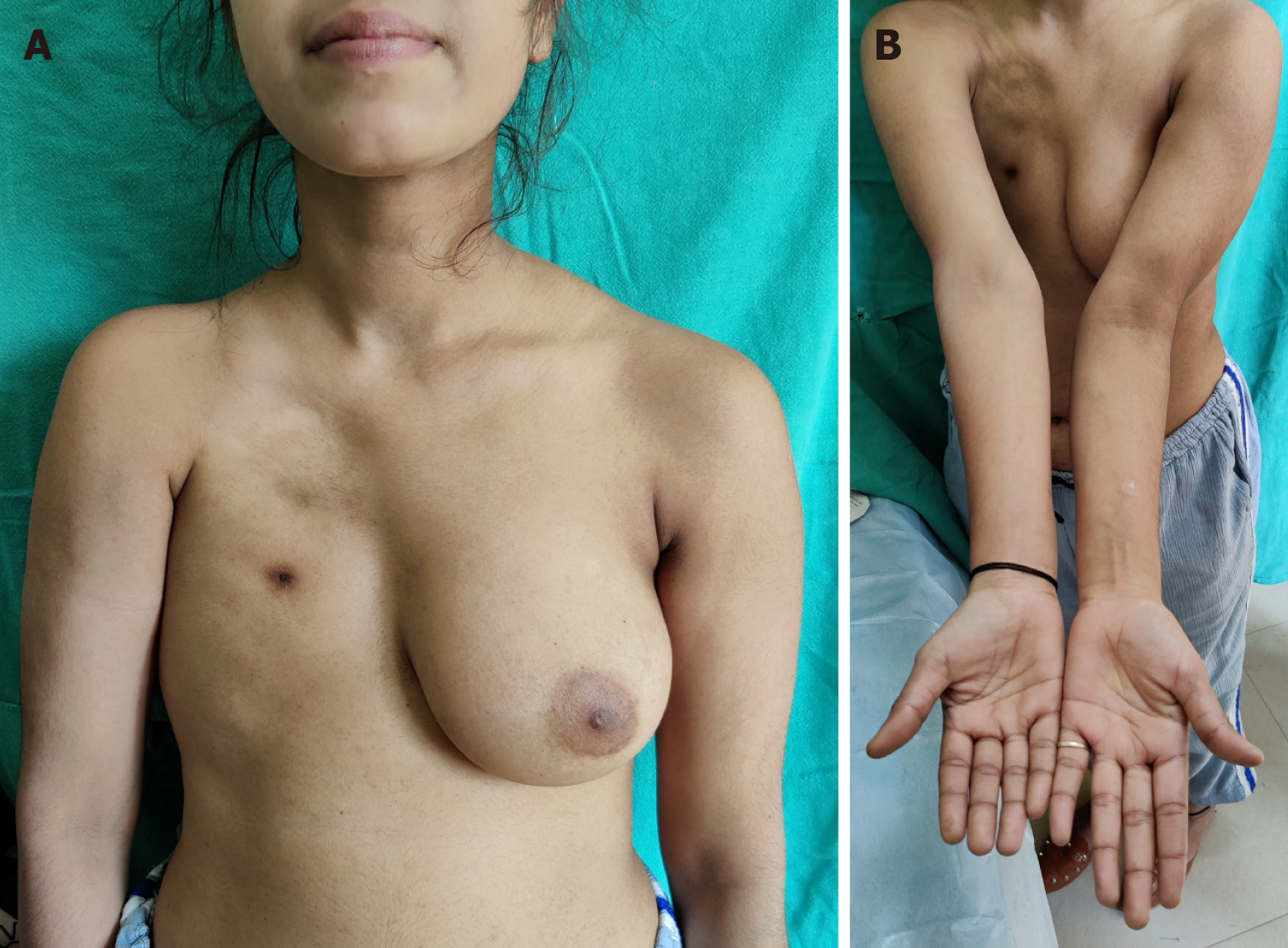
Poland’s syndrome is a rare congenital anomaly characterized by unilateral chest wall hypoplasia and ipsilateral hand abnormalities. In a classical case, there is unilateral symbrachydactyly with ipsilateral hypoplastic anterior chest and anterior axillary fold, diminished bulk of infraclavicular subcutaneous tissue, absence of the costosternal portion of the pectoralis major muscle, lack of the pectoralis minor muscle, aplasia or deformity of the costal cartilages/ribs, hypoplasia of nipple-areola complex and finally hypoplasia of the breast at the time of thelarche[71]. The extent of these deformities in a given case are variable. Extremely rarely, the syndrome can involve structures bilaterally. Apart from these, an extremely large number of other abnormalities have also been described in these patients[72].
The major anomalies requiring operative intervention are those of the hand and the anterior chest (including breast)[73,74]. The child is usually brought for hand deformities as chest wall deformity may not be obvious in an infant or toddler (Figures 8 and 9). In the latter cases, the surgeon brings to the notice of parents about the hypoplastic chest wall and the surgical interventions which may be required after puberty, especially in girls. There is a wide range of spectrum of hand deficiencies and surgery may have to be started from 6 month onwards in patients who require multiple stages or need to separate first web space while those requiring a single stage may be taken up at 18 months or so[73,75]. Reconstruction of breast needs to be done only after the contralateral breast has developed, usually in late teens (Figure 10). Teenaged and adult male patients may ignore chest wall hypoplasia, if not very severe and may not opt for any surgical treatment.
Figure 8 Poland syndrome in a 3-year-old girl child.
A: The chest can be reconstructed only after complete development of the opposite normal breast; B: Symbrachydactyly in the left hand.
Figure 9 Poland syndrome in a 6-year-old male child brought to the out-patient department for right hand deformity.
A: The parents did not notice the mild hypoplastic right chest and nipple areola complex. He may not need or desire a chest reconstruction at all later in life; B: Right hand deformity.
Figure 10 Poland syndrome in a girl in her late teens.
A: Right chest hypoplasia, absent breast seen. Reconstruction of right breast can be done with the left as a template; B: Short humerus on the affected side.
Syndactyly/polydactyly/thumb hypoplasia/cleft hand
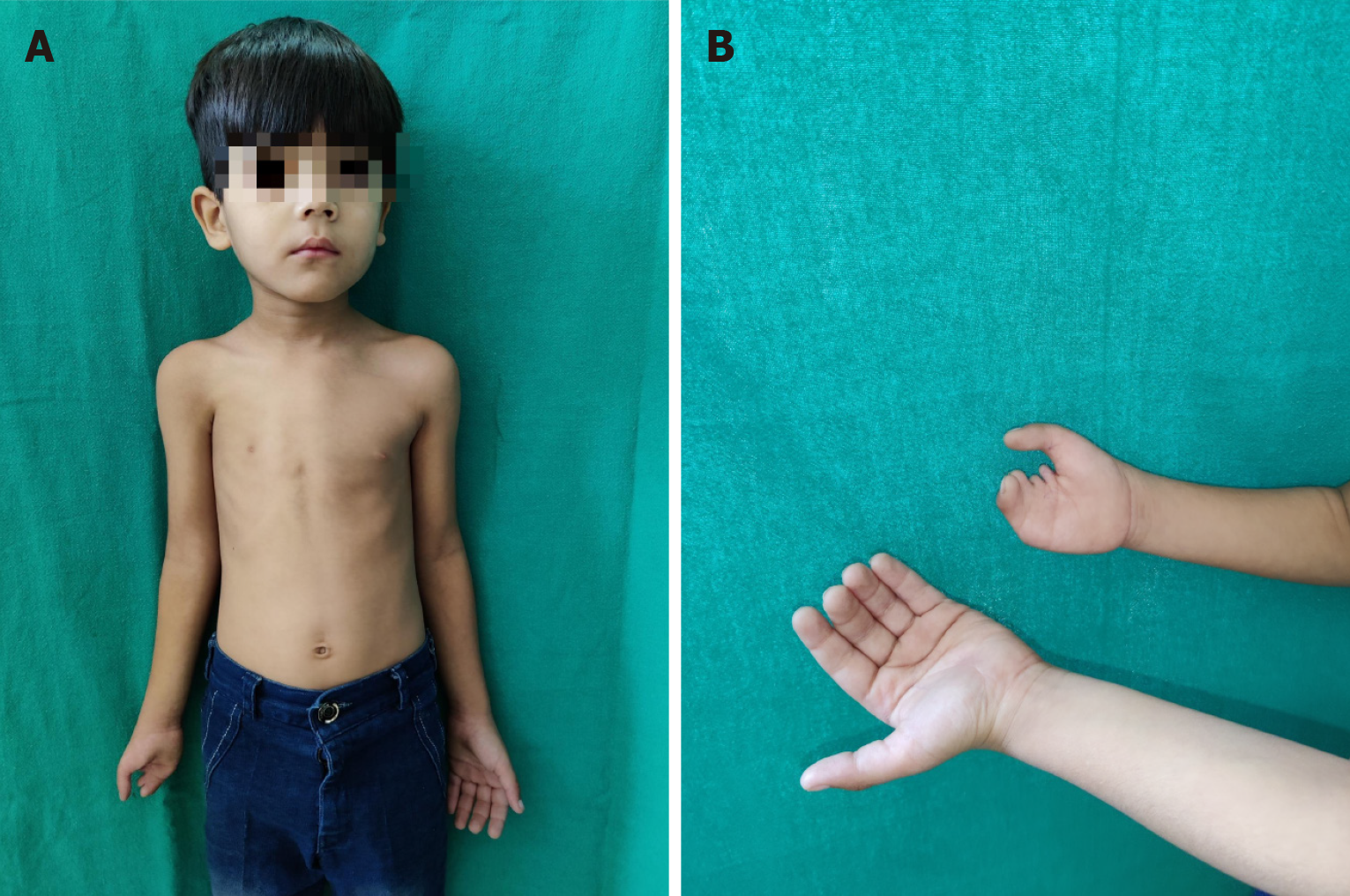
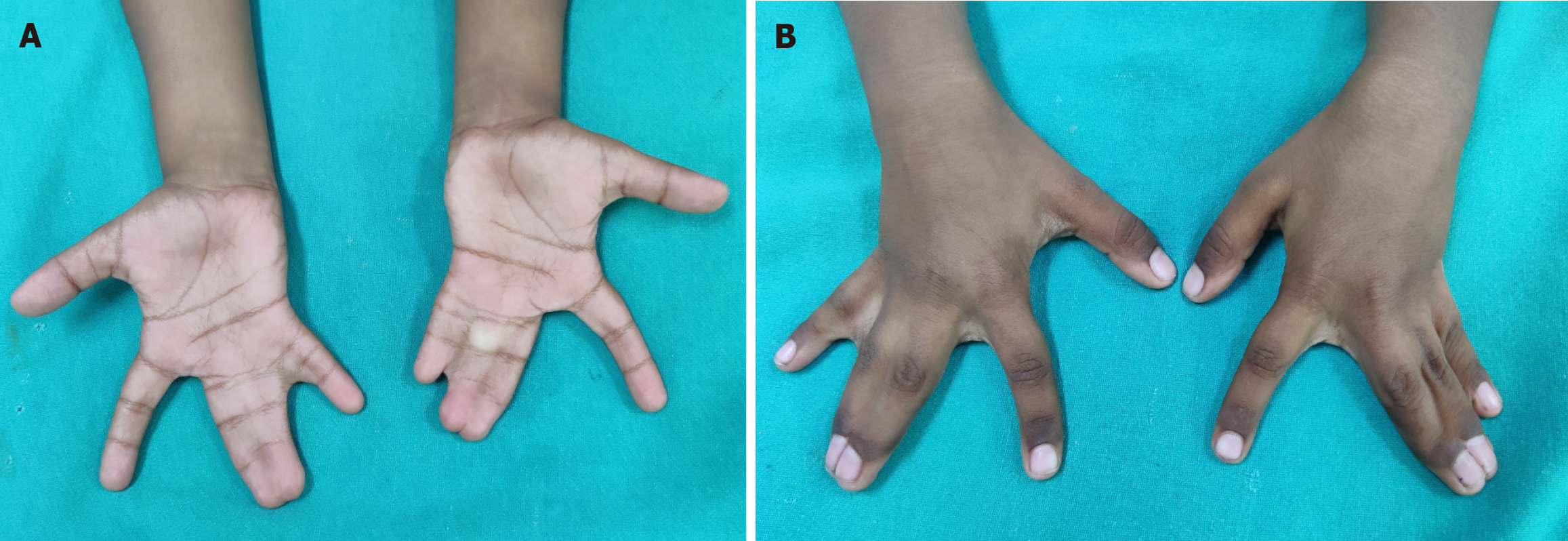
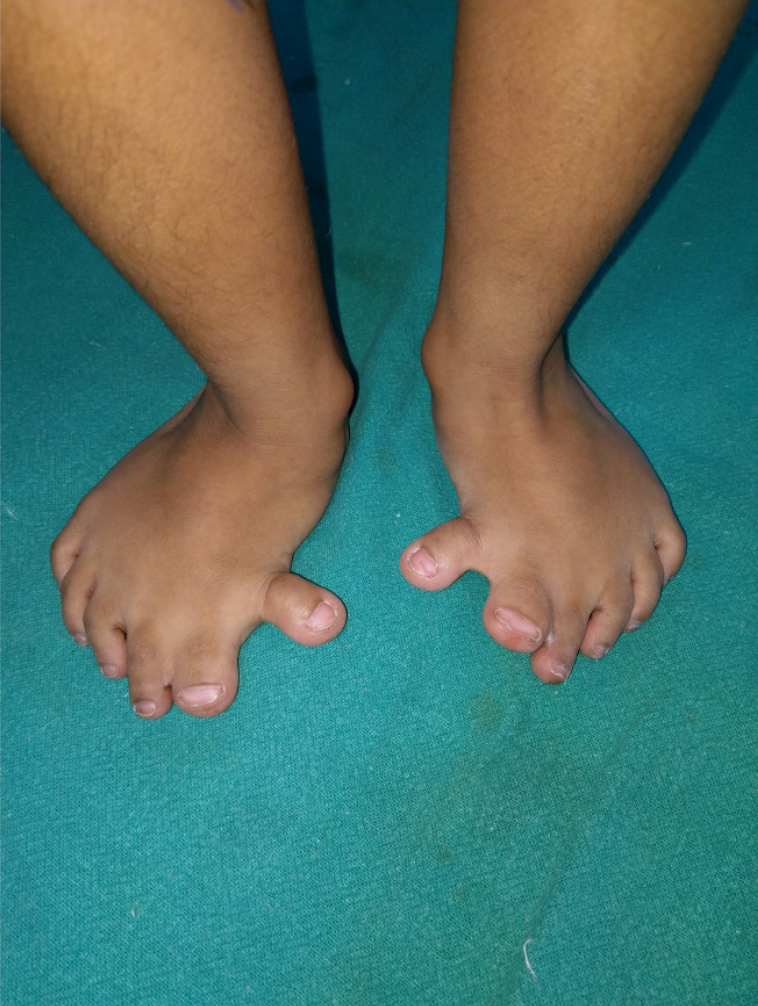
Syndactyly and polydactyly are the most common of congenital malformations affecting limbs. While it is obvious that many parents with minor problems may not opt for treatment, it is also true that many cases of syndactyly or polydactyly may have no functional/psychological problems to the child and hence no treatment desired. Many parents/grown-up children even consider supernumerary digit as bringing good luck. Hence, actual prevalence may not be known. They may be isolated or part of syndromes[76]. Both show a great spectrum of deformities and may be uni- or bilateral and can involve hands and/or feet. Thus, there may be partial fusion of skin between two adjacent fingers or all the fingers and thumb may be united upto whole length to give rise to a mitten hand (Figure 11). There may be bony fusion, extra or missing phalangeal bones or placed erratically. When fusion is upto finger tips, the nails are fused to each other despite the different length of digits and this causes deformities of fingers in a growing hand, prevention of which is an important consideration for an early operation. Additionally, when more than two adjoining fingers are joined, all of them cannot be released in one operation because of risk of damage to neurovascular bundles. A waiting period of at least 3 months should be given which also allows time for massage, exercises and some maturation of scar. Similarly, polydactyly may be as insignificant as a nubbin to as severe as a duplication of hand (mirror hand). There may be polysyndactyly, pre- or post-axial polydactyly and other severe deformities.
Figure 11 Syndactyly.
A: Complete syndactyly of bilateral 3rd web spaces and incomplete syndactyly 4th webspace (palmar view); B: Dorsal view.
It is thus, clear that the surgical release/amputation has to be based more on functional considerations (before the development of fine motor skills) apart from aesthetic considerations. Patterns of prehensile function are established by the age of 2 years. Timing of operation on fingers is also decided by their size as there is a risk of damaging minute neurovascular bundles, difficulty in multiple dressings required post operatively, care of the skin graft used almost as a routine for provision of skin cover after release of syndactyly and physio-occupational therapy. Administration of anaesthesia to infants is another consideration. Surgery can usually be done in most cases by 12-18 months or so. However, in cases requiring multiple operations, interventions may have to be started earlier.
In patients of syndactyly where the fused digits have significant difference in length or there are abnormal bones or bony fusions, intervention should be started by 6-9 months lest functional deformity results due to asymmetrical growth (Figure 12). This is especially true for syndactyly between the ring and little finger and between the thumb and index finger. In bilateral cases, surgery can be done simultaneously on both hands by two teams as the child is dependent on parents for all his daily needs[76]. Operative blood loss is not a consideration as these operations are always done under tourniquet control. It is ideal that the child goes to school with normal anatomy and function which is so important for development of their fine motor skills and psychosocial development. In mitten hand (e.g., Apert syndrome), the first and third web spaces may be separated first and the second and fourth web spaces may be done in second stage. First web is most important so that the child starts using the thumb in a normal way. First surgery must be started by 6 months of age. Last but not the least, the congenital anomalies of the hand are sometimes part of various syndromes and cardiovascular anomalies which may be life-threatening and demand prior correction. Consequently, usual timelines may have to be delayed. Children with partial simple syndactyly can be delayed without any consequences.
Figure 12
Complex complicated syndactyly with aberrant transverse bones and bony fusions.
In patients of polydactyly, planning is required to decide the digits to be kept and the ones to be amputated. Simple ablative surgery alone, especially in duplication of thumb, can result in instability and malalignment of the remaining thumb[77]. The main goal of operative intervention in these cases is restoration and maintenance of function and pinch activity along with improved appearance. While a nubbin can even be amputated under local analgesia at 1-2 months of age, a more developed supernumerary digit needs removal under general anaesthesia in a preschool child. Even polydactyly of the feet should be treated prior to school so that the child can wear normal shoes (Figure 13).
Figure 13
Bilateral feet pre-axial polydactyly and syndactyly in a teenager.
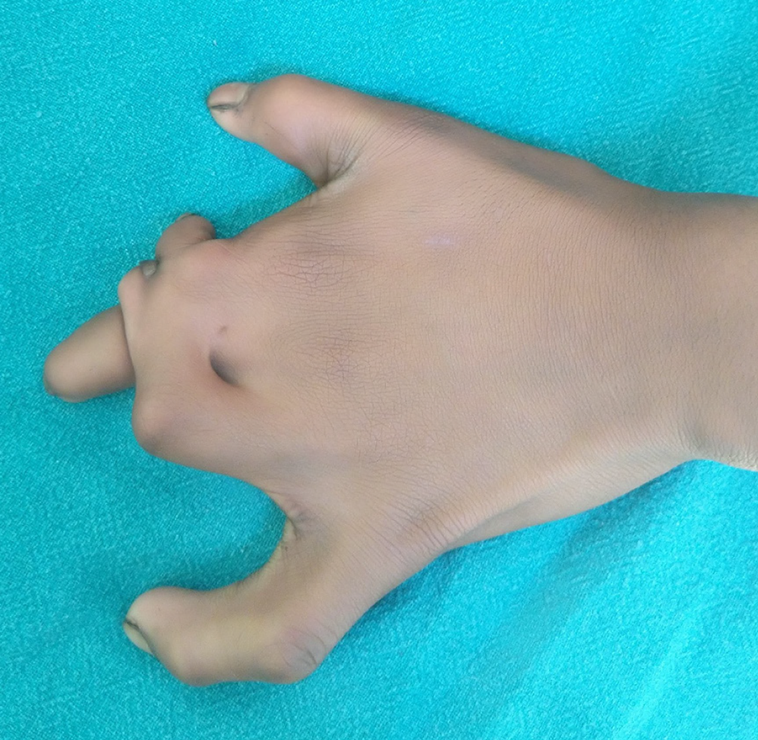
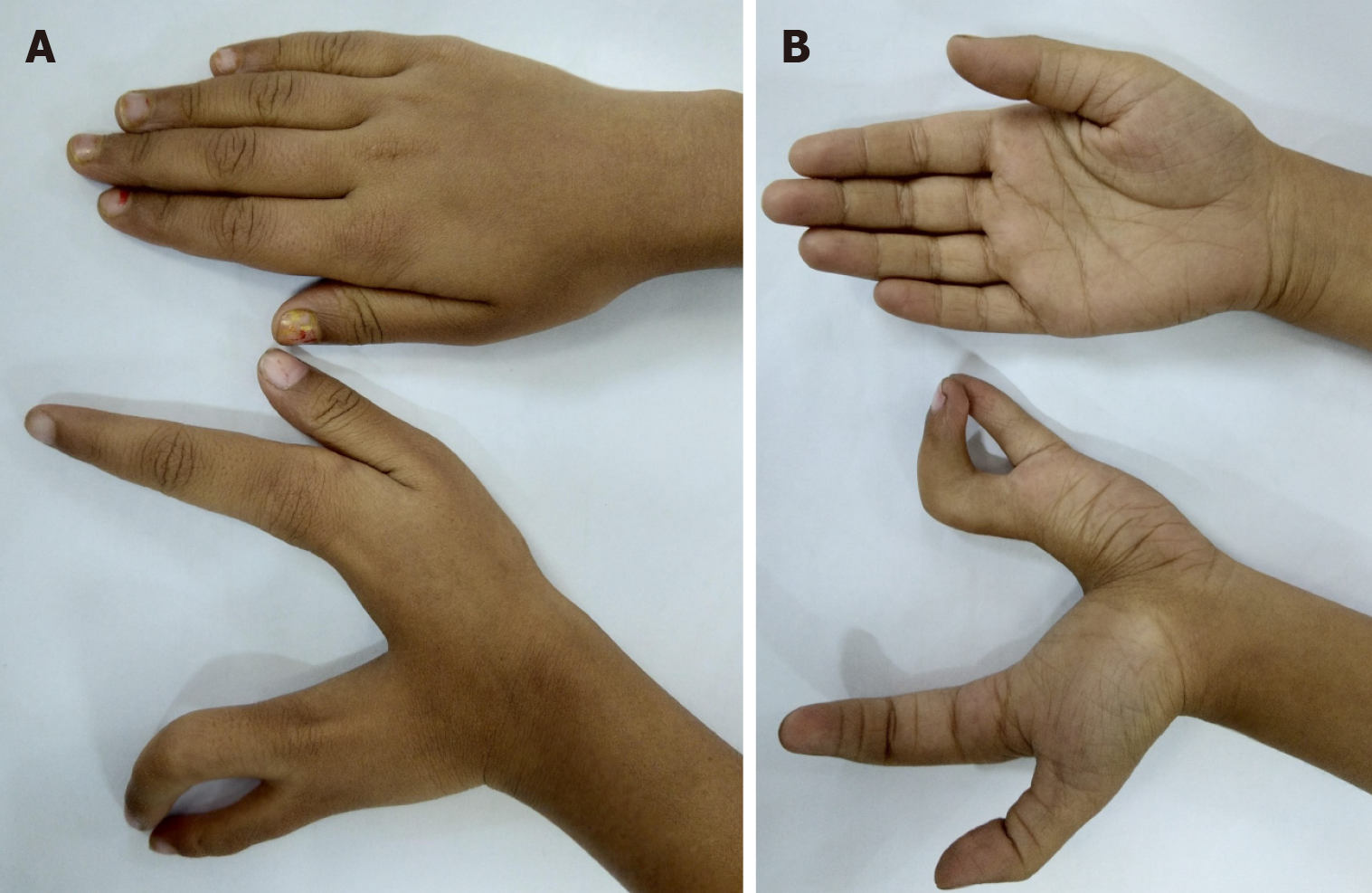
Cleft hand has been described as ‘a functional triumph, but a social disaster’ as many function well without correction (Figure 14)[78]. However, those with a progressive deformity (e.g., transverse bones, syndactyly, etc.) or absence of first web space or thumb need early intervention in early infancy. Other cases may be delayed till 1-2 years of age.
Figure 14 Left cleft hand with satisfactory function but poor aesthetic appearance.
A: Palmar view; B: Dorsal view.
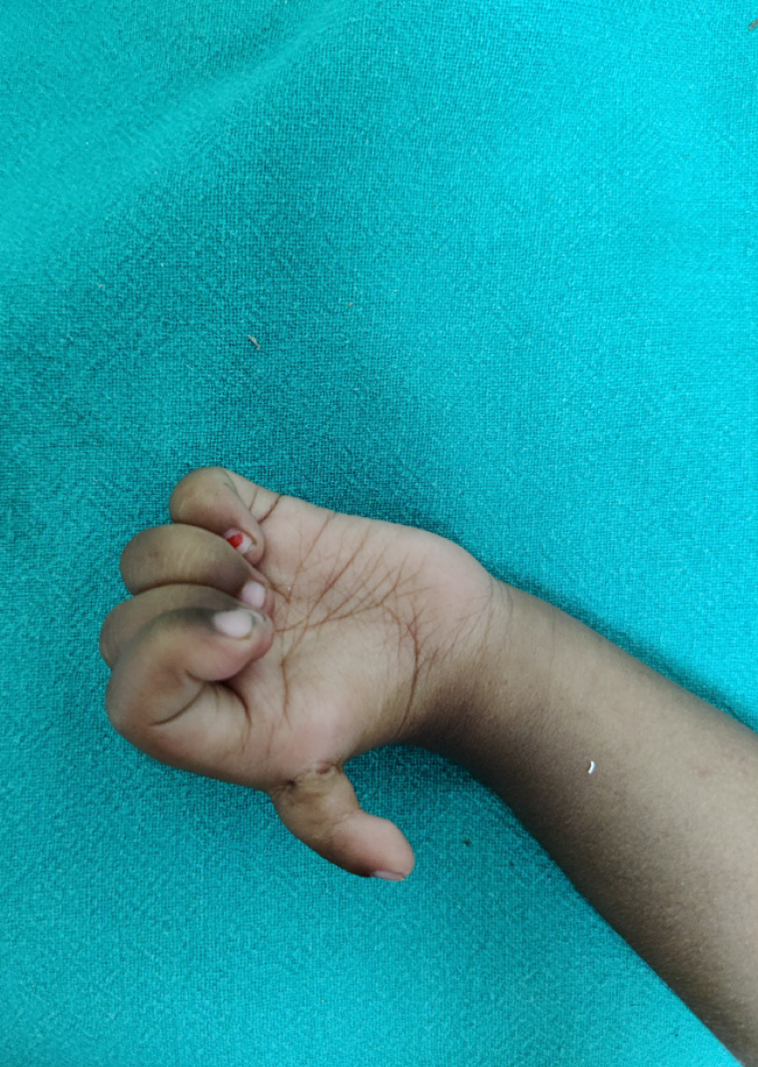
Thumb hypoplasia of lesser severity may not warrant any intervention but the most severe needs pollicization (Figure 15). Cortical plasticity and motor relearning are said to be of critical importance in functional development following pollicization[79]. There is a large region of the sensorimotor cortex dedicated to hand with significant chunk devoted to thumb. The cortical plasticity unveils formerly quiescent connections with sprouting of active afferents from cortical regions nearby. The changes within the sensorimotor cortex are amplified with practice by the child. Advantages of an early surgery are lessened by the very small size of the nerves, blood vessels and musculotendinous tissues. Pollicization from 1-2 years of age gives bigger child to the anaesthetist, bigger hand to work on by the surgeon and yet takes advantage of plasticity of brain of the infant/toddler. Older children and teenagers presenting late are, however, not denied the reconstructive surgery. Similar considerations apply in duplication of hand (mirror hand) where surgical intervention is needed to move one digit in place of thumb.
Figure 15 ‘Pouce flottant’ thumb dysplasia should be operated with a correct balance between cortical plasticity and adequate size of tissues.
In many cases, if the thumb of the opposite side is normal, the child does not use the reconstructed thumb even after pollecization.
Constriction ring syndrome
Congenital constriction ring syndrome[80], also called amniotic band syndrome, is a non-genetic condition resulting from entrapment of different fetal parts, most commonly limbs including digits (lower more commonly than upper limbs) in a fibrous band while the fetus is still in utero. There are skin indentations or grooves varying in depth and circumference (Figure 16A). A wide variety of rare presentations can be seen with involvement of trunk, viscera and face[81]. There may be associated cleft lip and palate, craniofacial anomalies, club foot, polydactyly, neural tube defects etc. The most common problems which concern a plastic surgeon may range from a cutaneous constriction ring which is not very tight with no distal effects to the one with very tight circumferential involvement leading to distal deformities including lymphedema, acrosyndactyly/syndactyly and even intrauterine amputation (Figure 16B). The limb proximal to constriction ring is normal. There are aesthetic considerations as well. The considerations for timing for surgery on consequences of amniotic bands on extremities include extent of compromise of blood supply to the part distal to constriction as severe hypoperfusion can lead to gangrene with risk of sepsis and threat to life of the child. This calls for an immediate surgical intervention. In other cases, general considerations of risks of surgery and anaesthesia-related complications and other associated life-threatening congenital anomalies requiring earlier management come into play. Thus, such children may have surgery done after 6 months of age. To prevent distal circulatory compromise, traditionally two stage surgery (Z-plasty of half the constriction ring in first stage followed by remaining half after 3 months) has been advocated. One-stage surgery with the obvious advantage of avoiding one more operation, is being reported with comparable results in the long-term follow-up[82]. Patients with superficial bands not affecting circulation and no/minimal lymphedema have cosmetic indications only and may be delayed. Presence of severe distal lymphedema demands an earlier intervention. It improves easily as there is no induration by scar tissue. Craniofacial and body wall anomalies should be approached by surgery based on clinical practice guidelines individually for each case. The routine use of ultrasound of the fetus in the prenatal period has allowed some observations on the prenatal natural course[83-85]. The outcomes have ranged from spontaneous resolution without any long-term consequences to progressive limb strangulation with subsequent amputation. There are reports of successful fetoscopic surgery for tackling amniotic bands in utero in animals as well as human[83-85].
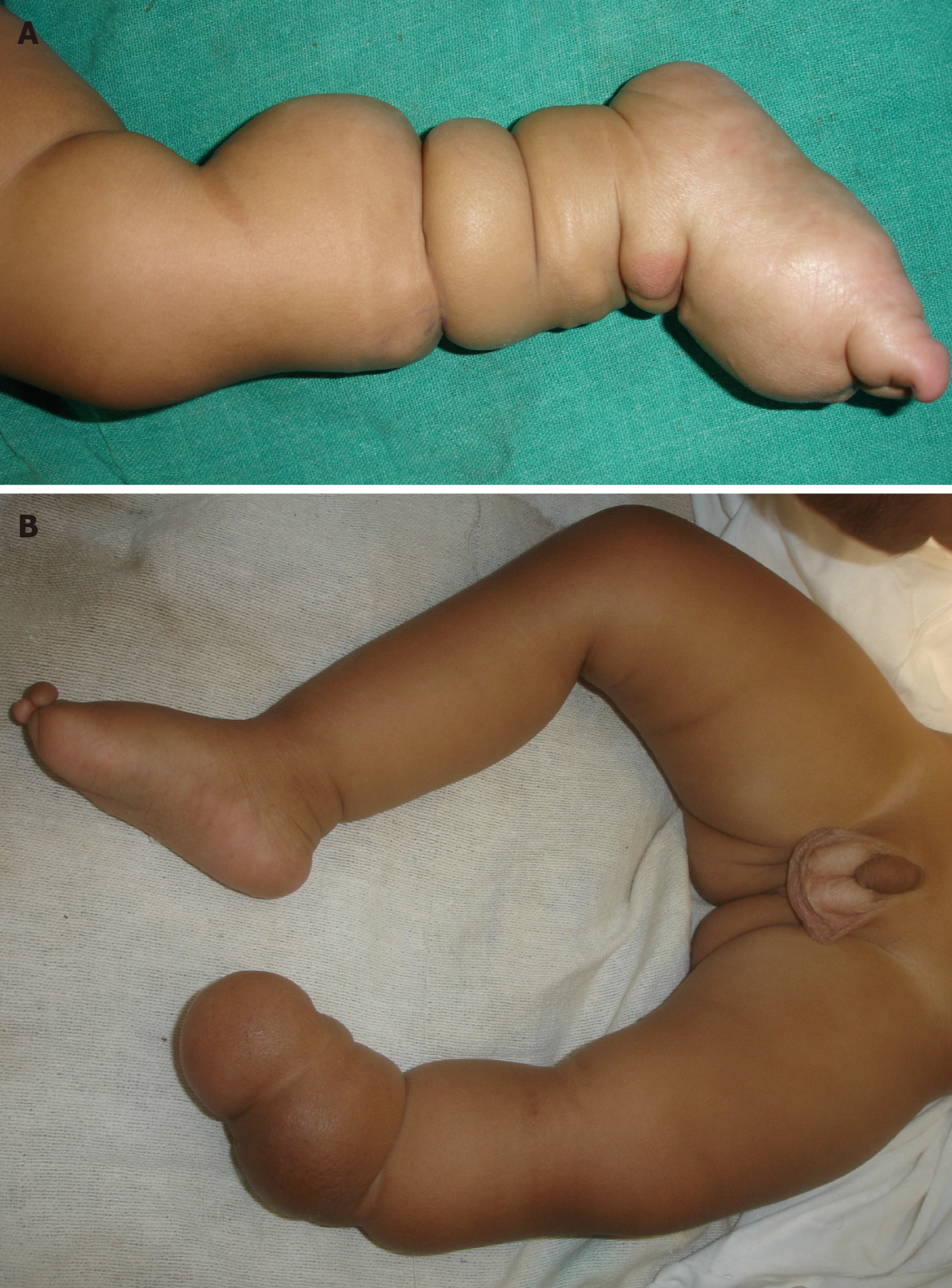
Figure 16 Constriction Ring Syndrome.
A: Multiple constriction rings in the lower limbs with lymphedema foot; B: Intrauterine amputation at level of distal left leg with proximal constriction rings seen.
Hypospadias
Hypospadias is a very common congenital anomaly affecting male children. The external urethral meatus, instead of being situated at the penile tip, terminates proximal to normal site on the under surface anywhere upto perineum. In addition, there is ventral bending or curvature of the penis, known as chordee (Figure 17). More proximal the opening, greater is the difficulty in passing urine in standing position without spoiling clothes. Similarly, greater the chordee, greater the ventral curvature of penis, especially during penile erection and thus, affecting sexual function in adults. Similar to other congenital anomalies, hypospadias may be an isolated problem or else may be associated with other congenital surgical anomalies like inguinal hernia, undescended testis or even intersex states[86]. The timing for operative intervention is controversial[87,88]. The considerations for operative timing arise from the functional reasons. Child becomes a subject of ridicule by the peers once he starts going to school and has to squat for urination. This has a lot of influence on psychosocial development of the child[89]. Gender identity, i.e., concept of being male or female, is well established by the age of 3-4 years with some awareness as early as 18 months[90]. Hence, corrective surgery must be over by 3 years of age. Early surgery also reduces the psychological stress of parents. The parents must be also be told that they should not get circumcision done because the preputial skin is required for urethroplasty. In adulthood, aesthetic problems may arise due to tell-tale evidence of surgical intervention or its deficiencies. Over a period of last several decades, there have been several improvements in surgical techniques. Psychological effects of operating on male genitalia after the age of memory recall (6 months) have also been studied greatly. Thus, the aim is an operation by single stage technique by 6-12 months of age[91,92]. In addition to above issues, another issue of great importance is that of surgeon being comfortable in performing surgery on very small tissues of genitalia, so-called technical considerations. Use of magnifying loupes, availability of finer micro instruments and sutures by some expert surgeons have made this technically feasible[93]. Many surgeons still prefer to wait till the age of 18-24 months (or even more) for performing urethroplasty so that larger tissues are available. The penis grows only moderately during the preschool period. Any delay beyond 3 years has no benefits to the surgeon during operation[94]. A few children may have associated meatal stenosis and may need meatotomy very early (may be at 3 months of age), if they are symptomatic (straining during micturition) and surgeon’s assessment finds meatal size to be the cause of problem.
Figure 17
Mid penile hypospadias with chordee in an infant.
Even after successful management, these patients must be followed till the adult age because the now grown-up patient may find some problems like scarred or tight skin, abnormal shape of glans, psychological issues etc. many of which may be treatable at this stage and improve the quality of life of the patient[95,96].
Vascular anomalies
The definitions, classification and treatment of vascular anomalies (vascular tumours and vascular malformations) has tremendously evolved over the last three decades[97]. They can occur in all parts of the body and are most common in head and neck region (Figure 18A and B). There is a very vast list of diseases which come under the category of vascular anomalies and obviously their treatment and need for surgical intervention cannot be same. Moreover, there is an extremely vast spectrum of severity with some just innocuous small lesions (Figure 18C) requiring no treatment to ones which have great morbidity and even risk to life. Hence, timing of surgical intervention has to be assessed by the surgeon taking all the factors into consideration. They are found in the internal organs as well. Thus, specialists of multiple disciplines like general surgery, plastic surgery, dermatology, neurosurgery and otorhinolaryngology etc. treat them and need coordination in their management[98]. The child needs treatment not only to minimize the physical side effects of vascular anomalies but also for aesthetic reasons.
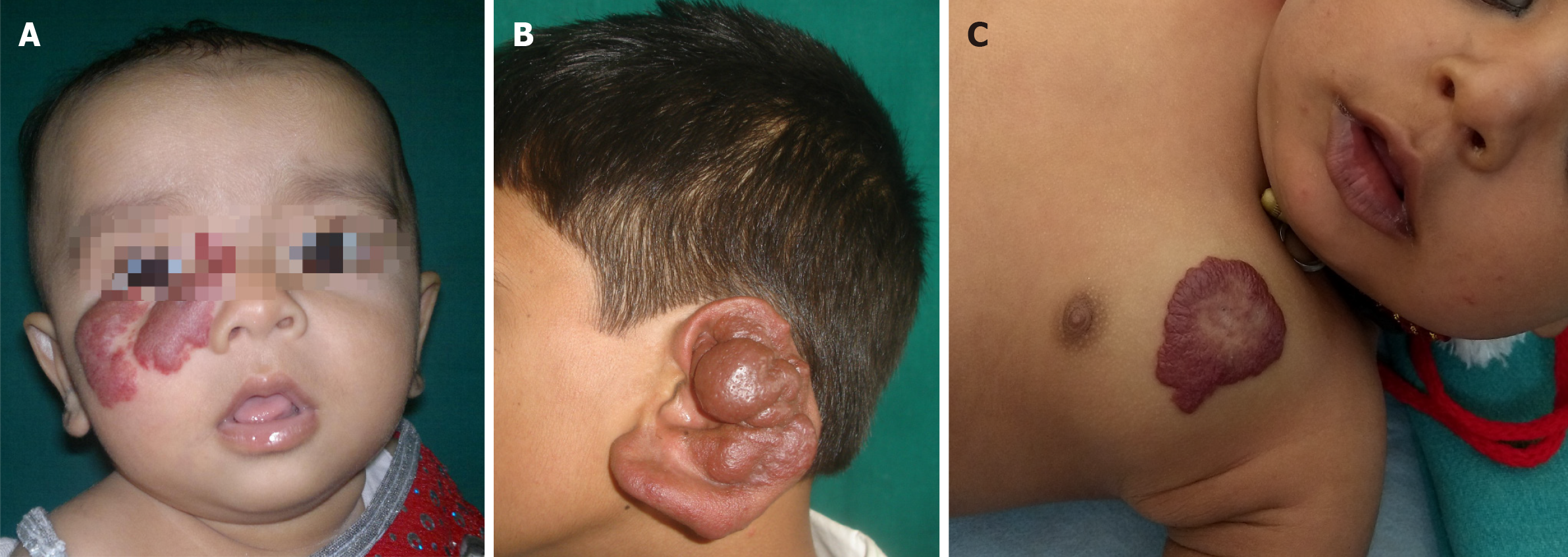
Figure 18 Vascular anomalies.
A: A hemangioma on the face of an infant. Proliferation of the lesion can cause obstruction to vision in the right eye, may need propranolol therapy to expedite involution; B: A pulsatile arterial malformation of the ear in a teenager, increasing in size- needs embolization and surgical intervention; C: An innocuous hemangioma on the chest- can follow the wait and watch policy for such a lesion.
Classically, infantile haemangiomas have been seen to proliferate initially over 9-12 months of age and then regress or involute over a period of time in almost 70% of children by 7 years of age. In those resolved completely of their own, some evidence is left in the form of atrophic scar etc. Thus, traditionally they have been managed with ‘wait and watch policy’ or the so-called ‘masterly inactivity’[99]. However, over a period of last few decades, several effective medical therapies have come up which are being used by surgeons to expedite this maturation and involution process[98,100]. During this waiting period stretching over several years, the parents of these children also need to be counselled and photographic records of the lesion taken at regular intervals be kept by the clinician as well as parents to see the progress (Figure 19). At the same time, the surgeon must show the pictures of children under his/her care showing spontaneous resolution to the parents to gain their confidence. Thus, at present, ‘masterly inactivity’ only refers to non-surgical management. Some children do need surgical intervention earlier because of functional reasons or recurrent ulceration/bleeding episodes. Those in the oral cavity and respiratory tract may obstruct airways and be life-threatening. Those present over the periorbital region may obstruct vision and lead to deprivation amblyopia. Nostrils may also get blocked. Laser therapy is effective in the management of many cases. Surgical intervention is best done for residual deformities and scars left after spontaneous resolution or laser treatment. Surgical excision is also sometimes done for very small lesions or those which may be better or equally well treated instead of expectant policy[101,102], especially with respect to resulting scar/residual lesion.
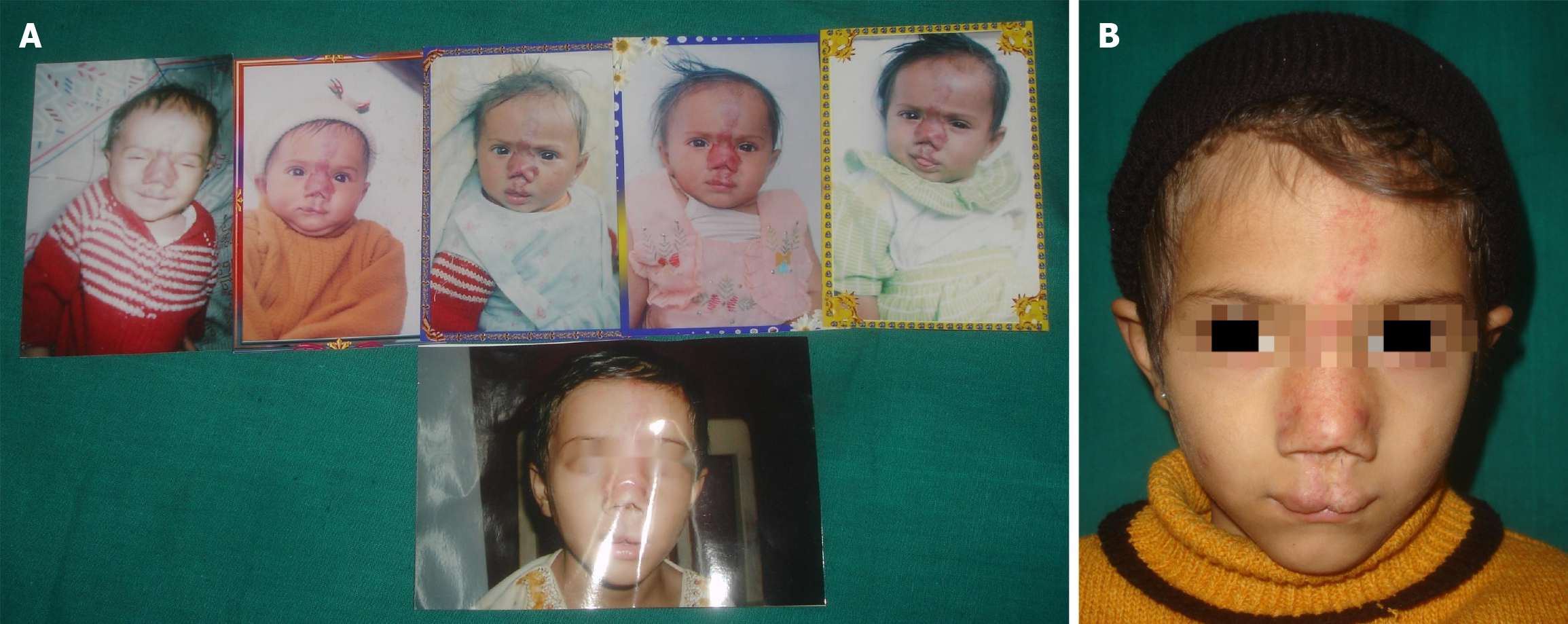
Figure 19 Congenital infantile hemagioma.
A: Serial photographic record of a child with vascular lesion on the face; B: Final photograph at 10 years of age with residual scarring.
There is a very wide spectrum of vascular anomalies from those with purely cosmetic considerations (e.g., Port-wine stain) to massive arteriovenous malformations with functional consequences and risk of massive haemorrhage. A very large spectrum of treatment modalities, e.g., sclerosant therapy and lasers etc. are available. Interventional radiology has been used to block the feeder vessels by embolization followed by surgical excision.
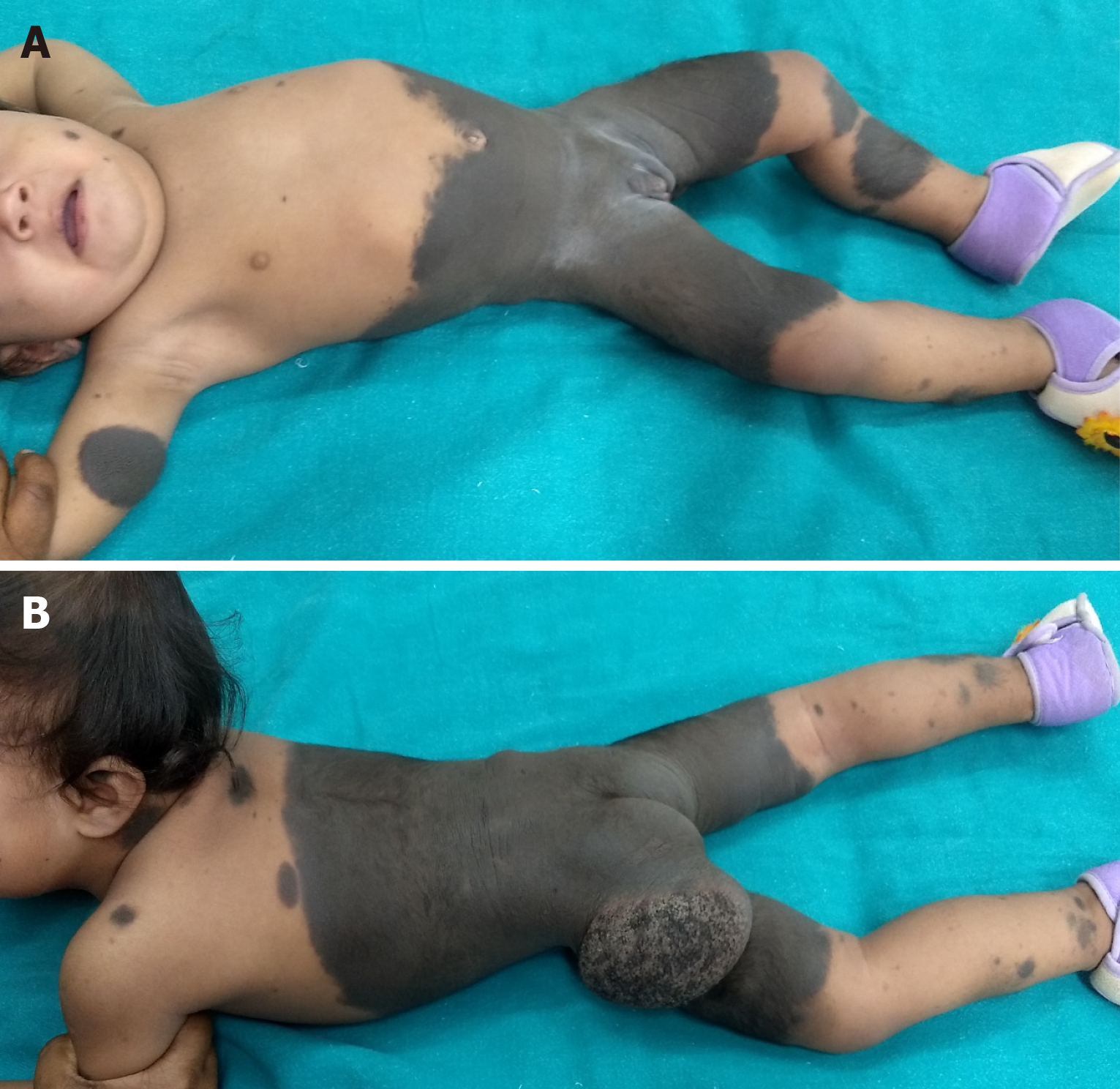
Giant hairy/non-hairy Nevi
Giant congenital hairy/non-hairy nevi are melanocytic nevi which are rare but very important not only because of their association with risks of malignant transformation (malignant melanoma) and central nervous system involvement but also because of their major psychosocial impact on the child and his/her parents due to their ghastly appearance (Figure 20). These children become a subject of ridicule by their peers in school who often call them ‘bear’ because of their skin colour. Although skin of any part of body can be involved, these are frequently seen in trunk, followed by extremities and head and neck. More than one body region may be affected. In addition, there may be multiple satellite lesions of varying sizes all over the body (Figure 21). Some peculiar locations have led to various terms like ‘bathing trunk nevus’ (Figure 22), ‘coat sleeve nevus’ etc. Various definitions of their size have been given by different workers[103]. Thus, giant nevi present aesthetic as well as risk to malignant change as considerations for timing of surgical intervention.
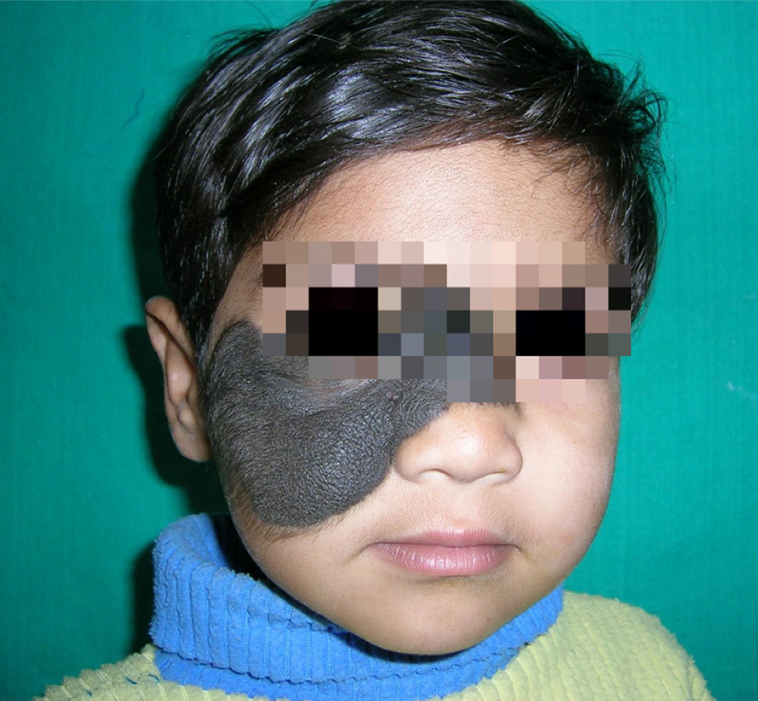
Figure 20
Congenital hairy nevus in a preschool toddler with a bear-like appearance- a huge stigma.
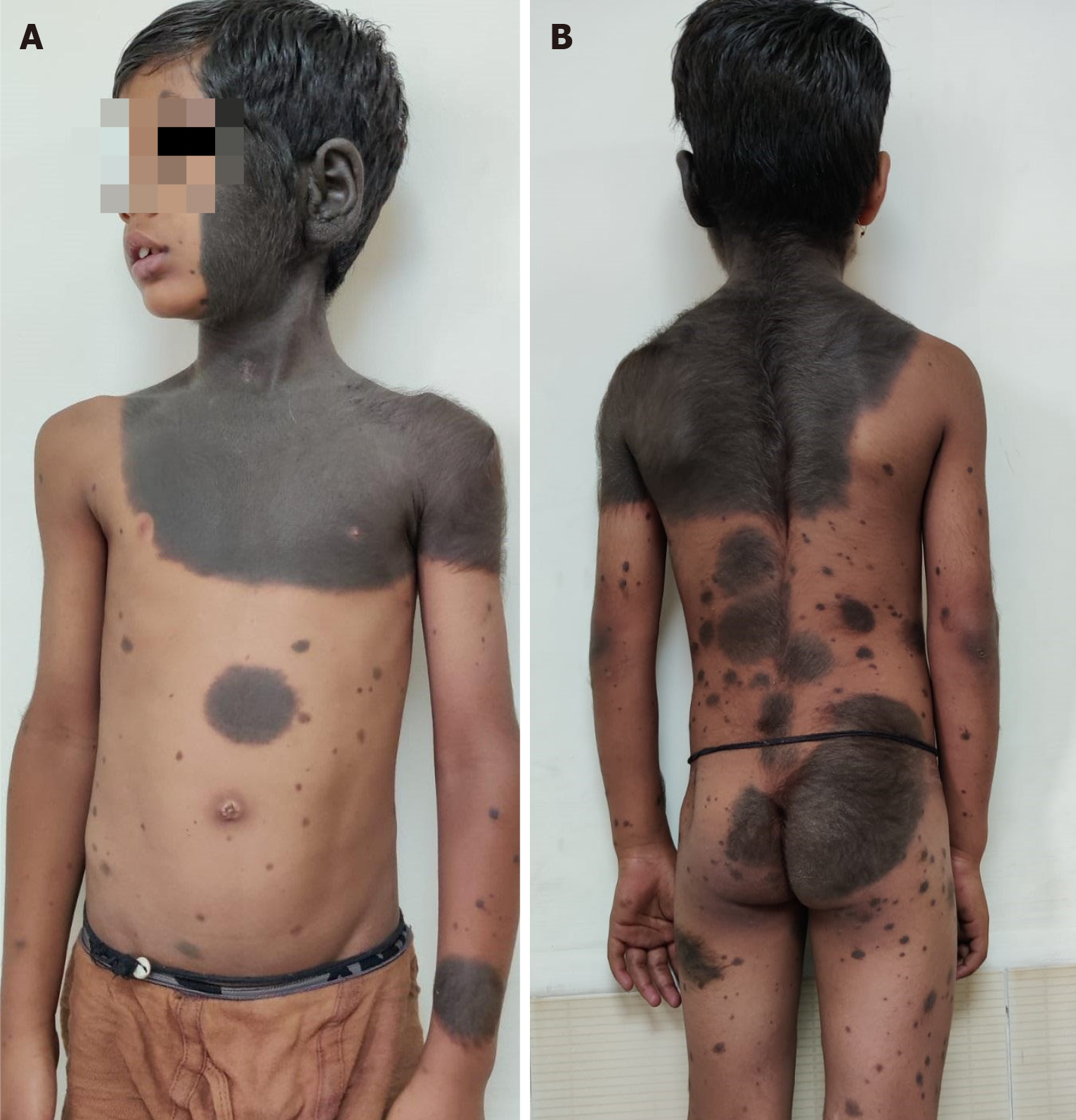
Figure 21 Giant hairy nevus.
A: A child with extensive involvement of the head and neck, trunk with multiple small and large satellite hairy nevi; B: Back, buttocks and limbs also have extensive involvement.
Figure 22 Bathing trunk nevus with satellite lesions.
There is not enough donor area for complete excision and resurfacing. A: Supine view; B: Prone view.
The exposed parts of body constitute an absolute indication for their excision to restore psychological well-being. Development of nodularity, repeated excoriation, ulceration and infection are also indications for excisional therapy. Complete armamentarium of a plastic surgeon is required in their management depending on their size, site and depth of involvement etc[104,105]. Some of the treatment modalities, e.g., dermabrasion, skin curettage, shave excision or lasers improve the appearance but since the complete burden of nevus cells is not taken care of, the risk of malignant change persists[106-108]. Hence, these patients must be under constant observation of clinician and must be taught to look for abnormal features and report them as soon as possible for confirmation or otherwise.
The risks of malignant change are different in different population groups. It has been seen to be very high in Caucasians while it is practically nil in Indian subcontinent. This is a very important consideration for surgical intervention. The risk of malignancy, which some workers have found to be 50% within first 3 years of life and 70% by 13 years in Caucasians, is the most contentious issue in management and demands complete excision of all the lesion as soon as possible[109]. However, the massive size of the lesion and the paucity of donor skin areas may not allow complete excision. In low/minimal risk population, these lesions need excision for their aesthetic considerations alone. In such a scenario, one would definitely like to make the child lesion free before going to school. However, for several massive lesions it may not be practically possible as they require multiple stages. Partial excisions in non-exposed areas may be acceptable as residual lesion is not likely to turn malignant. The first operation may be done at 6 months. It must be remembered that some of the best techniques of resurfacing after excision may not be possible in very small children. Thus, the lesion may have to be excised completely preventing risk of malignancy and removing the title of ‘bear’ to the child and cover provided with split skin graft before the child goes to school. The definitive resurfacing with tissue expansion or appropriate free flap can be provided later in teenage/adulthood. Once malignant melanoma is diagnosed, appropriate anti-cancer therapy needs to be instituted.