Published online Mar 9, 2024. doi: 10.5409/wjcp.v13.i1.89091
Peer-review started: October 20, 2023
First decision: November 23, 2023
Revised: December 4, 2023
Accepted: January 4, 2024
Article in press: January 4, 2024
Published online: March 9, 2024
Processing time: 138 Days and 23.6 Hours
Pediatric inflammatory bowel disease (IBD) is a chronic inflammatory disorder, with increasing incidence and prevalence worldwide. There have been recent advances in imaging and endoscopic technology for disease diagnosis, treatment, and monitoring. Intestinal ultrasound, including transabdominal, transperineal, and endoscopic, has been emerging for the assessment of transmural bowel inflammation and disease complications (e.g., fistula, abscess). Aside from surgery, IBD-related intestinal strictures now have endoscopic treatment options including through-the-scope balloon dilatation, injection, and needle knife stricturotomy and new evaluation tools such as endoscopic functional lumen imaging probe. Unsedated transnasal endoscopy may have a role in patients with upper gastrointestinal Crohn’s disease or those with IBD with new upper gastrointestinal symptoms. Improvements to dysplasia screening in pediatric patients with longstanding colonic disease or primary sclerosing cholangitis hold promise with the addition of virtual chromoendoscopy and ongoing research in the field of artificial intelligence-assisted endoscopic detection. Artificial inte
Core Tip: Recent advances to imaging and endoscopic techniques and technology have improved the diagnosis, treatment, and monitoring of pediatric patients with inflammatory bowel disease. Options are now less invasive and can help avoid the repeat need for general anesthesia during endoscopy and imaging. Point-of-care ultrasound (transabdominal, transperineal, endoscopic), through-the-scope imaging (endoscopic functional lumen imaging probe) and treatment tools (balloon dilatation, injection, knife stricturotomy), unsedated transnasal endoscopy, virtual chromoendoscopy, and artificial intelligence were summarized in this current review.
- Citation: Hudson AS, Wahbeh GT, Zheng HB. Imaging and endoscopic tools in pediatric inflammatory bowel disease: What’s new? World J Clin Pediatr 2024; 13(1): 89091
- URL: https://www.wjgnet.com/2219-2808/full/v13/i1/89091.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v13.i1.89091
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder consisting of Crohn’s disease (CD), ulcerative colitis (UC), and IBD unclassified[1,2]. The incidence of IBD continues to rise, including pediatric-onset disease, with most recent estimates approaching 1.5-2.0 per 10000 person years in areas with the highest rates of disease (Europe, North America)[3]. The youngest children, being diagnosed with very early onset IBD (< 6 years), are the fastest growing diagnosed population in Canada[4]. In the United States, nearly 1% of children and adults are living with IBD[5]. Recently, westernized countries, such as Asia and South America where IBD was rarely diagnosed, have seen a surge in newly diagnosed cases[3].
The gold standard of confirming a diagnosis of IBD includes macroscopic findings on endoscopy and microscopic findings on histopathology[6,7]. Imaging is a helpful additional tool, particularly in assessing bowel that is unable to be reached by upper and lower endoscopy. In addition to the initial diagnostic phase, endoscopy and imaging are essential tools in the ongoing monitoring and reassessment of disease activity in response to treatment. Disease monitoring has become a critical part of IBD patient care, particularly as mucosal healing has been identified as an important patient outcome to achieve[8].
The field of IBD has seen ongoing advancements in imaging and endoscopy over recent years. This is particularly of interest for pediatric patients, where better access to more noninvasive diagnostic, therapeutic, and monitoring tools could reduce the need for repeat general anesthesia for endoscopy and help alter the natural history of the disease by identifying when a treatment change is needed. Endoscopic advances may also now allow for the role of therapeutic endoscopy in place of surgery. Given how quickly technology has advanced in this area in the past several years, it is an important topic to review for training and practicing pediatric gastroenterologists. While there have been a few reviews of imaging or endoscopy in pediatric IBD (discussed below), there is a need to combine these topics and review all recent technology advances as a comprehensive approach in pediatric IBD. This review summarized these recent advances in imaging and endoscopy. This is not a systematic review but rather a focused review intended to summarize new changes and provide important clinical context.
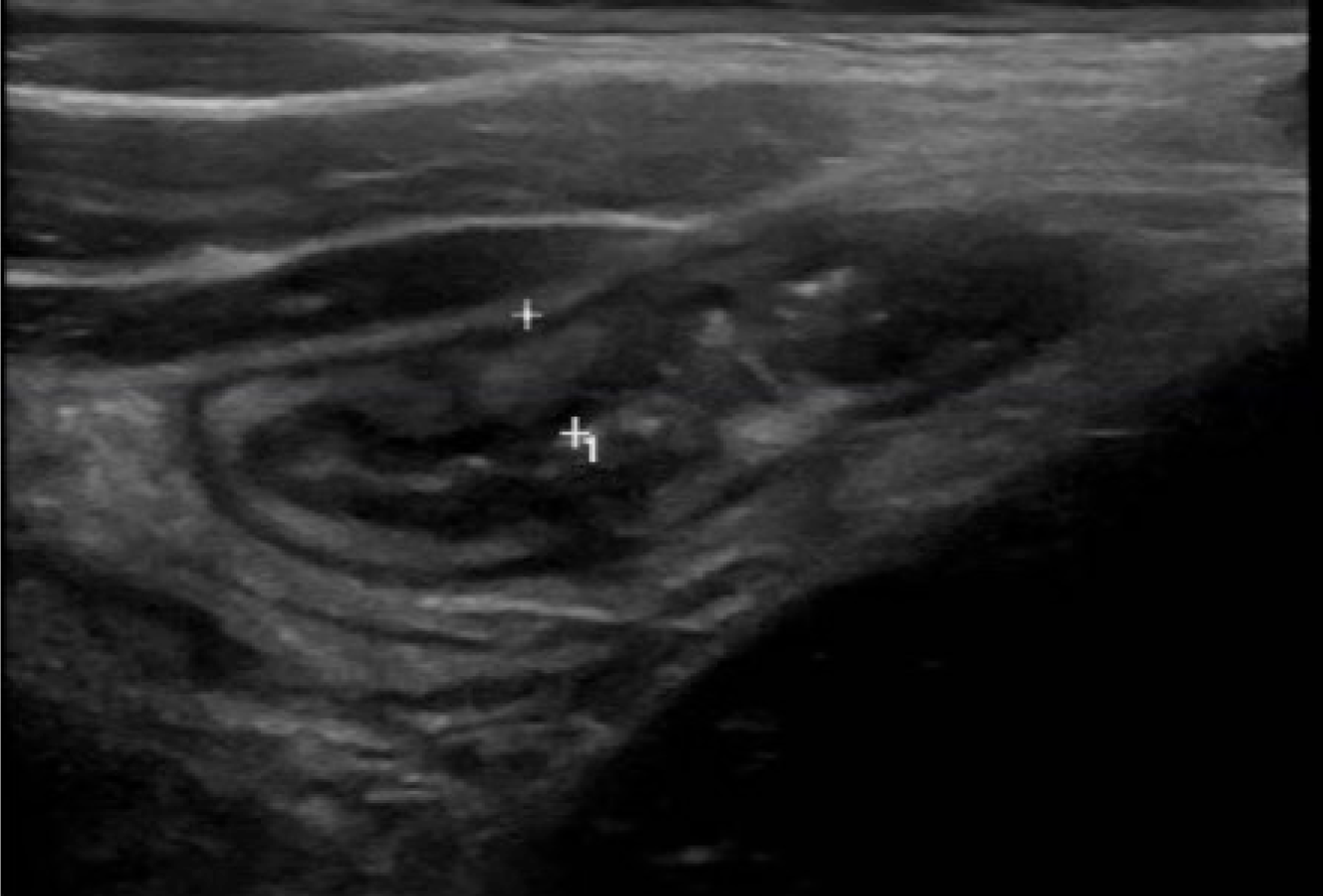
Until recently, magnetic resonance (MR) and computed tomography enterography have been the mainstay of IBD imaging[9]. Gastroenterologist-performed point of care intestinal ultrasound (IUS) is now becoming more accessible in pediatric gastroenterology clinics worldwide, particularly since the implementation of the standardized International Bowel Ultrasound Group curriculum and available IUS scoring systems[10]. Bowel wall thickness, hyperemia, echogenicity, bowel wall stratification, and surrounding fat proliferation are some of the items that can be measured to assess bowel inflammation, with bowel wall thickness being one of the most relevant (Figure 1). Wall thickness has been shown to correlate well with MR and endoscopy[10]. It also allows for transmural assessment of the bowel, which endoscopy is unable to do. Importantly, IUS is well-received by pediatric patients and their caregivers, preferring it over other investigation modalities[11]. The optimal use of IUS in the decision-making tree during a patient’s treatment course is under investigation. Whether or not IUS can replace repeat endoscopy is an important ongoing research question.
Over a quarter of pediatric patients with IBD will have perianal disease in the form of fistulas and abscesses, most often associated with CD[12]. MR imaging (MRI) pelvis and exams under anesthesia (EUA) by a general surgeon are currently the only options for diagnosis and follow-up of both simple and complex perianal abscesses and fistula. Transperineal ultrasound, using microconvex and microlinear probes against the perineum, is a tool that is being explored in clinical practice for this use. Its use in clinic or at the same time as endoscopy may be a valuable means of perianal disease monitoring. Just like IUS, transperineal ultrasound (US) is more accessible than MR and avoids the general anesthesia associated with endoscopy and EUA, making it very favorable and accessible for repeated use in pediatric patients.
Another evolving utility is to use the transperineal probe to assess rectum mucosal disease activity in UC, an area that is very difficult to see on transabdominal IUS[13]. A recent study in pediatric patients with UC found transperineal US accuracy to be comparable to endoscopy[13]. It has also been shown to be able to distinguish between active IBD proctitis compared to non-IBD proctitis in children by detecting thicker bowel walls in those with inflammation[14]. Therefore, combining transperineal and transabdominal US would allow for a more complete assessment in UC, colonic CD, and IBD-associated perianal disease.
Mainly used for adult assessments, endoscopic ultrasound (EUS) has thus far been focused on pancreatic and biliary tree disease. The use of EUS in IBD has been limited mainly to assess bowel wall thickness[15] as well as perianal fistula tracts[9,16,17]. Similar to perineal US, its use to assess perianal fistulas and transmural inflammation, with the advantage of timing during colonoscopy, is an important area for further exploration. It may also be a helpful adjunct in guiding a surgical EUA and avoiding delay of the EUA by skipping the need for a preoperative pelvic MRI[17,18]. Future research is also needed to learn more about the EUS measurement of bowel wall thickness, similarly to IUS, and if this could help risk stratification or prognostication of patients at the time of their endoscopy.
Upper and lower endoscopy is an essential diagnostic and assessment tool in pediatric IBD[6]. It is the only IBD tool we have to assess mucosal disease both macroscopically and microscopically. Over recent years, there have been improvements in the quality of endoscopes (e.g., more high-definition images) as well as available endoscopic tools such as balloon dilators and endoscopic needles[16,18].
Approximately 10% of pediatric IBD patients will present with an intestinal stricture at IBD diagnosis, with even more experiencing a stricture later in the disease course (inflammatory or fibrotic) or as a postsurgical anastomotic stricture[19]. In addition to surgery, through-the-scope balloon dilatation has become a therapeutic option for short (< 4 cm), single intestinal strictures in the setting of treated IBD (Figure 2). In the 1st year post-dilatation, surgery-free rates over 80% have been reported, although up to one-third may need repeat dilatation[6,20,21]. The European Society of Pediatric Gastroenterology, Hepatology, and Nutrition recently published a position paper on the use of endoscopic balloon dilatation for pediatric stricturing CD and highlighted the importance of an experienced endoscopist performing the dilation on short strictures (up to 5 cm) in the duodenum, terminal ileum, or colon with no associated fistula, phlegmon, or abscess[22]. Fluoroscopy at the same time could be considered but is not necessary for all patients. Both primary and postsurgical anastomotic strictures as well as inflammatory vs fibrotic strictures have had similar success rates with endoscopic dilatation[22].
After dilatation disruption of strictured intestinal tissue, there is resulting inflammation that can lead to fibrosis and potentially reformation of the stricture. In an effort to reduce this inflammation and risk of re-stricturing, endoscopic intralesional anti-inflammatory medication injections (e.g., steroids, infliximab) have been studied with mixed results[6]. One small pediatric randomized controlled trial found that compared to placebo injection, intrastricture injection of corticosteroid after endoscopic balloon dilatation had increased time free of re-dilatation and surgery[23]. Topical mitomycin, an antiproliferative agent, application post-dilatation has been reported for refractory esophageal strictures with mixed results[24] but is not yet widely explored in intestinal IBD strictures. Due to lack of sufficient evidence, intralesional injections and topical medication application during endoscopic balloon dilatation in pediatric IBD is not currently recommended[6].
Endoscopic needle knife stricturotomy performed by an experienced endoscopist is an alternative for the treatment of persistent IBD strictures despite attempts at endoscopic balloon dilatation[25]. Circumferential or radial incisions are carefully made at the stricture site with the through-the-scope needle knife. One recent study of IBD patients with anastomotic strictures reported an improvement rate of 20% with this technique, with over 80% not needing surgical resection of the stricture[25]. Endoscopic stricturotomy has been reported in CD strictures, post-IBD surgery anastomotic strictures, and ileal-pouch strictures[26]. Comparison between stricturotomy and balloon dilatation in CD anastomotic strictures in adult patients found that stricturotomy appeared to be more effective, although both carry perforation and bleeding risks[27]. This has not yet been widely studied in pediatric IBD patients.
Endoscopic functional lumen imaging probe (EndoFLIP) is a newer tool that is used at the time of endoscopy to assess lumen distensibility and stiffness, most commonly in the esophagus. It uses impedance planimetry to measure the cross-sectional area, lumen diameter, distension pressure, and overall motility[28]. Its reported uses have been in patients with achalasia, eosinophilic esophagitis (EoE), and pre-esophageal and postesophageal dilatation. The role of EndoFLIP in IBD-related intestinal strictures as well as preintestinal and postintestinal dilatation has not been widely described. A recent case report described its use in a 31-year-old IBD patient with a CD ileocolonic anastomotic stricture, demon
Unsedated transnasal endoscopy (TNE) has been gaining popularity in the last few years, particularly for follow-up of EoE[30]. Being able to perform awake TNE in the gastroenterology clinic allows for faster patient access and the avoidance of a general anesthetic. It also can help ease wait times for endoscopy and improve patient access. Its images and pathology specimens have been found to be comparable to standard peroral esophagogastroduodenoscopy[30]. Its use in IBD has not yet been explored. Approximately one-quarter of new pediatric IBD patients will have upper gastrointestinal (GI) tract involvement[19]. Esophageal disease and stricturing due to CD are rare but a possible disease complication, reported in up to 10% of adult patients with IBD[31]. In addition, patients with IBD have an increased risk of developing EoE[32]. Therefore, unsedated TNE may be a helpful clinic-based assessment tool in pediatric IBD patients presenting with dysphagia or upper GI symptoms or in the reassessment of known complex upper GI tract CD.
The development of virtual chromoendoscopy (using a filter rather than a spray dye during endoscopy) has allowed for improved visual enhancement of the mucosal architecture during endoscopy. The majority of the literature has focused on its use in dysplasia detection, including colorectal adenocarcinoma[33]. Although rare in pediatric patients, colonoscopy for dysplasia surveillance is recommended annually starting at the time of diagnosis in patients with concurrent primary sclerosing cholangitis as well as 8-10 years post-diagnosis of IBD affecting the colon (UC or colonic CD/IBD unclassified)[34]. Given the increasing rates of very early onset IBD (< 6-years-old at diagnosis), these patients will start undergoing dysplasia screening while still under the care of pediatric gastroenterology specialists. Increasing accessibility and training of this technology in pediatric IBD would be highly valuable.
There has been a recent emerging role of artificial intelligence (AI) in gastroenterology to be able to improve endoscopic disease detection, diagnosis, and severity grading due to high endoscopic inter-rater variability that currently exists[35]. It has also been proposed as a potential IBD research tool to replace the need for a central endoscopy reader, which could help with the speed of trial completion[36]. In addition to diagnostic endoscopy, the use of AI to help detect small bowel ulceration and nonobstructive bowel stenosis in video capsule endoscopy is a developing field[37]. Furthermore, similarly to virtual chromoendoscopy, there is much interest in the use of AI to detect premalignant and malignant lesions in long-term IBD patients[38].
AI and its machine learning capabilities also holds promise in helping predict treatment response[38]. With the rapidly increasing number of available IBD biologics and small molecule medications, there is an important need for the development of personalized IBD care with the use of predictive markers. A recent systematic review of AI and machine learning in IBD identified 78 studies that have used clinical and microbiome data sets to aid in IBD diagnosis, disease course, and disease severity[39]. The number of recent publications on this subject has nearly doubled in the past 3 years, highlighting the growing interest[37]. Integrating AI at the time of patient diagnosis to help inform a treatment path with the highest likelihood of success would be of particular interest.
There have been exciting advances in imaging and endoscopic technology in pediatric IBD in recent years. Further development of less invasive diagnostic and therapeutic tools is always important for the pediatric population. There is new technology emerging from EoE and motility disorders that have not yet been explored in IBD (e.g., EndoFLIP, unsedated transnasal endoscopy). The nature of a non-systematic review is a limitation of this current review. There is also a paucity of available pediatric literature given that the majority of research has focused on adult IBD patients.
The future of IBD will certainly benefit from diagnostic, assessment, and therapeutic tools that can aid in more personalized treatment to help establish early and sustained clinical, biochemical, and endoscopic remission. Future research should include prospective studies assessing efficacy, safety, and patient/caregiver satisfaction with these new imaging and endoscopic tools. It would also be of interest to identify if any of these tools are able to aid in the development of a treatment decision tree and eliminate the need for repeat sedated endoscopy or MRI in the pediatric IBD patient. Given the chronicity of IBD and with young pediatric patients being the fastest growing population with newly diagnosed IBD, there is a need to continue to develop these tools for use in patients that will live with this disease and potential disease-related complications for multiple decades.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain S-Editor: Qu XL L-Editor: Filipodia P-Editor: Qu XL
| 1. | Bouhuys M, Lexmond WS, van Rheenen PF. Pediatric Inflammatory Bowel Disease. Pediatrics. 2023;151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 2. | El-Matary W, Carroll MW, Deslandres C, Griffiths AM, Kuenzig ME, Mack DR, Wine E, Weinstein J, Geist R, Davis T, Chan J, Khan R, Matthews P, Kaplan GG, Windsor JW, Bernstein CN, Bitton A, Coward S, Jones JL, Lee K, Murthy SK, Targownik LE, Peña-Sánchez JN, Rohatinsky N, Ghandeharian S, Im JHB, Goddard Q, Gorospe J, Verdugo J, Morin SA, Morganstein T, Banning L, Benchimol EI. The 2023 Impact of Inflammatory Bowel Disease in Canada: Special Populations-Children and Adolescents with IBD. J Can Assoc Gastroenterol. 2023;6:S35-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 3. | Kuenzig ME, Fung SG, Marderfeld L, Mak JWY, Kaplan GG, Ng SC, Wilson DC, Cameron F, Henderson P, Kotze PG, Bhatti J, Fang V, Gerber S, Guay E, Kotteduwa Jayawarden S, Kadota L, Maldonado D F, Osei JA, Sandarage R, Stanton A, Wan M; InsightScope Pediatric IBD Epidemiology Group, Benchimol EI. Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology. 2022;162:1147-1159.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 325] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 4. | Benchimol EI, Bernstein CN, Bitton A, Carroll MW, Singh H, Otley AR, Vutcovici M, El-Matary W, Nguyen GC, Griffiths AM, Mack DR, Jacobson K, Mojaverian N, Tanyingoh D, Cui Y, Nugent ZJ, Coulombe J, Targownik LE, Jones JL, Leddin D, Murthy SK, Kaplan GG. Trends in Epidemiology of Pediatric Inflammatory Bowel Disease in Canada: Distributed Network Analysis of Multiple Population-Based Provincial Health Administrative Databases. Am J Gastroenterol. 2017;112:1120-1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 5. | Lewis JD, Parlett LE, Jonsson Funk ML, Brensinger C, Pate V, Wu Q, Dawwas GK, Weiss A, Constant BD, McCauley M, Haynes K, Yang JY, Schaubel DE, Hurtado-Lorenzo A, Kappelman MD. Incidence, Prevalence, and Racial and Ethnic Distribution of Inflammatory Bowel Disease in the United States. Gastroenterology. 2023;165:1197-1205.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 157] [Article Influence: 78.5] [Reference Citation Analysis (1)] |
| 6. | Oliva S, Thomson M, de Ridder L, Martín-de-Carpi J, Van Biervliet S, Braegger C, Dias JA, Kolacek S, Miele E, Buderus S, Bronsky J, Winter H, Navas-López VM, Assa A, Chong SKF, Afzal NA, Smets F, Shaoul R, Hussey S, Turner D, Cucchiara S. Endoscopy in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto IBD Group of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67:414-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 7. | Walsh CM, Lightdale JR, Mack DR, Amil-Dias J, Bontems P, Brill H, Croft NM, Fishman DS, Furlano RI, Gillett PM, Hojsak I, Homan M, Huynh HQ, Jacobson K, Leibowitz IH, Lerner DG, Liu QY, Mamula P, Narula P, Oliva S, Riley MR, Rosh JR, Tavares M, Utterson EC, Ambartsumyan L, Otley AR, Kramer RE, Connan V, McCreath GA, Thomson MA; PEnQuIN Working Group. Overview of the Pediatric Endoscopy Quality Improvement Network Quality Standards and Indicators for Pediatric Endoscopy: A Joint NASPGHAN/ESPGHAN Guideline. J Pediatr Gastroenterol Nutr. 2022;74:S3-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1595] [Article Influence: 398.8] [Reference Citation Analysis (1)] |
| 9. | Rosen MJ, Moulton DE, Koyama T, Morgan WM 3rd, Morrow SE, Herline AJ, Muldoon RL, Wise PE, Polk DB, Schwartz DA. Endoscopic ultrasound to guide the combined medical and surgical management of pediatric perianal Crohn's disease. Inflamm Bowel Dis. 2010;16:461-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Kellar A, Dolinger M, Novak KL, Chavannes M, Dubinsky M, Huynh H. Intestinal Ultrasound for the Pediatric Gastroenterologist: A Guide for Inflammatory Bowel Disease Monitoring in Children: Expert Consensus on Behalf of the International Bowel Ultrasound Group (IBUS) Pediatric Committee. J Pediatr Gastroenterol Nutr. 2023;76:142-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (1)] |
| 11. | Hudson AS, Huynh HQ, Novak KL, Ma H, Kuc A, Kim J, Almeida P, Carroll MW, Wine E, Isaac DM. Pediatric Patient and Caregiver Satisfaction With the Use of Transabdominal Bowel Ultrasound in the Assessment of Inflammatory Bowel Diseases. J Pediatr Gastroenterol Nutr. 2023;76:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (2)] |
| 12. | de Zoeten EF, Pasternak BA, Mattei P, Kramer RE, Kader HA. Diagnosis and treatment of perianal Crohn disease: NASPGHAN clinical report and consensus statement. J Pediatr Gastroenterol Nutr. 2013;57:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Jimbo K, Hosoi K, Suzuki M, Kyodo R, Maruyama K, Arai N, Sato M, Miyata E, Hoshino E, Kudo T, Shimizu T. Accuracy of Transperineal Ultrasonography for Assessing Rectal Lesions in Paediatric Ulcerative Colitis: A Prospective Study. J Crohns Colitis. 2023;17:1122-1127. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Tokushima K, Jimbo K, Suzuki M, Endo Y, Hibio M, Maruyama K, Kashiwagi K, Arai N, Sato M, Kudo T, Hoshino E, Ohtsuka Y, Shimizu T. Differentiation of Active Ulcerative Colitis vs Noninflammatory Bowel Disease Proctitis by Transperineal Superb Microvascular Imaging. Inflamm Bowel Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Ellrichmann M, Wietzke-Braun P, Dhar S, Nikolaus S, Arlt A, Bethge J, Kuehbacher T, Wintermeyer L, Balschun K, Klapper W, Schreiber S, Fritscher-Ravens A. Endoscopic ultrasound of the colon for the differentiation of Crohn's disease and ulcerative colitis in comparison with healthy controls. Aliment Pharmacol Ther. 2014;39:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Lerner DG, Mencin A, Novak I, Huang C, Ng K, Lirio RA, Khlevner J, Utterson EC, Harris BR, Pitman RT, Mir S, Gugig R, Walsh CM, Fishman D. Advances in Pediatric Diagnostic Endoscopy: A State-of-the-Art Review. JPGN Rep. 2022;3:e224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Statie RC, Florescu DN, Gheonea DI, Ungureanu BS, Iordache S, Rogoveanu I, Ciurea T. The Use of Endoscopic Ultrasonography in Inflammatory Bowel Disease: A Review of the Literature. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Carman N, Picoraro JA. Advances in Endoscopy for Pediatric Inflammatory Bowel Disease. Gastrointest Endosc Clin N Am. 2023;33:447-461. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Dhaliwal J, Walters TD, Mack DR, Huynh HQ, Jacobson K, Otley AR, Debruyn J, El-Matary W, Deslandres C, Sherlock ME, Critch JN, Bax K, Seidman E, Jantchou P, Ricciuto A, Rashid M, Muise AM, Wine E, Carroll M, Lawrence S, Van Limbergen J, Benchimol EI, Church P, Griffiths AM. Phenotypic Variation in Paediatric Inflammatory Bowel Disease by Age: A Multicentre Prospective Inception Cohort Study of the Canadian Children IBD Network. J Crohns Colitis. 2020;14:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 20. | Morini S, Hassan C, Lorenzetti R, Zullo A, Cerro P, Winn S, Giustini M, Taggi F. Long-term outcome of endoscopic pneumatic dilatation in Crohn's disease. Dig Liver Dis. 2003;35:893-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Ferlitsch A, Reinisch W, Püspök A, Dejaco C, Schillinger M, Schöfl R, Pötzi R, Gangl A, Vogelsang H. Safety and efficacy of endoscopic balloon dilation for treatment of Crohn's disease strictures. Endoscopy. 2006;38:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Ledder O, Homan M, Furlano R, Papadopoulou A, Oliva S, Dias JA, Dall'oglio L, Faraci S, Narula P, Schluckebier D, Hauser B, Nita A, Romano C, Tzivinikos C, Bontems P, Thomson M. Approach to Endoscopic Balloon Dilatation in Pediatric Stricturing Crohn Disease: A Position Paper of the Endoscopy Special Interest Group of ESPGHAN. J Pediatr Gastroenterol Nutr. 2023;76:799-806. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 23. | Di Nardo G, Oliva S, Passariello M, Pallotta N, Civitelli F, Frediani S, Gualdi G, Gandullia P, Mallardo S, Cucchiara S. Intralesional steroid injection after endoscopic balloon dilation in pediatric Crohn's disease with stricture: a prospective, randomized, double-blind, controlled trial. Gastrointest Endosc. 2010;72:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Ley D, Bridenne M, Gottrand F, Lemale J, Hauser B, Lachaux A, Rebouissoux L, Viala J, Fayoux P, Michaud L. Efficacy and Safety of the Local Application of Mitomycin C to Recurrent Esophageal Strictures in Children. J Pediatr Gastroenterol Nutr. 2019;69:528-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Zhang LJ, Lan N, Wu XR, Shen B. Endoscopic stricturotomy in the treatment of anastomotic strictures in inflammatory bowel disease (IBD) and non-IBD patients. Gastroenterol Rep (Oxf). 2020;8:143-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Shen B, Lian L, Kiran RP, Queener E, Lavery IC, Fazio VW, Remzi FH. Efficacy and safety of endoscopic treatment of ileal pouch strictures. Inflamm Bowel Dis. 2011;17:2527-2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Lan N, Shen B. Endoscopic Stricturotomy Versus Balloon Dilation in the Treatment of Anastomotic Strictures in Crohn's Disease. Inflamm Bowel Dis. 2018;24:897-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 28. | Desprez C, Roman S, Leroi AM, Gourcerol G. The use of impedance planimetry (Endoscopic Functional Lumen Imaging Probe, EndoFLIP(®) ) in the gastrointestinal tract: A systematic review. Neurogastroenterol Motil. 2020;32:e13980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Ismail MS, Akshintala V, Kumbhari V, Selaru F. Impedance planimetry for Crohn’s disease anastomotic stricture characterization: A predictor of treatment response? ACG Case Rep J. 2022;9:e00729. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Friedlander JA, DeBoer EM, Soden JS, Furuta GT, Menard-Katcher CD, Atkins D, Fleischer DM, Kramer RE, Deterding RR, Capocelli KE, Prager JD. Unsedated transnasal esophagoscopy for monitoring therapy in pediatric eosinophilic esophagitis. Gastrointest Endosc. 2016;83:299-306.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Laube R, Liu K, Schifter M, Yang JL, Suen MK, Leong RW. Oral and upper gastrointestinal Crohn's disease. J Gastroenterol Hepatol. 2018;33:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Moore H, Wechsler J, Frost C, Whiteside E, Baldassano R, Markowitz J, Muir AB. Comorbid Diagnosis of Eosinophilic Esophagitis and Inflammatory Bowel Disease in the Pediatric Population. J Pediatr Gastroenterol Nutr. 2021;72:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 922] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 34. | El-Matary W, Guthery SL, Amir AZ, DiGuglielmo M, Draijer LG, Furuya KN, Gupta N, Hochberg JT, Horslen S, Kerkar N, Koot BGP, Laborda TJ, Loomes KM, Mack C, Martinez M, Miethke A, Miloh T, Mogul D, Mohammed S, Moroz S, Ovchinsky N, Perito ER, Rao G, Ricciuto A, Sathya P, Schwarz KB, Shah U, Singh R, Soufi N, Valentino PL, Zizzo A, Deneau MR. Colorectal Dysplasia and Cancer in Pediatric-Onset Ulcerative Colitis Associated With Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol. 2021;19:1067-1070.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Sundaram S, Choden T, Mattar MC, Desai S, Desai M. Artificial intelligence in inflammatory bowel disease endoscopy: current landscape and the road ahead. Ther Adv Gastrointest Endosc. 2021;14:26317745211017809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Ahmad HA, East JE, Panaccione R, Travis S, Canavan JB, Usiskin K, Byrne MF. Artificial Intelligence in Inflammatory Bowel Disease Endoscopy: Implications for Clinical Trials. J Crohns Colitis. 2023;17:1342-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 37. | Stidham RW, Takenaka K. Artificial Intelligence for Disease Assessment in Inflammatory Bowel Disease: How Will it Change Our Practice? Gastroenterology. 2022;162:1493-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 38. | Le Berre C, Sandborn WJ, Aridhi S, Devignes MD, Fournier L, Smaïl-Tabbone M, Danese S, Peyrin-Biroulet L. Application of Artificial Intelligence to Gastroenterology and Hepatology. Gastroenterology. 2020;158:76-94.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 323] [Article Influence: 64.6] [Reference Citation Analysis (1)] |
| 39. | Stafford IS, Gosink MM, Mossotto E, Ennis S, Hauben M. A Systematic Review of Artificial Intelligence and Machine Learning Applications to Inflammatory Bowel Disease, with Practical Guidelines for Interpretation. Inflamm Bowel Dis. 2022;28:1573-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |