Published online Mar 9, 2024. doi: 10.5409/wjcp.v13.i1.89580
Peer-review started: November 27, 2023
First decision: December 17, 2023
Revised: December 26, 2023
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: March 9, 2024
Processing time: 96 Days and 19.2 Hours
Eosinophilic esophagitis is a newly recognized disease first described about 50 years ago. The definition, diagnosis, and management have evolved with new published consensus guidelines and newly approved treatment available to pediatricians, enabling a better understanding of this disease and more targeted treatment for patients. We describe the definition, presentation, and diagnosis of eosinophilic esophagitis including management, challenges, and future directions in children. The definition, diagnosis, and management of eosinophilic esopha
Core Tip: Eosinophilic esophagitis is a newly recognized disease described in the last 50 years. The definition, diagnosis, and management have evolved with new guidelines and medications available to allow for better understanding and treatment of pediatric patients. We describe the definition, presentation, diagnosis, and management including new biological treatment, long-term follow-up and the challenges of eosinophilic esophagitis in children. We discuss new management strategies and new future directions of monitoring eosinophilic esophagitis in children.
- Citation: Elghoudi A, Zourob D, Al Atrash E, Alshamsi F, Alkatheeri M, Narchi H, Bitar R. Evolving strategies: Enhancements in managing eosinophilic esophagitis in pediatric patients. World J Clin Pediatr 2024; 13(1): 89580
- URL: https://www.wjgnet.com/2219-2808/full/v13/i1/89580.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v13.i1.89580
Eosinophilic esophagitis (EoE) is a persistent inflammatory disorder of the esophagus that arises in individuals with a genetic predisposition and is not associated with immunoglobulin E (IgE) mediation[1]. This condition ranks as the second most commonly encountered etiology of chronic esophagitis, behind gastroesophageal reflux disease. Furthermore, it is the primary culprit responsible for dysphagia among the pediatric and young adult population[2].
The initial instances of this condition were documented in the late 1970s and early 1980s[3-5]. Subsequently, the inaugural consensus guidelines outlining the diagnostic and therapeutic approaches for EoE were introduced in 2007, with subsequent updates provided in 2011[1,6]. The current guidelines establish the criteria for EoE as a persistent clinicopathological condition marked by the presence of eosinophils within the esophageal epithelium at a density of 15 or more eosinophils per high-power field (hpf), coupled with symptoms of esophageal dysfunction, after excluding other potential causes of esophageal eosinophilia[1].
In recent decades, EoE has emerged as a prevalent source of morbidity within the upper gastrointestinal (GI) tract, affecting both pediatric and adult populations. The epidemiology of EoE has experienced a substantial upswing, closely mirroring the heightened awareness and understanding of this condition. Most epidemiological data stem from population-based studies, primarily conducted in North America and Europe[2,7,8]. In developed nations, the incidence rates and prevalence of EoE in children exhibit geographic variation[7]. Incidence figures span from 2.1 cases per 100000 individuals annually in the Netherlands to 12.8 cases per 100000 individuals annually in Ohio, United States[9,10]. EoE can manifest at any age and is more prevalent in males[11]. It exhibits a strong connection with other atopic conditions including asthma, eczema, rhinitis, and food allergies.
EoE has a substantial effect on the daily lives of affected individuals. The symptoms have a noteworthy impact on school performance and participation in educational activities. While therapies may result in a better quality of life with reduced symptom severity, they may also introduce challenges in terms of dietary restrictions, potentially influencing overall quality of life.
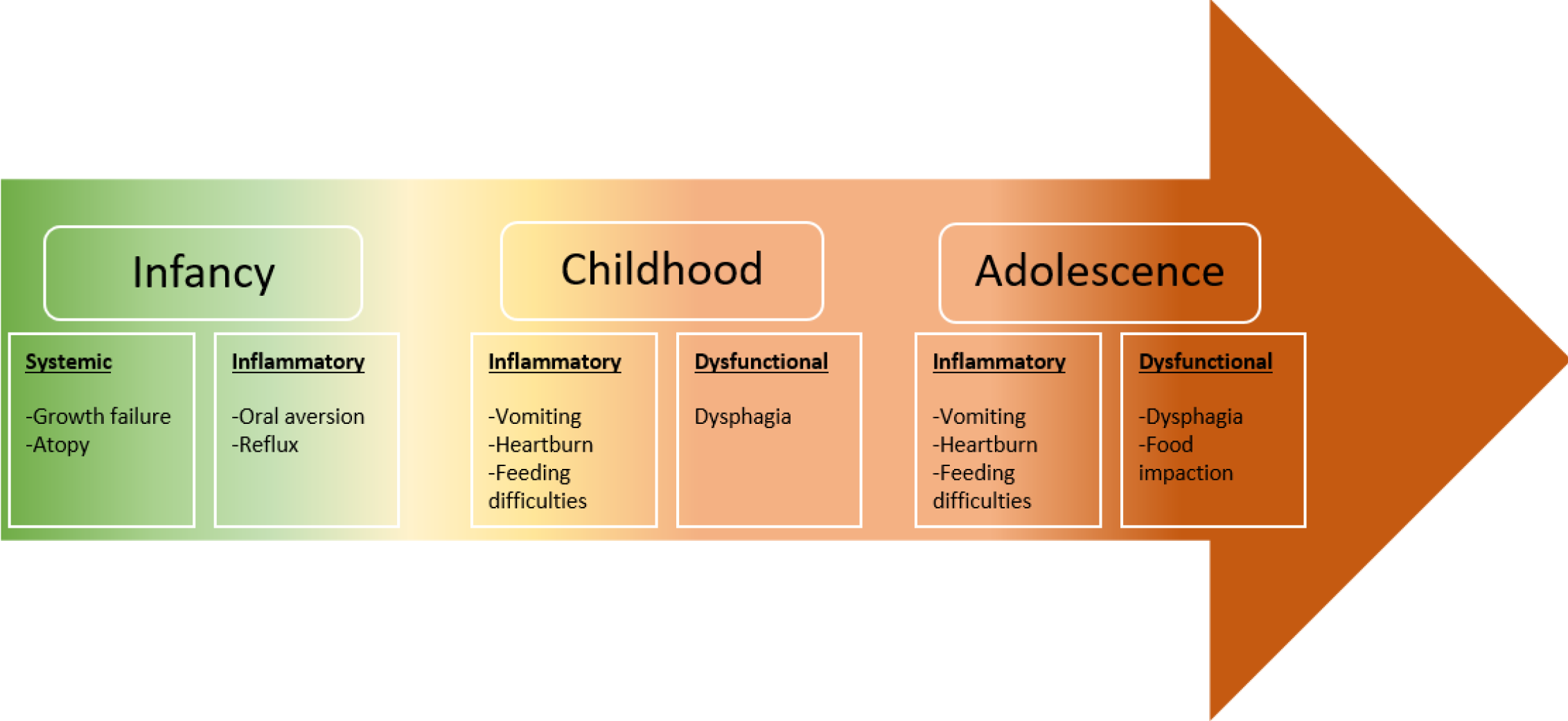
Symptomatology in EoE can be widely variable, reflecting esophageal dysfunction and inflammation[12]. The esophagus is an obscure organ within the thoracic cavity, manifesting its ailments as vague symptoms and rarely results in specific physical signs[13]. The developmental stage of the child impacts the clinical manifestations; while adolescents typically exhibit symptoms akin to those observed in adults, such as dysphagia to solids and food impaction, younger patients tend to manifest more general and nonspecific complaints. These include abdominal or chest discomfort, vomiting, feeding difficulties, or extreme failure to thrive[12-14]. Interestingly, most children develop coping mechanisms, so feeding difficulty tends to be subtle and requires careful history to elicit positive findings[12,14]. Therefore, refusal to eat, inability to progress from liquids to solids, selective avoidance of hard consistencies after successfully tolerating a variety of solids, exaggerated chewing, prolonged meal time, or drinking excessive water to facilitate swallowing are all well-recognized patterns of coping mechanisms[12,13]. Another point worth discussing is that progressive symptoms are common and correlate strongly with transitioning from an inflammatory phenomenon in early childhood to a fibrostenotic remodeling process that significantly hinders esophageal compliance and function[13]. The relationship between symptomology progression and age is outlined in Figure 1.
Multiple symptoms can coexist, and some overlap with other diseases such as gastroesophageal reflux[15]. Subse
The expanding research and literature available on EoE in the past 20 years has shaped our better conceptualization of the pathophysiology and natural history of EoE. Nevertheless, due to the nonspecific clinical picture in young children, EoE remains a diagnostic challenge to pediatricians who need a vigilant correlation between symptoms and endoscopic and histologic findings[12,13,15,16]. Consequently, an average 3 years to 5 years delay between symptoms onset and diagnosis was reported in one review, which was associated with a parallel increase in fibrostenotic complication rates[12]. Accordingly, guidelines have been revised several times to guide clinicians and help bridge this gap[12,17].
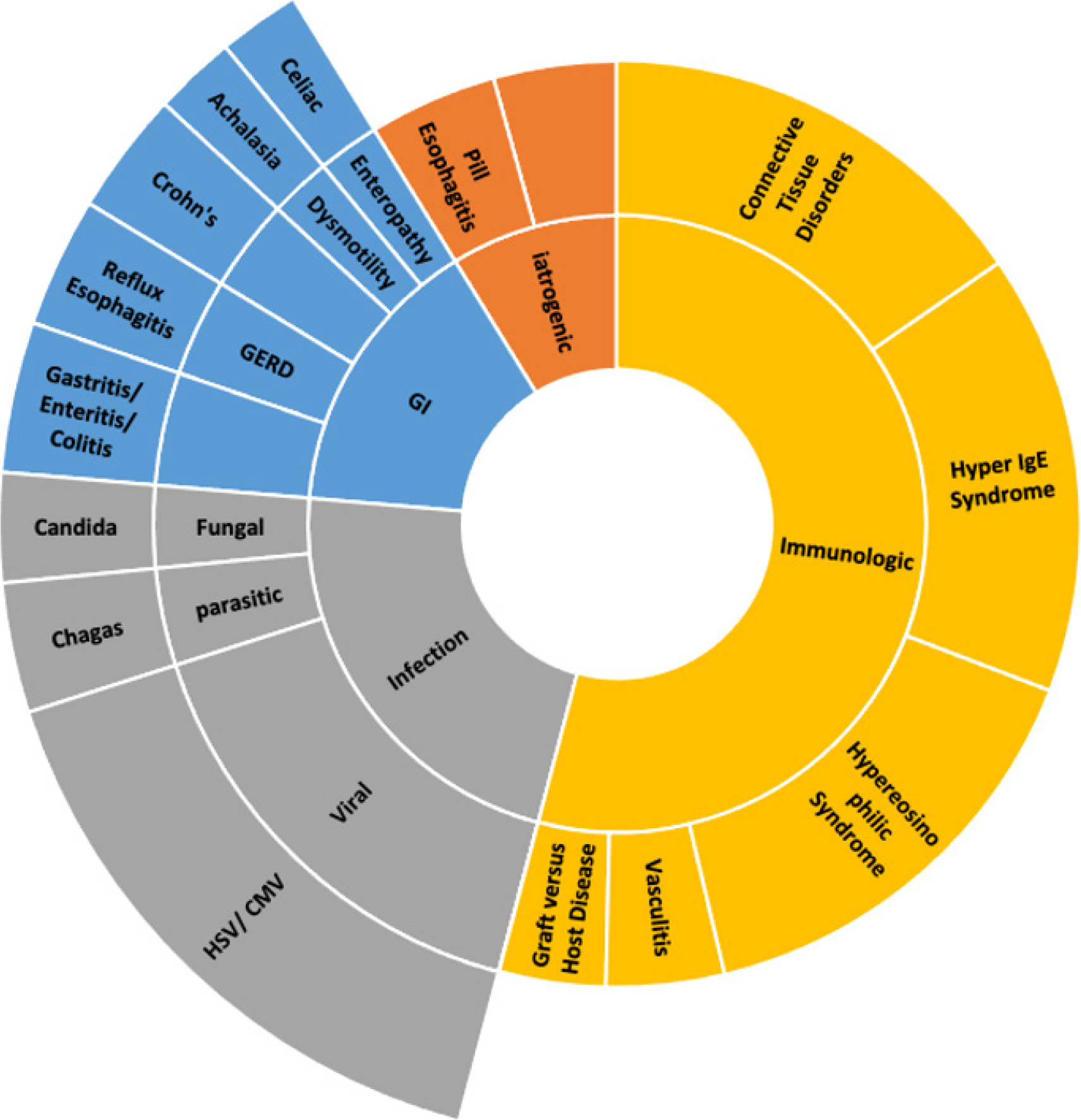
The consensus outlines the definition of EoE as a clinicopathologic entity, with evident esophageal impaired function, combined with histologic proof of esophageal eosinophilic predominant inflammation after ruling out other causes of eosinophilia (Figure 2)[12,13,15]. Upper GI endoscopy is the modality of choice for the gross examination of characteristic mucosal changes, obtaining biopsies for histological confirmation, and excluding other pathologies of the esophagus[12,13,15,17]. Endoscopically, features of inflammation or fibrotic transformation features such as furrows, exudate, edema, rings, trachealization, and stricture, can be observed. The endoscopic findings of Edema, Rings, Furrows, Exudate, and Stricture (ERFES) are given a grade and have been grouped into a scoring system called EoE ERFES scoring system to aid in the diagnosis of EoE and monitoring patients’ disease progression. However, confirmation requires histologic evaluation[14]. It is crucial to remember that as many as 17% of individuals with EoE may exhibit normal endoscopic findings. Therefore, it is recommended that for all pediatric patients with nonspecific upper GI symptoms severe enough to necessitate endoscopic examination, esophageal biopsies need to be collected[12,15]. A minimum of six biopsies should be obtained from various levels spanning from the proximal to the distal esophagus, focusing on areas of inflammation to enhance the likelihood of positive findings in the microscopic examination[12,13,15].
In histopathological assessment, eosinophilic infiltration is confirmed when it attains or surpasses a threshold of 15 eosinophils per hpf, excluding eosinophilia in the stomach or duodenum is a pre-requisite for diagnosing EoE[12,15,17]. Apart from mucosal esophageal eosinophil density, noteworthy histological characteristics in EoE encompass basal cell hyperplasia and the presence of eosinophilic layering[12].
The British Society of Pediatric Gastroenterology, Hepatology, and Nutrition revised its guidelines in 2022. It is recommended to stop PPI, if prescribed previously for the patient, for at least 3 wk before endoscopy[15]. The objective is to identify a subgroup of EoE patients referred to as PPI-responsive eosinophilia[12,15]. To elaborate further, PPI is uniquely anti-inflammatory in EoE by blocking interleukin 13 (IL-13)-mediated inflammatory cascade, masking the significant histological changes[12,15].
Additional laboratory testing, such as serum IgE levels and eosinophilic count, might serve as a clue to the atopic tendency of the patient. However, it has no diagnostic weight. Allergic testing could be sought and guided by an allergist based on initial assessment[15].
The multifactorial pathophysiology of EoE implicates a role in genetic susceptibility. Supporting evidence for the former can be found in the elevated risk observed among first-degree relatives of individuals with EoE in developing the condition[13]. Therefore, researchers have been adamant about mapping novel genetic loci involved in EoE[13,17]. A breakthrough finding revealed a distinctive genetic expression pattern in esophageal biopsies of EoE patients, involving 96 genes, differentiating their biopsies from non-EoE[13,17]. This work and later research confirmed that the degree of genetic expression is directly related to the abundance of inflammatory cells and the presentation of its relevant genetic material in biopsy samples[12,13].
Genotyping can also be utilized in stratifying patients with EoE into inflammatory predominant or fibrostenotic phenotype[13]. However, evidence has yet to be validated through future research to guide the implementation of such a sophisticated molecular testing strategy[13,16].
Treatment options for EoE include dietary elimination, and drug treatment in addition to esophageal dilatation in some advanced cases. Some scholars came up with the abbreviation of 3 ”D” S, representing Drugs, Diet, and Dilatation[16]. Over the past year, dupilumab, a new biologic, a human monoclonal antibody that targets the IL-4 receptor alpha subunit of heterodimeric IL-4 and IL-13 receptors, has become the first United States Food and Drug Administration (FDA)-approved biologic treatment for EoE in adult and adolescent populations aged 12 years and older. EoE is one of those diseases that needs an individualized treatment plan for every patient. The decision of which treatment to start depends on the clinical picture, severity, practicality/suitability, other comorbid allergic disease, and the patient or parents' choice. Clinicians usually decide on the treatment on a case-by-case basis. Hence, the treatment formulation will rely on mutual agreement between the child or the child’s family and the treating clinicians. Efficient treatment will help ease the signs and symptoms and reverse inflammatory damage to the esophagus and help the clinician liberate the child’s diet, which could positively impact the nutritional status and well-being of the child[18]. Patients will benefit from being cared for by a multidisciplinary team, which should include the treating physician or the gastroenterologist, allergist, psychologist, general pediatrician, and dietitian to support patient care, avoid any conflicts regarding the advice given, and reduce the number of clinic visits. Gastroenterologists will lead the investigations, endoscopies, and treatments and perform esophageal dilation when needed. Allergists work with gastroenterologists to provide comprehensive care for patients with EoE and recommend elimination diets and treatments. Allergists also investigate the atopic co-morbidities common with EoE and provide additional treatment as necessary[15]. Dietitians are vital in arranging individualized dietary plans, providing replacements for the eliminated food groups, and ensuring that patients receive appropriate nutritional intake. General pediatricians can follow the child's growth, nutritional status, and general well-being. Nevertheless, a psychologist can help patients and their families manage the psychological concerns of the disease.
Dietary therapy is based on EoE being a non-IgE-mediated food allergy. Thus, eliminating certain allergenic foods could induce disease remission. Three different methods are used in dietary therapy, full elemental diet, six food exclusion diet, and gradual food elimination diet. An elemental diet is administered by providing an elemental liquid formula; this formula should encompass all essential nutrients including carbohydrates, fats, vital minerals, and vitamins[13]. A systematic review by Rank et al[19] involving 431 patients, concluded that histological remission was achieved in 93.65% of patients on elemental diet, in contrast to the control groups in the studies where only 13.3% attained remission. This approach is generally not preferred for most children and care givers, because elemental milk has an unfavorable taste and can be relatively expensive. However, it remains a good option in infants with EoE especially that they are mostly dependent on milk for the 1st few months of their life. It is important to note that children with non-IgE-mediated food allergies have a potential risk of developing IgE-mediated allergies to the food eliminated, especially if food is excluded from their diet for an extended duration[19]. Therefore, allergy testing is required before introducing the common allergenic foods[20].
The most commonly recommended dietary therapy eliminates the six common allergenic foods commonly linked to food allergy in EoE. These foods are ranked based on their allergenicity as follows: cow's milk, wheat, egg, soy, peanuts/tree nuts, and fish. As expected, this six-food elimination is more effective than gradual food elimination, where one or two foods are eliminated for up to 12 wk, followed by endoscopy. However, six-food elimination is very challenging, especially for children, where compliance becomes an issue. Gradual food elimination diet focuses on excluding one or two types of food, which are thought to be the expected foods causing the patients eosinophilic esophagitis after thorough assessment by the pediatric allergist and gastroenterologist while considering starting with the most allergenic foods such as cow’s milk and wheat followed by egg and Soya and finally peanuts/tree nuts and fish. It is vital to explain to the patient/carer that exclusive elemental diet, six-food elimination or gradual elimination required repeated endoscopy to document remission before food(s) are reintroduced in a reverse order of elimination based on the level of allergenicity. In the six-food elimination diet, fish and shellfish are introduced first, while cow’s milk is usually the last to be reintroduced[21]. It is essential that a pediatric dietician is involved in the patient medical care to perform a comprehensive nutritional assessment and replacement of all deficient nutrients in the child’s diet.
The medications that can be used for controlling inflammation in EoE include PPIs, corticosteroids, and dupilumab. PPIs are used for 8 wk as a therapeutic option for treatment. Numerous studies have substantiated its effectiveness as a primary therapeutic choice for managing EoE. In addition, it is used by some clinicians to rule out conditions such as reflux esophagitis, especially in children whose endoscopy is not done or delayed for different reasons. In a systematic review by Rank et al[19] with 1051 patients proved PPI's therapeutic effectiveness and efficacy.
Corticosteroids are known for their potent anti-inflammatory effect. They can be swallowed as a thickened solution or from the metered dose inhaler device. A systematic review encompassing trials demonstrated that topical corticosteroids led to histological remission in 64% of patients, a significant contrast to the 13.3% remission rate observed in the control groups of the study [confidence interval (CI:) 0.85-1.19][19]. Topical corticosteroids are proven to be very safe in children. Transient oral candidiasis is probably the most reported side effect reported. This can be avoided by mouth washing after administering swallowed corticosteroid treatment.
In May 2022, dupilumab was approved by the FDA as the first and only drug specific for treating EoE for children aged 12 and above[22]. The biological drug is still not commonly used in children, and it is not clear where it fits in the stepwise approach for treating EoE in children. Nevertheless, the FDA's endorsement of it as the initial sanctioned treatment for EoE supports its consideration as a first-line medication. On the other hand, as it is a novel drug, certain healthcare providers contemplate its usage in instances where traditional therapeutic agents fall short in achieving both clinical and histological remission. Dupilumab is not a new medication; it has an established role in the management of moderate to severe asthma, challenging cases of atopic dermatitis, and chronic sinusitis with polyps in children. The Joint Task Force of The American Academy of Allergy, Asthma, and Immunology, American College of Allergy, Asthma, and Immunology, along with the American Gastroenterology Association, have recently issued expert opinion guidelines for the treatment of EoE. This paper guides healthcare professionals on using dupilumab in treating patients with EoE. It emphasizes the significance of the FDA approving dupilumab as the initial and sole pharmaceutical intervention for EoE management[20]. Furthermore, it states that dupilumab is to be considered in patients who refuse or are unsuitable for food elimination and swallowed corticosteroids, patients with esophageal dilatation, those who developed side effects to ongoing treatment, patients with strictures or narrow caliber esophagus, and patients with refractory EoE[22]. Dupilumab is currently licensed for 12-year-old children and above, and it has received FDA approval for use in Children over 1 year old with eosinophilic esophagitis in January 2024. However, in atopic dermatitis, it is licensed for treating children from 6 mo of age.
Mepolizumab is a biologic agent developed to treat asthma. It represents a humanized monoclonal antibody of immunoglobulin G1 κ type, which targets human IL-5 and thus prevents its interaction with the α-chain of the IL-5 receptor. A larger multicenter parallel group trial also demonstrated reductions in both peak and mean esophageal eosinophil counts of greater than 50% after receiving three doses of mepolizumab in up to 77% of patients. In addition, peripheral blood eosinophil counts were also decreased. However, most mucosal biopsies did not return completely to normal (defined as fewer than four eosinophils per hpf for this study)[15]. Although it has shown promise in reducing both peak and mean esophageal eosinophil counts it has not been FDA-approved for treating eosinophilic esophagitis in children. Reslizumab was approved by the FDA in March 2016, and is indicated as an add-on maintenance therapy for adults with severe asthma with an eosinophilic phenotype. Reslizumab is a humanized monoclonal antibody that occupies the region glutamic acid, arginine, arginine, and arginine corresponding to amino acids 89–92 on IL-5, which is a region critical for its interaction with the IL-5 receptor on the eosinophil surface. Although reslizumab improves patients’ clinical symptoms, the effect on lowering esophageal eosinophil level is modest. Other biologic agents including malizumab, cendaakimab, lirentelimab are potential treatments that may be used in the future to treat patients with eosinophilic esophagitis.
With the progression of eosinophilic esophagitis, children may develop esophageal narrowing, loss of elasticity, and stricture formation, especially when the inflammatory component of the disease advances to fibrosis. If the narrowing is not improved with medical treatment or is severe, esophageal dilatation may be required to improve patient symptoms. In addition, dilatation can also be used to release food bolus obstruction in the acute setting. Although esophageal dilatation can offer immediate symptom relief of dysphagia, esophageal dilatation cannot be considered an isolated treatment for EoE and must be used with appropriate medical therapy. Esophageal dilatation can be performed with balloon dilators or bougie dilators and is advised to be performed gradually with serial endoscopic sessions. There is not much data on esophageal dilatation in children, and most evidence is extrapolated from adult studies. The meta-analysis conducted by Dougherty and colleagues encompassed 37 studies, comprising 3 focused on pediatric populations and 2 encompassing children and adults[23].
A total of 977 patients underwent a combined 2034 dilations using either balloon or bougie dilators. Of these patients, 87% reported ameliorating dysphagia symptoms following esophageal dilation, although no concurrent improvement was observed in esophageal eosinophilia. Nine perforations were recorded during the procedures at a rate of 0.033% (95%CI: 0%-0.226%) per procedure. Notably, none of these perforations necessitated surgical intervention or led to any mortalities. Furthermore, a comprehensive systematic review and meta-analysis, encompassing 27 studies involving 87 pediatric patients of 845 with EoE, collectively underwent 1820 esophageal dilations[24]. This group's median number of dilations was three (range: 1-35). Clinical improvement was observed in 95% of patients in 17 studies. Notably, perforation occurred in 0.38% (95%CI: 0.18%-0.85%; I2: 0% based on data from 27 studies), and hemorrhage was documented in 0.05% (95%CI: 0%-0.3%; I2: 0% from 18 studies). Encouragingly, there were no reported fatalities. Therefore, esophageal dilatation in EoE is considered safe by using balloon or bougie dilators and improves dysphagia in patients with esophageal strictures. Dilatation will reduce the narrowing of the esophageal diameter. Still, it will not treat the inflammation and thus needs to be combined with medical treatment such as medication or food exclusion.
The understanding of eosinophilic esophagitis is becoming increasingly evident in its natural progression. Pathogenesis starts with mucosal inflammation, leading to remodeling and fibrosis[17]. In a single center in Switzerland, two related studies were conducted on the progression of EoE in adults; these studies showed the persistence of dysphagia and eosinophilic inflammation, primarily observed in children, which subsequently progresses into subepithelial fibrosis in adults[25,26]. Therefore, EoE is classified as a chronic disease that can be persistent or relapsing, necessitating a comprehensive long-term monitoring and treatment plan.
The primary objectives in treating EoE are to effectively manage symptoms, mitigate the risk of complications associated with fibrostenotic remodeling, and improve patient quality of life[27]. To ensure treatment efficacy, it is advisable to schedule a follow-up endoscopy with esophageal biopsies within 6 to 12 wk after initiating therapy to evaluate the histological findings and adjust the treatment accordingly[1,28].
Given that the diagnosis relies on histology, symptoms, and endoscopic findings, a system has been implemented to evaluate the progress of patients within a spectrum that includes complete normalization, partial response, and nonresponse[29]. Patients are categorized to: (1) Nonresponders with persistent eosinophilia ≥ 15 eosinophils/hpf; (2) partial responders with reduced eosinophilia of 7-14 eosinophils/hpf OR 1-6 eosinophils/hpf; and (3) complete normalization with normal biopsy with < 1 eosinophils/hpf[29].
Clinicians and patients may face different challenges when managing eosinophilic esophagitis patients. Although diet therapy offers nonpharmacologic options for disease management, it can be difficult for patients to follow. This can result in noncompliance, isolation from peers, the extra cost of exceptional food alternatives, cross-contamination while preparing meals, or possibly nutritional deficiencies in poorly planned diets. In addition, repeated hospital visits and multiple endoscopies to identify the triggers during the elimination diet can lead to extra financial and psychological burdens on patients and their families. Symptoms do not fully reflect disease activity, which might demotivate patients during the lengthy period of food elimination and re-introduction. Also, clinicians may find difficulty in interpreting biopsies of refractory disease vs noncompliance to the elimination diet[30].
The potential consequences of untreated or undertreated eosinophilic inflammation includes the development of fibrosis, thickened esophageal walls, and the formation of strictures. Ultimately, these structural and functional changes can damage the esophagus[31] resulting in complications such as esophageal stenosis, food impaction, esophageal perforation, and malnutrition. These complications will certainly affect patient QoL[17]. EoE harms the health-related QoL (HRQoL) of patients and their families while also imposing a significant burden on the healthcare system. Although there are limited data available, the currently accessible treatments seem to positively impact HRQoL[32]. Therefore, it is crucial to incorporate psychosocial support into patient care by actively engaging both the patient and their family in group support. Additionally, it is essential to ensure that they are well-informed about the appropriate resources available for EoE.
In addition to the well-established and approved biological treatment, dupilumab, some other drugs are now being explored for managing EoE and targeting type 2 inflammatory response with different cytokine pathways associated with EoE pathogenesis. Hirano et al[33] investigated anti-IL-13 with half of the patients having steroid-refractory disease. The trial demonstrated reduced eosinophil counts and the patient’s reported disease severity with improvement in dysphagia symptoms, but it lacked statistical significance.
Also, a randomized control trial with chemoattractant receptor-homologous molecule expressed on Th2 cells antagonist showed a statistically significant reduction in eosinophil count compared to placebo[34]. Compared to placebo, anti-IgE monoclonal antibody (omalizumab) showed no substantial improvement in clinical symptoms or decrease in eosinophil count[35].
Other new therapeutic drugs under investigation include Siglec-8 blockers (lirentelimab). The KRYPTOS trial (Phase 2/3) showed that lirentelimab achieved a statistically significant eosinophil reduction. Despite improving dysphagia symptoms compared to placebo, it did not meet the primary endpoint of the change in daily Dysphagia Symptom Questionnaire score[36].
The new therapeutic options highlight the need for further comprehensive trials. Nonetheless, it provides hope for potentially treating many atopic conditions, including EoE, with a single medication.
EoE necessitates multiple endoscopies for diagnosing and monitoring the disease. Less invasive monitoring tools are needed. New minimally invasive methods are now being studied to assess EoE disease activity. Cytosponge is a sponge-containing capsule after being swallowed it collects esophageal tissue as it is pulled back, offering an easy method to assess EoE inflammatory activity[37]. Another monitoring tool currently being assessed is the Esophageal String Test, which assesses eosinophil-derived granule proteins from secretions for the esophagus that stick to the string as it is removed[38].
Additionally, a new way to perform serial endoscopies is through a transnasal endoscopy. It provides an effective and less invasive test that can be done without sedation while maintaining a visual assessment of the esophagus and histopathologic testing[39]. These investigations are still in the initial stages of assessment and need further validation and evaluation of effectiveness.
EoE is a persistent clinicopathological condition distinguished by eosinophilic infiltration within the esophageal epithelium, typically exceeding 15 eosinophils per hpf. This manifestation of EoE is associated with esophageal dysfunction symptoms, following the exclusion of alternative causes of esophageal eosinophilia[1]. Thorough clinical assessment and histological endoscopic biopsy remain the mainstay of diagnosis. Treatment is best achieved in a multidisciplinary setting after appropriate counseling. There is hope for targeted biologic therapy; these treatments are still in their infantile stages and more studies are required. New minimally invasive methods are being evaluated to aid in the monitoring of patients. However, endoscopy and tissue histology remain the only methods to assess response to treatment and monitor disease.
EoE continues to be a fascinating disease for clinicians. Despite the consensus and guidelines published on managing children with EoE[1,15,19,20,30], many unanswered questions still need to be answered, such as: What is the actual prevalence of the disease? What is the disease's natural clinical progression, and is complete recovery possible for patients? Which factors contribute to the development of fibrotic disease? What constitutes the most effective treatment approach? Lastly, what potential long-term side effects are associated with current biologic therapy? We hope to have readily available biomarkers that can help assess patients for response to treatment. Much research is still needed to answer all the remaining questions related to EoE.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Sheikh Khalifa Medical City, 10106; British Society of Pediatric Gastroenterology, M1458.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Arab Emirates
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozen H, Turkey S-Editor: Liu JH L-Editor: Filipodia P-Editor: Zhao YQ
| 1. | Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES, Dohil R, Falk GW, Gonsalves N, Gupta SK, Katzka DA, Lucendo AJ, Markowitz JE, Noel RJ, Odze RD, Putnam PE, Richter JE, Romero Y, Ruchelli E, Sampson HA, Schoepfer A, Shaheen NJ, Sicherer SH, Spechler S, Spergel JM, Straumann A, Wershil BK, Rothenberg ME, Aceves SS. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3-20.e6; quiz 21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1605] [Cited by in RCA: 1485] [Article Influence: 106.1] [Reference Citation Analysis (1)] |
| 2. | Straumann A, Schoepfer A. Update on basic and clinical aspects of eosinophilic oesophagitis. Gut. 2014;63:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Landres RT, Kuster GG, Strum WB. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology. 1978;74:1298-1301. [PubMed] |
| 5. | Shiflett DW, Gilliam JH, Wu WC, Austin WE, Ott DJ. Multiple esophageal webs. Gastroenterology. 1979;77:556-559. [PubMed] |
| 6. | Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME; First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1253] [Cited by in RCA: 1154] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 7. | Dellon ES, Erichsen R, Baron JA, Shaheen NJ, Vyberg M, Sorensen HT, Pedersen L. The increasing incidence and prevalence of eosinophilic oesophagitis outpaces changes in endoscopic and biopsy practice: national population-based estimates from Denmark. Aliment Pharmacol Ther. 2015;41:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Giriens B, Yan P, Safroneeva E, Zwahlen M, Reinhard A, Nydegger A, Vavricka S, Sempoux C, Straumann A, Schoepfer AM. Escalating incidence of eosinophilic esophagitis in Canton of Vaud, Switzerland, 1993-2013: a population-based study. Allergy. 2015;70:1633-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | van Rhijn BD, Verheij J, Smout AJ, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil. 2013;25:47-52.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Falk GW. Eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:xiii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940-941. [PubMed] |
| 12. | Barni S, Arasi S, Mastrorilli C, Pecoraro L, Giovannini M, Mori F, Liotti L, Saretta F, Castagnoli R, Caminiti L, Cianferoni A, Novembre E. Pediatric eosinophilic esophagitis: a review for the clinician. Ital J Pediatr. 2021;47:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Muir A, Falk GW. Eosinophilic Esophagitis: A Review. JAMA. 2021;326:1310-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 14. | Biedermann L, Straumann A, Greuter T, Schreiner P. Eosinophilic esophagitis-established facts and new horizons. Semin Immunopathol. 2021;43:319-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Dhar A, Haboubi HN, Attwood SE, Auth MKH, Dunn JM, Sweis R, Morris D, Epstein J, Novelli MR, Hunter H, Cordell A, Hall S, Hayat JO, Kapur K, Moore AR, Read C, Sami SS, Turner PJ, Trudgill NJ. British Society of Gastroenterology (BSG) and British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) joint consensus guidelines on the diagnosis and management of eosinophilic oesophagitis in children and adults. Gut. 2022;71:1459-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 16. | Gómez-Aldana A, Jaramillo-Santos M, Delgado A, Jaramillo C, Lúquez-Mindiola A. Eosinophilic esophagitis: Current concepts in diagnosis and treatment. World J Gastroenterol. 2019;25:4598-4613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (5)] |
| 17. | Gomez Torrijos E, Gonzalez-Mendiola R, Alvarado M, Avila R, Prieto-Garcia A, Valbuena T, Borja J, Infante S, Lopez MP, Marchan E, Prieto P, Moro M, Rosado A, Saiz V, Somoza ML, Uriel O, Vazquez A, Mur P, Poza-Guedes P, Bartra J. Eosinophilic Esophagitis: Review and Update. Front Med (Lausanne). 2018;5:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Papadopoulou A, Dias JA. Eosinophilic esophagitis: an emerging disease in childhood - review of diagnostic and management strategies. Front Pediatr. 2014;2:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Rank MA, Sharaf RN, Furuta GT, Aceves SS, Greenhawt M, Spergel JM, Falck-Ytter YT, Dellon ES; AGA Institute. Electronic address: clinicalpractice@gastro.org; Joint Task Force on Allergy-Immunology Practice Parameters collaborators. Electronic address: drdanawallace@gmail.com; AGA Institute; Joint Task Force on Allergy-Immunology Practice Parameters collaborators. Technical Review on the Management of Eosinophilic Esophagitis: A Report From the AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters. Gastroenterology. 2020;158:1789-1810.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 20. | Hirano I, Chan ES, Rank MA, Sharaf RN, Stollman NH, Stukus DR, Wang K, Greenhawt M, Falck-Ytter YT; AGA Institute Clinical Guidelines Committee; Joint Task Force on Allergy-Immunology Practice Parameters. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology. 2020;158:1776-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 231] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 21. | Votto M, De Filippo M, Lenti MV, Rossi CM, Di Sabatino A, Marseglia GL, Licari A. Diet Therapy in Eosinophilic Esophagitis. Focus on a Personalized Approach. Front Pediatr. 2021;9:820192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Aceves SS, Dellon ES, Greenhawt M, Hirano I, Liacouras CA, Spergel JM. Clinical guidance for the use of dupilumab in eosinophilic esophagitis: A yardstick. Ann Allergy Asthma Immunol. 2023;130:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 57] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 23. | Dougherty M, Runge TM, Eluri S, Dellon ES. Esophageal dilation with either bougie or balloon technique as a treatment for eosinophilic esophagitis: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:581-591.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Moawad FJ, Molina-Infante J, Lucendo AJ, Cantrell SE, Tmanova L, Douglas KM. Systematic review with meta-analysis: endoscopic dilation is highly effective and safe in children and adults with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2017;46:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 25. | Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, Schoepfer A, Simon HU. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2011;9:400-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 26. | Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003;125:1660-1669. [PubMed] |
| 27. | Khokhar D, Marella S, Idelman G, Chang JW, Chehade M, Hogan SP. Eosinophilic esophagitis: Immune mechanisms and therapeutic targets. Clin Exp Allergy. 2022;52:1142-1156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Laserna-Mendieta EJ, Casabona S, Guagnozzi D, Savarino E, Perelló A, Guardiola-Arévalo A, Barrio J, Pérez-Martínez I, Lund Krarup A, Alcedo J, de la Riva S, Rey-Iborra E, Santander C, Arias Á, Lucendo AJ; EUREOS EoE CONNECT Research group. Efficacy of proton pump inhibitor therapy for eosinophilic oesophagitis in 630 patients: results from the EoE connect registry. Aliment Pharmacol Ther. 2020;52:798-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 29. | Dellon ES, Gupta SK. A Conceptual Approach to Understanding Treatment Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2019;17:2149-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 30. | Chang JW, Kliewer K, Haller E, Lynett A, Doerfler B, Katzka DA, Peterson KA, Dellon ES, Gonsalves N; Consortium of Eosinophilic Gastrointestinal Disease Researchers. Development of a Practical Guide to Implement and Monitor Diet Therapy for Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023;21:1690-1698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU, Straumann A. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230-6.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 572] [Article Influence: 47.7] [Reference Citation Analysis (1)] |
| 32. | Mukkada V, Falk GW, Eichinger CS, King D, Todorova L, Shaheen NJ. Health-Related Quality of Life and Costs Associated With Eosinophilic Esophagitis: A Systematic Review. Clin Gastroenterol Hepatol. 2018;16:495-503.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 33. | Hirano I, Collins MH, Assouline-Dayan Y, Evans L, Gupta S, Schoepfer AM, Straumann A, Safroneeva E, Grimm M, Smith H, Tompkins CA, Woo A, Peach R, Frohna P, Gujrathi S, Penenberg DN, Li C, Opiteck GJ, Olson A, Aranda R, Rothenberg ME, Dellon ES; HEROES Study Group. RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology. 2019;156:592-603.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 34. | Straumann A, Hoesli S, Bussmann Ch, Stuck M, Perkins M, Collins LP, Payton M, Pettipher R, Hunter M, Steiner J, Simon HU. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68:375-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 35. | Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, Lowichik A, Chen X, Emerson L, Cox K, O'Gorman MA, Peterson KA. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 36. | Dellon E, Chehade M, Genta RM, Leiman DA, Peterson KA, Spergel J, Wechsler J, Bortey E, Chang AT, Hirano I. S446 Results from KRYPTOS, a Phase 2/3 Study of Lirentelimab (AK002) in Adults and Adolescents With EoE. Am J Gastroenterol. 2022;117:e316-e317. [DOI] [Full Text] |
| 37. | Katzka DA, Smyrk TC, Alexander JA, Geno DM, Beitia RA, Chang AO, Shaheen NJ, Fitzgerald RC, Dellon ES. Accuracy and Safety of the Cytosponge for Assessing Histologic Activity in Eosinophilic Esophagitis: A Two-Center Study. Am J Gastroenterol. 2017;112:1538-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 38. | Ackerman SJ, Kagalwalla AF, Hirano I, Gonsalves N, Katcher PM, Gupta S, Wechsler JB, Grozdanovic M, Pan Z, Masterson JC, Du J, Fantus RJ, Alumkal P, Lee JJ, Ochkur S, Ahmed F, Capocelli K, Melin-Aldana H, Biette K, Dubner A, Amsden K, Keeley K, Sulkowski M, Zalewski A, Atkins D, Furuta GT. One-Hour Esophageal String Test: A Nonendoscopic Minimally Invasive Test That Accurately Detects Disease Activity in Eosinophilic Esophagitis. Am J Gastroenterol. 2019;114:1614-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 39. | Nguyen N, Lavery WJ, Capocelli KE, Smith C, DeBoer EM, Deterding R, Prager JD, Leinwand K, Kobak GE, Kramer RE, Menard-Katcher C, Furuta GT, Atkins D, Fleischer D, Greenhawt M, Friedlander JA. Transnasal Endoscopy in Unsedated Children With Eosinophilic Esophagitis Using Virtual Reality Video Goggles. Clin Gastroenterol Hepatol. 2019;17:2455-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |