Published online Mar 9, 2024. doi: 10.5409/wjcp.v13.i1.87866
Peer-review started: August 30, 2023
First decision: November 1, 2023
Revised: November 11, 2023
Accepted: November 29, 2023
Article in press: November 29, 2023
Published online: March 9, 2024
Processing time: 189 Days and 10 Hours
Childhood bronchial asthma (BA) is a chronic inflammatory respiratory disease. Nutritional conditions, including zinc deficiency, can affect such allergic dis
To outline the difference in serum zinc levels between asthmatic children and healthy controls.
A cross-sectional study was carried out at Children’s Hospital, Cairo University, investigating serum zinc levels in children with BA (n = 40) and healthy children
Children with BA had higher levels of zinc, yet the difference was not significant (P = 0.115). Serum ferritin and IgE levels were significantly higher in asthmatic children (P = 0.006 and 0.001, respectively), yet their levels did not differ significantly by severity (P = 0.623 and 0.126, respectively). There was a nonsignificant weak correlation between serum ferritin levels and both serum iron and Hb levels.
Serum zinc levels do not seem to differ between asthmatic children and healthy children. Serum ferritin levels may be a marker of asthma control. Serum IgE levels are not markers of asthma severity.
Core Tip: Serum zinc levels were higher in asthmatic children than in nonasthmatic children. However, the difference was not significant. Serum ferritin levels were significantly higher in asthmatic children, which may be due to its immunosuppressive properties. Serum ferritin should not be considered in the diagnosis of iron deficiency anemia in asthmatic children. Serum immunoglobulin E should not be applied to diagnose the severity of childhood asthma. Further studies that track biomarkers such as ferritin during asthma progression are needed.
- Citation: Atef Abdelsattar Ibrahim H, Mohsen M, Salep Aziz Hanna B, Mahmoud D, Mohamed Abdelhamid El-Khashab K. Childhood asthma biomarkers including zinc: An exploratory cross-sectional study. World J Clin Pediatr 2024; 13(1): 87866
- URL: https://www.wjgnet.com/2219-2808/full/v13/i1/87866.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v13.i1.87866
The most prevalent chronic respiratory condition in children is bronchial asthma (BA). BA is a chronic inflammatory disease of the lungs that causes airway inflammation and bronchial hyperreactivity; it may also be described as intermittent, reversible airway blockages[1]. Due to its immune-modulating properties, zinc has attracted much attention in relation to asthma and airway inflammation. Zinc is a crucial trace element for human metabolism and helps regulate gene expression, enzyme activity, and protein structure. Additionally, it is crucial for immune system regulation and functions as an antioxidant, anti-inflammatory, and antiapoptotic agent[2].
In the presence of continuous inflammation, free serum ferritin levels are elevated; in addition, free serum ferritin has a protective function in redox biology and iron homeostasis. In contrast, new research reveals that ferritin may have a causal role in the inflammatory pathology of illness, including rheumatologic, immunologic, neoplastic, and infectious diseases, and ferritin levels may be fundamental in the pathology of disease and help in predicting prognosis in addition to tracking disease activity[3].
Since the beginning of the 20th century, it has been known that immunoglobulin E (IgE) is different from other immunoglobulin isotypes in that it may both trigger extremely rapid pathological reactions and serve as a highly sensitive immunological amplifier. Furthermore, it is well known that patients with atopic diseases have higher IgE levels and that IgE serves as a vital link between the adaptive immune system's role in antigen recognition and the effector functions of mast cells and basophils at mucosal and cutaneous sites of environmental exposure. Due to these roles, IgE has become a desirable target for pharmacological intervention, and IgE blocking has clinical potential in a wide range of therapeutic fields[4,5].
Our study aimed to identify the difference in serum zinc levels between asthmatic children and healthy controls. Additionally, other labs of interest, such as serum ferritin and IgE levels, were studied. Moreover, the possible role of these findings as markers of controlled asthma was investigated.
This exploratory cross-sectional study was carried out at Children’s Hospital Cairo University between May 2022 and October 2022. Sixty-one children were enrolled [40 cases (asthmatic), 21 controls (non-asthmatic)]. The control group consisted of healthy children of comparable age and sex to the cases who had no disease based on history and physical examination and no history of BA or zinc deficiency. All asthmatic children who were attending in the asthma clinic, aged from 5-12 years, and whose parents or caregivers approved participation were included. Owing to the absence of the reliability of lung function tests in children under five, as they are rarely practical[6], children aged less than 5 years were excluded. Data on sociodemographic and clinical characteristics such as body mass index (BMI) and degree of asthma severity were collected. In addition, laboratory findings such as serum zinc, albumin, ferritin, and IgE levels were recorded and compared between cases and controls.
The diagnosis of BA was considered using the medical history, family history, clinical examination, and symptoms including episodic dyspnea, coughing, wheezing, and tightness in the chest, as well as laboratory findings. The results of pulmonary function tests allowed for the confirmation of the diagnosis of BA and a determination of the severity and reversibility of airflow restriction[7,8]. Malnutrition was defined using World Health Organization definitions[9-12].
As our primary outcome was to compare cases and controls with regard to zinc levels, we essentially excluded participants with a drug history of zinc supplementation. We also excluded cases with a drug history of iron therapy, as our secondary outcome was to examine the difference between the two groups with regard to iron hemostasis.
Children admitted to the hospital were excluded because in-hospital admission could negatively affect their nutritional status and zinc level. Likewise, children with comorbidities that could affect their nutritional status were excluded.
The primary objective of the current study was to compare serum zinc levels between asthmatic children and healthy controls. Umar et al[13] reported that the mean serum zinc level in BA patients was 79.63 ± 9.62 µg/dL, while it was 93.27 ± 12.21 µg/dL in healthy controls. G*Power software (version 3.1.9.2) was used to estimate the required sample size. The alpha was set as 0.05, the power (1-β) was set as 0.99, and the case-to-control ratio was set as 2:1. Considering a nonparticipation rate of 10%, the minimum required sample size for the study was 60 patients, including 40 cases, and 20 controls.
All procedures were carried out in line with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 2013 and were approved by the Research Ethics Committee of the Faculty of Medicine, Cairo University. The ethical approval number is MS-587-2021.
Data are statistically described in terms of the mean ± SD, the median and interquartile range, or frequencies (number of cases) and percentages when appropriate. Tests of normality were performed for all the numerical variables of interest using the Kolmogorov-Smirnov/Shapiro-Wilk test. The comparison of numerical variables between cases and controls was performed using the independent samples t-test for the parametric data and the Mann-Whitney U test for nonparametric statistics. When the analyses between more than 2 groups (between children with mild, moderate, and severe asthma) were needed, the Kruskal-Wallis test for nonparametric data was performed (as in the comparison regarding ferritin and IgE levels). Cross-tabulation was applied to compare the categorical variables using chi-square and Fisher’s exact tests depending on whether more than 20% of the cells had expected cell counts less than 5. Spearman's rank correlation was applied if one or both of the numerical data of interest were not parametrically distributed, as in the case of the correlation between serum ferritin levels and both serum iron and hemoglobin levels. A two-sided P value less than or equal to 0.05 was considered statistically significant. A graphical presentation was also used to illustrate the difference between medians using a box plot and the difference between means using error plot graphs. Furthermore, a scattered plot was applied to clearly illustrate the possible linear relation between serum iron and ferritin levels. All statistical calculations were performed using the computer program IBM SPSS (Statistical Package for the Social Science; IBM Corp, Armonk, NY, United States) release 27 for Microsoft Windows.
Our study aimed to describe the difference in zinc levels between children with BA and healthy controls without BA.
Matching between cases and controls regarding age, sex, BMI, and the coexistence of malnutrition was performed (Table 1). Table 1 shows no significant differences, with P values more than 0.05
The normal distribution of the numerical variables of interest was tested using the Kolmogorov-Smirnov/Shapiro-Wilk test to identify the possible statistical methods of choice (Table 2). Age, serum ferritin levels, IgE levels, and BMI scores were not normally distributed. On the other hand, serum zinc, iron, hemoglobin (Hb) and albumin levels were normally distributed.
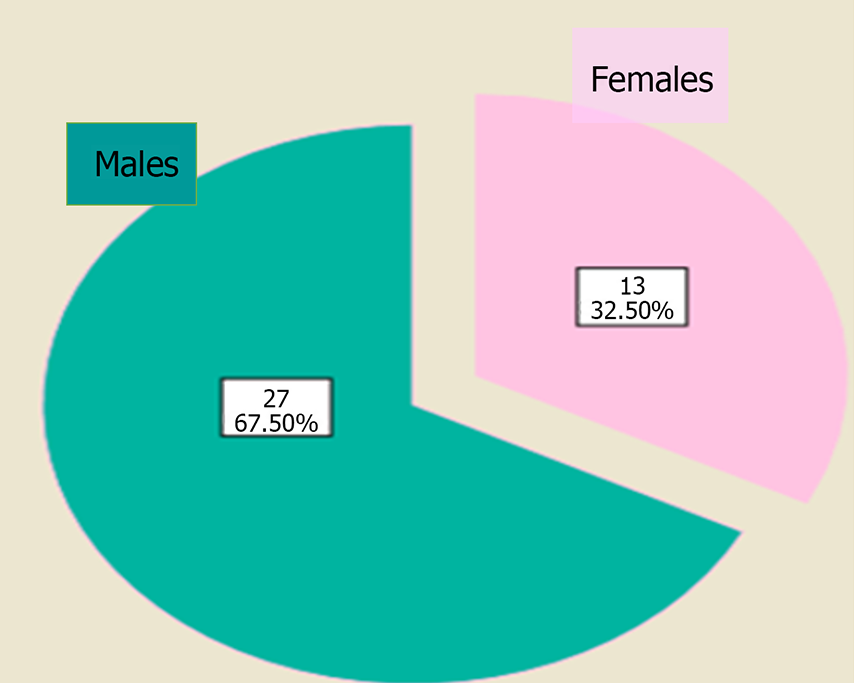
Table 3 illustrates the sociodemographic criteria of the study participants. Among the study participants, male sex generally predominated, specifically in children with BA (Figure 1).
| Sociodemographic criteria | |
| Age of the study participants, yr | |
| Median (IQR) | 7 (4) |
| Min-max | 5-12 |
| Age of children with BA, yr | |
| Median (IQR) | 7.4 (4) |
| Min-max | 5-12 |
| Sex distribution of the study, n = 61 (%) | |
| Males | 38 (62.3) |
| Females | 23 (37.7) |
| Sex distribution in children with BA, n = 40 (%) | |
| Males | 27 (67.5) |
| Females | 13 (32.5) |
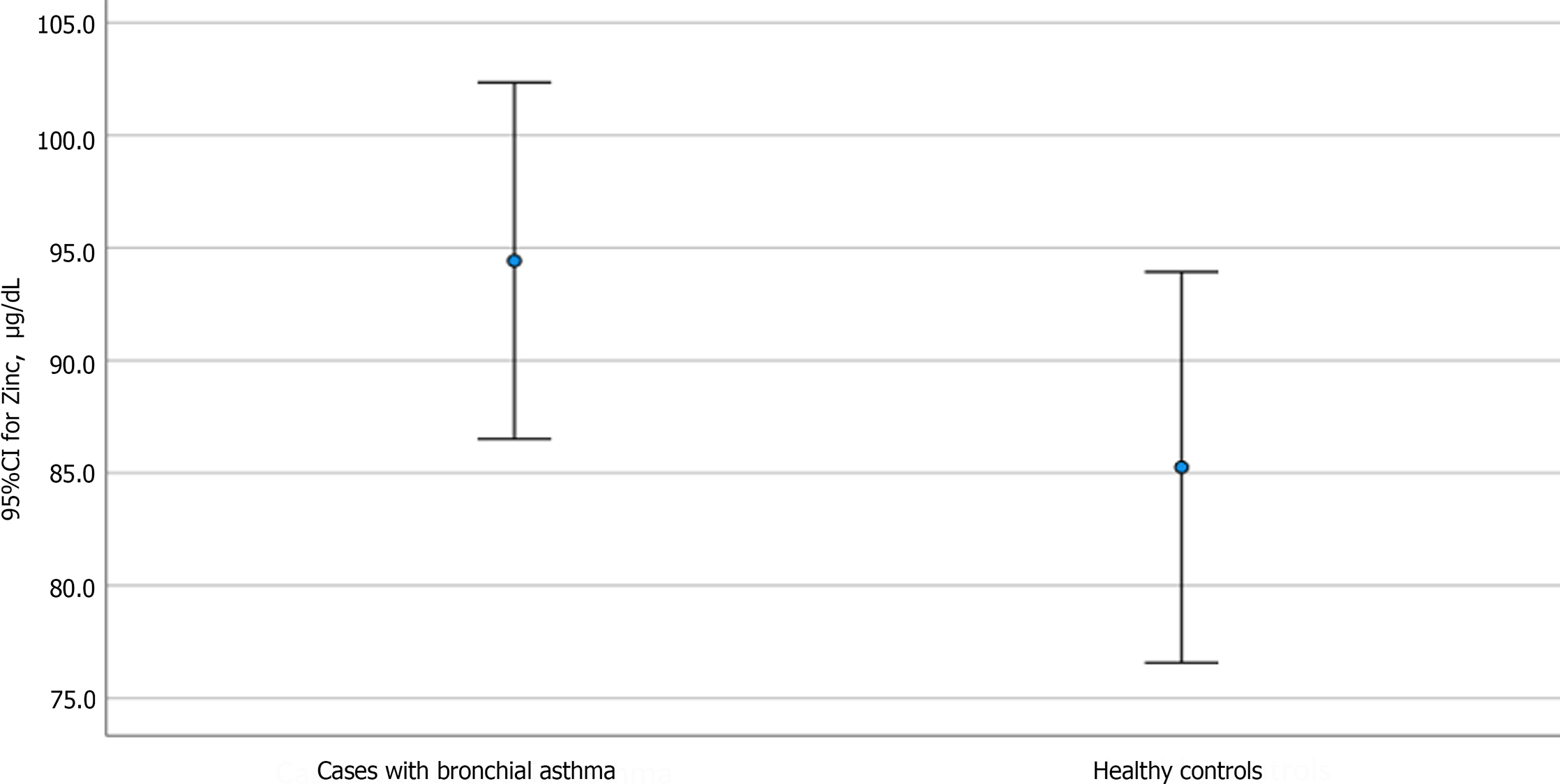
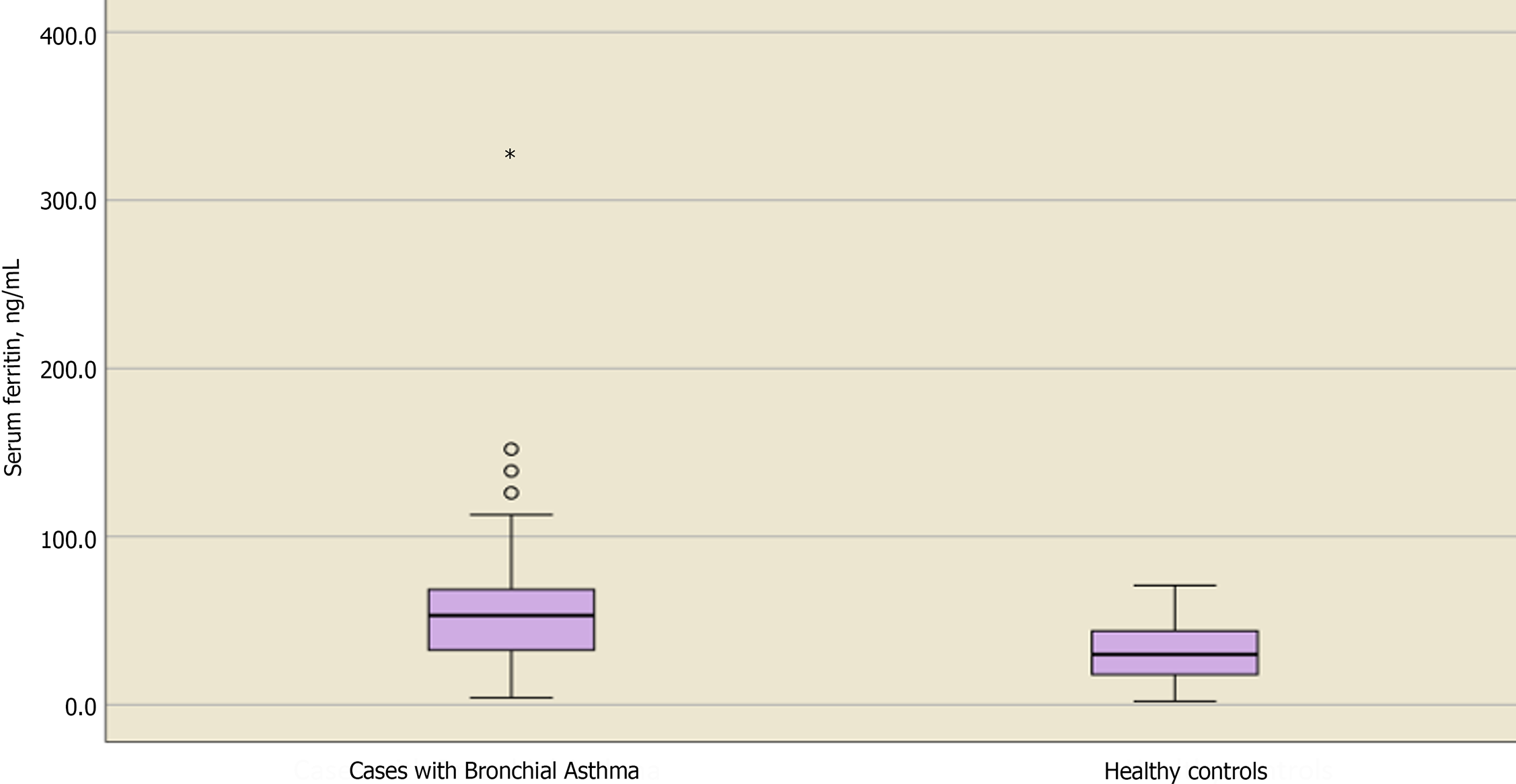
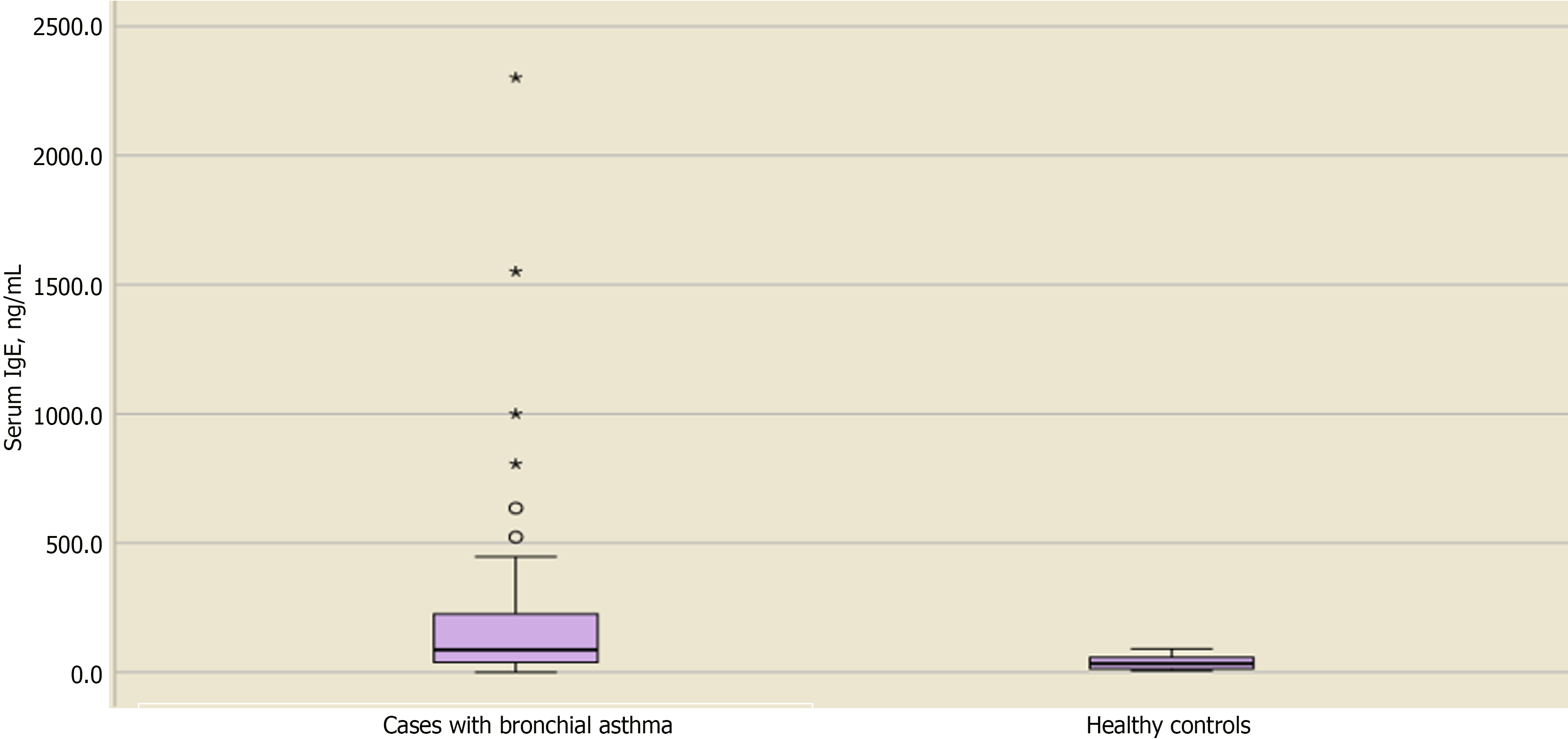
Table 4 shows the distribution and level of significance of the biochemical laboratory assessments between cases and controls. The mean ± SD serum zinc level for asthmatic vs nonasthmatic children was 94.4 ± 24.7 and 85.2 ± 19, respectively, with no significant difference (P = 0.115). Similarly, no significant differences were observed regarding serum iron, Hb, and albumin levels in cases vs controls (P = 0.389, 0.857, and 0.391, respectively). In contrast, serum levels of IgE and ferritin showed significant differences between cases and controls, with P values of 0.001 and 0.006, respectively.
| Children with BA | Healthy controls | P value | |
| Serum zinc Min-max | 94.4 ± 24.7 47-142 | 85.2 ± 19 47.3-112 | 0.1151 |
| Serum iron Min-max | 68.8 ± 28.8 18.7-136.8 | 62.4 ± 26.4 26.5-116.9 | 0.3891 |
| Serum ferritin, median (Q1-Q3) Min-max | 53.1 (68.6-32.2) 4.2-329.2 | 30 (46-17) 2-71 | 0.006a,2 |
| Serum Hb Min-max | 12 ± 0.8 9.9-13.7 | 12 ± 0.8 10.3-13.6 | 0.8571 |
| Serum albumin Min-max | 3.9 ± 0.2 3.5-4.6 | 3.9 ± 0.19 3.5-4.2 | 0.3911 |
| Serum IgE, median (Q1-Q3) Min-max | 264 (229-37) 0.1-2302 | 33 (60.5-12.7) 6.7-91 | 0.001a,2 |
Our primary objective was to compare zinc levels between asthmatic and nonasthmatic children. The mean difference was higher in asthmatic children, as shown in Figure 2.
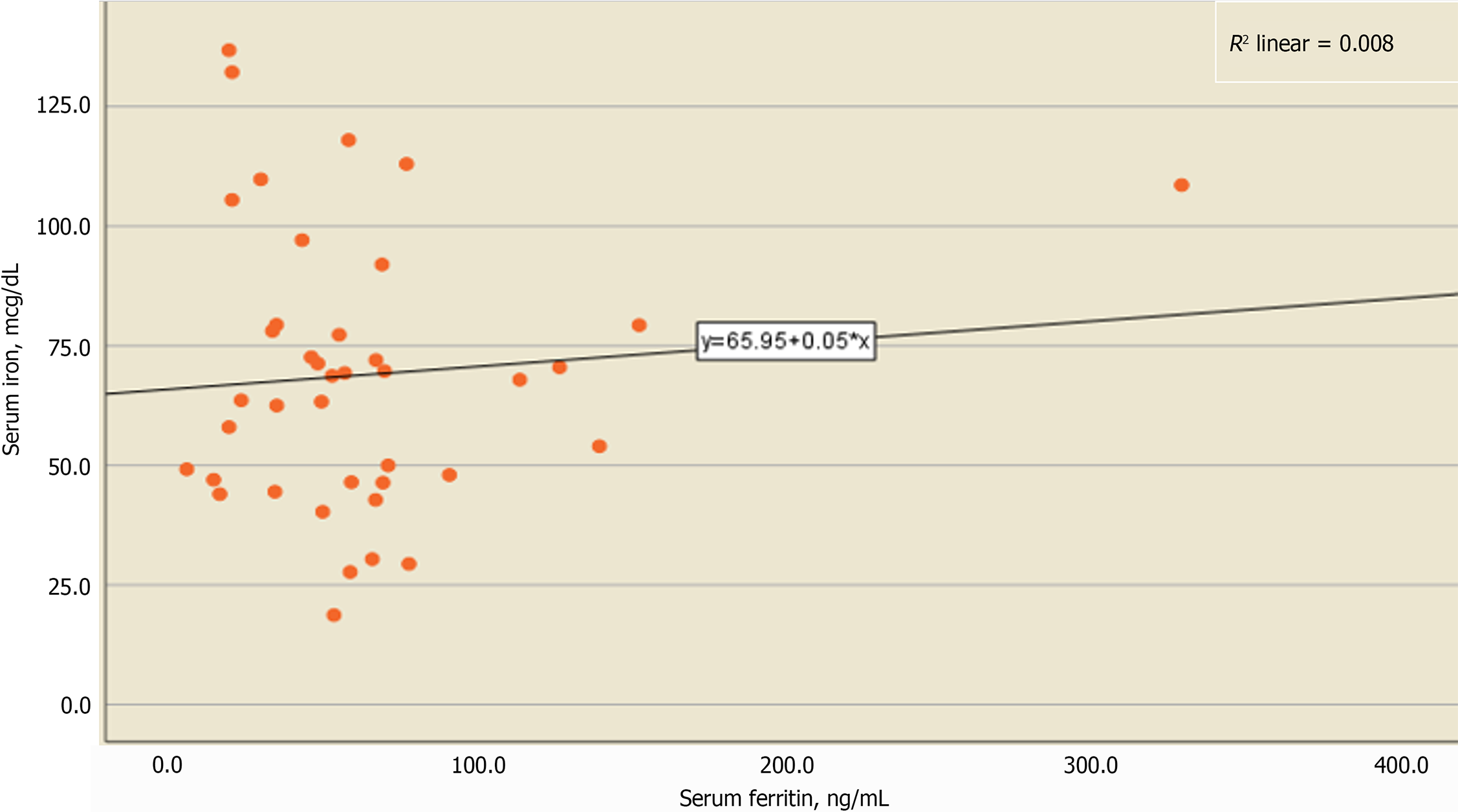
Upon determining the serum levels of ferritin, median differences were higher in children with BA, as shown in Figure 3. This may disclose its role in inflammation, as higher serum ferritin levels do not necessarily mean higher serum iron levels. Spearman's rank correlation was performed and revealed a weak nonsignificant correlation between both serum ferritin and iron levels (rs = -0.077, P = 0.637), as shown in Table 5, which also yielded a similar weak nonsignificant correlation between serum ferritin and Hb levels (rs = 0.204, P = 0.208). In addition, a weak relation was observed in the scatter plot for ferritin and iron levels, as shown in Figure 4.
| Studied covariates | Serum ferritin | |
| rs | P value | |
| Serum iron | -0.077 | 0.637 |
| Hb | 0.204 | 0.208 |
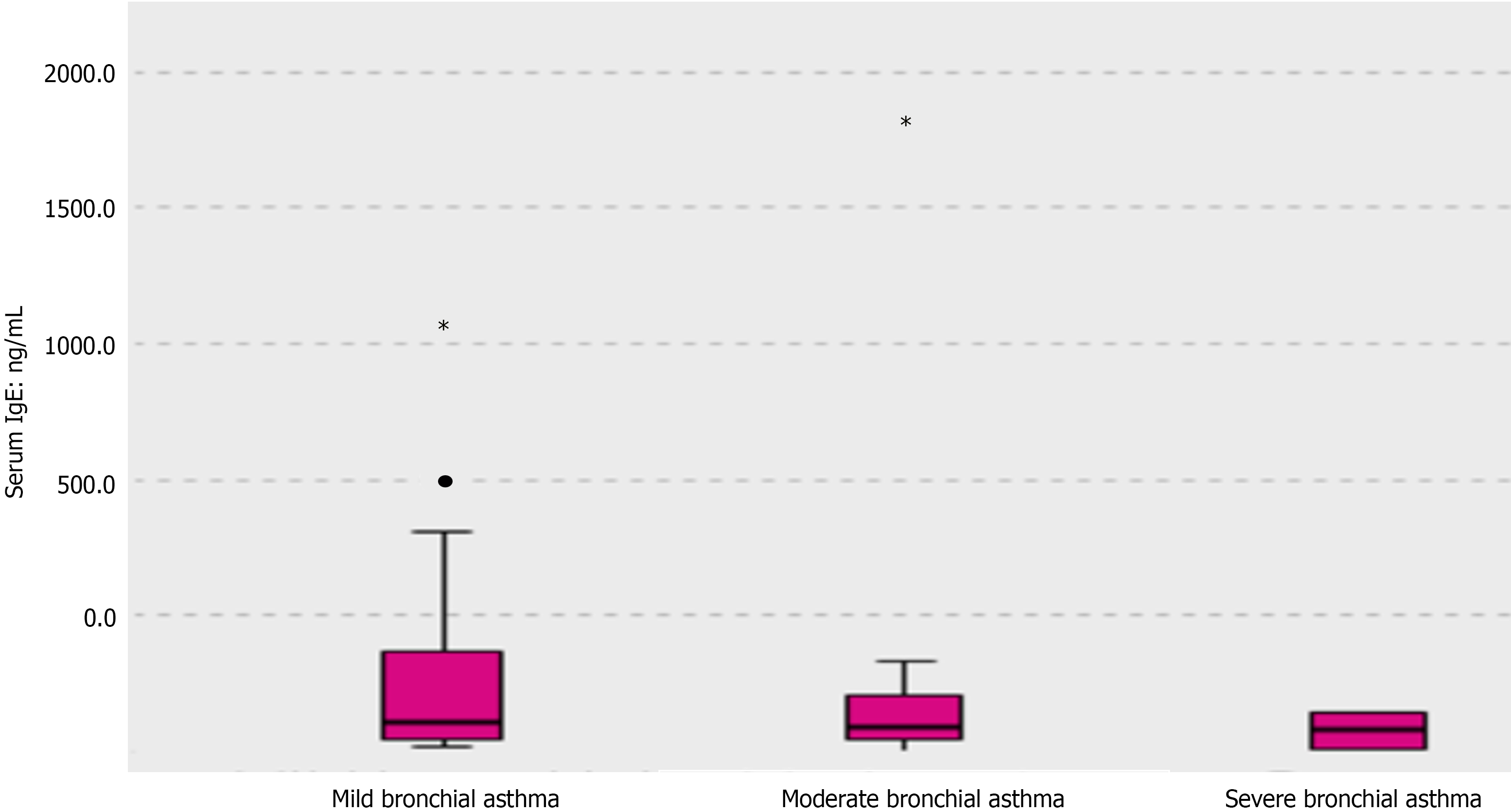
Upon checking the median difference in IgE levels, children with BA showed higher levels, as shown in Figure 5. However, serum IgE levels did not differ significantly in regard to the degree of asthma in children with BA, as shown in Table 6 and Figure 6. Likewise, serum ferritin levels did not show a significant difference regarding the grades of asthma severity (Table 6).
Our study included 61 children: 40 diagnosed with BA and 21 without BA. We examined zinc levels between the two groups. Yousef et al[14] detected significantly diminished levels in cases with BA in comparison to controls, and the P value was < 0.01. In addition, higher levels of serum zinc in control children were observed in another former study, yet the difference was not significant (P = 0.388)[15]. Our study showed a different result. For example, mean values of serum zinc were higher in cases with BA in our study. This difference may be because all cases were receiving asthma therapy such as inhaled steroids. Rahman et al[16] proposed that after steroid therapy, stimulation of glutathione (GSH) synthesis in the liver occurs due to a decrease in the generation of reactive oxygen species by neutrophils. Reduced GSH and GSH disulfide (GSSG) are critical modulators of both the rate of zinc transfer and the ultimate number of zinc atoms transferred. GSSG increases the rate of zinc transfer by 3-fold, and its concentration is the major determinant for efficient zinc transfer[17]. In another study, Raeve et al[18] showed that after corticosteroid therapy, macrophage oxidant production decreased, and the number of oxidant-generating cells present in the asthmatic airway mucosa also decreased; hence, the enhancement of GSH synthesis in the liver could subsequently occur. Considering that lower serum zinc levels indicate higher asthma severity[19], our finding of higher zinc levels in asthmatic children may suggest that lower serum zinc levels may indicate poor compliance with therapy and vice versa.
Other biochemical laboratory test results were assessed, all of which showed no significant difference between cases and controls except for serum ferritin and serum IgE levels. Serum ferritin appears to be a better biomarker for inflammation than iron status[20]. This may be the reason for the significantly elevated levels of ferritin in children with BA compared to controls in our study (P = 0.006). There is mounting evidence that circulating ferritin levels might not only reflect the acute phase response but also play a crucial role in inflammation. Its secretion is regulated via proinflammatory cytokines, and ferritin has immunosuppressive effects that are probably mediated by binding to its receptor. Although it is commonly accepted that circulating ferritin levels may reflect an acute phase response, the explanation for how and why serum ferritin is increased is unknown[21]. Higher ferritin does not essentially equal iron overload[22]. Ferritin can be a good biomarker of appropriate vs excessive inflammation, and previous research found that high ferritin in severe coronavirus disease 2019 pneumonia patients is associated with improved outcomes following steroid treatment[23]. Another study found that low ferritin levels in the course of steroid therapy were linked to greater mortality[24]. Therefore, our study may increase attention toward the possible use of ferritin as a marker of asthma control after steroid therapy. In addition, serum ferritin is not significantly correlated with Hb and iron. However, using serum ferritin as a marker of iron hemostasis or iron deficiency anemia in asthmatic children may be controversial.
Allergic diseases involving asthma are characterized by an increase in serum IgE levels[25,26]. Our study showed that there was a significant increase in serum IgE levels in patients with BA. However, there was no significant difference regarding the degrees of severity. Sandeep et al[27] reported a similar finding. It may be suggested that levels of IgE are quite high at the local inflammation site and that the serum levels do not essentially reflect the levels in the lungs or bronchus. Moreover, IgE is bound to mast cells with rather high affinity, and hence, circulating IgE may not provide conclusive evidence of the severity of inflammation[28].
Serum zinc levels did not show a significant difference between asthmatic children and nonasthmatic children. Serum ferritin may be a marker of controlled asthma. Serum IgE levels should not be used to stratify asthmatic children according to severity.
Zinc levels might differ in asthmatic children.
The possible role of the biochemical nutritional assessment including zinc to be a biomarker for asthma severity.
To outline the difference in zinc levels between asthmatic and healthy children.
A cross-sectional study was carried out investigating serum zinc levels in asthmatic and healthy children.
Zinc levels weren’t different. Ferritin levels were significantly higher in cases with bronchial asthma.
Ferritin could be used as a future biomarker for asthma controller therapy.
Further studies investigating the possible role of ferritin and other possible biomarkers for asthma severity should be outlined.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mogulkoc R, Turkey S-Editor: Zhang H L-Editor: A P-Editor: Cai YX
| 1. | Wang J, Yang L, Sun P, Guo C, Jin Y, Jing X. Expression patterns of serum miR-27a-3p and activating transcription factor 3 in children with bronchial asthma and their correlations with airway inflammation. Clin Respir J. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Rerksuppaphol S, Rerksuppaphol L. Zinc Supplementation in Children with Asthma Exacerbation. Pediatr Rep. 2016;8:6685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 391] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 4. | Ishizaka K, Ishizaka T, Hornbrook MM. Physicochemical properties of reaginic antibody. V. Correlation of reaginic activity wth gamma-E-globulin antibody. J Immunol. 1966;97:840-853. [PubMed] |
| 5. | Matucci A, Vultaggio A, Maggi E, Kasujee I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Respir Res. 2018;19:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 6. | Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, Sheikh A. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 264] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 7. | Wypych-Ślusarska A, Grot M, Kujawińska M, Nigowski M, Krupa-Kotara K, Oleksiuk K, Głogowska-Ligus J, Grajek M. Respiratory Symptoms, Allergies, and Environmental Exposures in Children with and without Asthma. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Martin J, Townshend J, Brodlie M. Diagnosis and management of asthma in children. BMJ Paediatr Open. 2022;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 9. | Atef Abdelsattar Ibrahim H, Abdallah Nasr R, Adel Salama A, Ahmed Amin A. Childhood malnutrition and hypo mineralized molar defects; a cross sectional study, Egypt. F1000Res. 2021;10:1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Atef H, Abdel-Raouf R, Zeid AS, Elsebaie EH, Abdalaleem S, Amin AA, Aboulghar H. Development of a simple and valid nutrition screening tool for pediatric hospitalized patients with acute illness. F1000Res. 2021;10:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Atef Abdelsattar Ibrahim H, Kaddah S, El-Asheer OM, Mahmoud M, Wishahy A. The Pattern of Nutritional and Inflammatory Parameters in Children with Acute Appendicitis. J Child Sci. 2023;13:e96-e103. [DOI] [Full Text] |
| 12. | Atef Abdelsattar Ibrahim H, Fouad Ahmed G, Mohamed Abdelhamid ElKhashab K, Amin AA, Farag Attia Elsebaey A, Sayed Abbas E. Prevalence of Overweight and Obesity in Children Diagnosed with Phenylketonuria. J Compr Ped. 2023;14:e136499. [DOI] [Full Text] |
| 13. | Umar MM, Ramachandran P, Vinoth PN. G507 (P) Zinc status in children with bronchial asthma. Arch Dis Child. 2019;104:A204-A205. [DOI] [Full Text] |
| 14. | Yousef AM, Elmorsy E. Serum zinc level in bronchial asthma. Egypt J Chest Dis Tuberc. 2017;66:1-4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | AbdulWahab A, Zeidan A, Avades T, Chandra P, Soliman A. Serum Zinc Level in Asthmatic and Non-Asthmatic School Children. Children (Basel). 2018;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 521] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 17. | Jiang LJ, Maret W, Vallee BL. The glutathione redox couple modulates zinc transfer from metallothionein to zinc-depleted sorbitol dehydrogenase. Proc Natl Acad Sci U S A. 1998;95:3483-3488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 227] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | De Raeve HR, Thunnissen FB, Kaneko FT, Guo FH, Lewis M, Kavuru MS, Secic M, Thomassen MJ, Erzurum SC. Decreased Cu,Zn-SOD activity in asthmatic airway epithelium: correction by inhaled corticosteroid in vivo. Am J Physiol. 1997;272:L148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Khanbabaee G, Omidian A, Imanzadeh F, Adibeshgh F, Ashayeripanah M, Rezaei N. Serum level of zinc in asthmatic patients: a case-control study. Allergol Immunopathol (Madr). 2014;42:19-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Khan A, Khan WM, Ayub M, Humayun M, Haroon M. Ferritin Is a Marker of Inflammation rather than Iron Deficiency in Overweight and Obese People. J Obes. 2016;2016:1937320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Rosário C, Zandman-Goddard G, Meyron-Holtz EG, D'Cruz DP, Shoenfeld Y. The hyperferritinemic syndrome: macrophage activation syndrome, Still's disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 327] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 22. | Adams P. Management of elevated serum ferritin levels. Gastroenterol Hepatol (N Y). 2008;4:333-334. [PubMed] |
| 23. | Papamanoli A, Kalogeropoulos AP, Hotelling J, Yoo J, Grewal P, Predun W, Jacob RP, Cao K, Marcos LA, Skopicki HA. Association of Serum Ferritin Levels and Methylprednisolone Treatment With Outcomes in Nonintubated Patients With Severe COVID-19 Pneumonia. JAMA Netw Open. 2021;4:e2127172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Papamanoli A, Kalogeropoulos A, Hotelling J, Yoo J, Grewal P, Predun W, Jacob R, Rawal S, Marcos L, Skopicki H. Admission Serum Ferritin Levels and Effect of Methyl&#173;prednisolone in Nonintubated Patients with Severe COVID-19 Pneumonia. Am J Respir Crit Care Med. 2021;203:A379. [DOI] [Full Text] |
| 25. | Peng Z. Vaccines targeting IgE in the treatment of asthma and allergy. Hum Vaccin. 2009;5:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Rage E, Jacquemin B, Nadif R, Oryszczyn MP, Siroux V, Aguilera I, Kauffmann F, Künzli N; Epidemiological Study on the Genetics Environment of Asthma (EGEA). Total serum IgE levels are associated with ambient ozone concentration in asthmatic adults. Allergy. 2009;64:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Sandeep T, Roopakala MS, Silvia CR, Chandrashekara S, Rao M. Evaluation of serum immunoglobulin E levels in bronchial asthma. Lung India. 2010;27:138-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Kindt TJ, Goldsby RA, Osborne B. Kuby Immunology. 6th ed. New York: WH Freeman and Company, 2007: 380-385. |