Published online Dec 9, 2023. doi: 10.5409/wjcp.v12.i5.350
Peer-review started: July 22, 2023
First decision: September 4, 2023
Revised: September 9, 2023
Accepted: September 26, 2023
Article in press: September 26, 2023
Published online: December 9, 2023
Processing time: 139 Days and 0.1 Hours
Type B lactic acidosis and hypoglycemia can occur in various pediatric conditions. In young children with a history of fasting preceding these metabolic derange
To identify the clinical course, treatment strategies, and outcomes of childhood hematologic malignancies with type B lactic acidosis.
We performed a comprehensive search of the PubMed, Scopus, and Cochrane databases without any time restriction but limited to English language articles. The databases were last accessed on July 1st, 2023.
A total of 20 publications were included in the analysis, all of which were case reports or case series. No higher quality evidence was available. Among children with hematologic malignancies and Warburg effect, there were 14 cases of acute lymphoblastic leukemia and 6 cases of non-Hodgkin’s lymphoma including our illustrative case. Lactic acidosis occurred in 55% of newly diagnosed cases and 45% of relapsed cases. The mean age was 10.3 ± 4.5 years, and 80% of cases were male. The mean serum lactate was 16.9 ± 12.6 mmol/L, and 43.8% of the cases had concomitant hypoglycemia. Lactic acidosis initially subsided in 80% of patients receiving chemotherapy compared to 60% in the contrast group. The mortality rate of newly diagnosed cases was 45.5%, while the relapsed cases represented a 100% mortality rate. All 8 patients reported before 2001 died from disease-related complications. However, patients described in reports published between 2003 and 2023 had a 54.5% rate of complete remission.
This complication has historically led to fatal outcome; however, patients who received chemotherapy showed a more favorable response. Therefore, it is crucial to promptly initiate specific treatment in this context.
Core Tip: In children with a history of fasting preceding lactic acidosis, inborn errors of metabolism (IEM) should be considered. However, we describe a case of 10-year-old boy with Burkitt leukemia who exhibited Warburg effect mimicking IEM. The most recent review on lactic acidosis in pediatric leukemia/lymphoma was published in the journal Cancer in 2001. All cases published to that date experienced worsening or recurrence of lactic acidosis, with a mortality rate of 100%. However, this updated systematic review has shown improved outcomes for children with this complication over the past two decades. Newly diagnosed patients and those who received chemotherapy displayed more favorable outcomes.
- Citation: Permtawee K, Tengsujaritkul M, Choed-Amphai C, Chanthong S, Mankhemthong K, Sathitsamitphong L, Natesirinilkul R, Charoenkwan P. Warburg effect mimicking inborn errors of metabolism in childhood hematologic malignancies: A case-based systematic review. World J Clin Pediatr 2023; 12(5): 350-358
- URL: https://www.wjgnet.com/2219-2808/full/v12/i5/350.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v12.i5.350
Leukemia and lymphoma are the most common childhood cancers worldwide[1]. These hematologic malignancies have been known to develop several complications, such as tumor lysis syndrome, hyperleukocytosis, and superior vena cava syndrome[2]. In terms of metabolic derangements, hyperkalemia, hyperuricemia, hyperphosphatemia, and hypocalcemia commonly occur in patients with active disease. In a minority of cases, another form of metabolic complication in children with cancer is lactic acidosis[3].
Normal lactic acid production is mainly derived from glucose metabolism via the glycolytic pathway, and its utilization primarily occurs in the liver. When abnormal lactate accumulates in the body, it can lead to metabolic acidosis. A serum lactate between 2 mmol/L and 5 mmol/L represents hyperlactatemia, whereas lactic acidosis is commonly found when lactate levels are greater than 5 mmol/L[4]. Lactic acidosis usually occurs when there is an imbalance between oxygen delivery and oxygen demand in type A lactic acidosis. In contrast, type B lactic acidosis results from an impairment of oxidative phosphorylation, which is associated with various conditions, including inborn errors of metabolism (IEM), exposure to drugs toxins, and malignancies. The latter form, which is presented in patients with cancer, could be defined as the “Warburg effect”.
Unlike normal cells, which primarily rely on mitochondrial oxidative phosphorylation to generate the energy required for cellular processes, most cancer cells instead rely on aerobic glycolysis[5]. Various factors influence this phenomenon such as oncogene activation, loss of function of tumor suppressors, and effects of transcription factors[6]. This is a rare and unusual metabolic complication in children with hematologic malignancies. Few pediatric case series and case reports of this condition have been published. A previous literature review in 2001 showed that cases with lactic acidosis were more commonly observed in relapsed disease with specific clinical manifestations related to bone marrow, hepatosplenic, or lymph node involvement, and were associated with a poor outcome[3]. However, we encountered a case of newly diagnosed Burkitt leukemia in a 10-year-old boy with hypoglycemia and lactic acidosis which mimicked an IEM disorder. The patient achieved complete remission after treatment with a combination of rituximab and multiagent chemotherapy. Furthermore, we performed an updated systematic review in order to identify the overall profile of clinical course, treatment strategies, and outcomes of childhood hematologic malignancies with type B lactic acidosis from recent decades.
A 10-year-old boy presented with poor appetite and vomiting that had persisted for 2 d. The physical examination did not show any masses, lymphadenopathy, or hepatosplenomegaly. Complete blood count and peripheral blood smear were within normal limits. A critical blood sample demonstrated the following values: Sodium of 142 mmol/L (normal range: 136-143 mmol/L); potassium of 4.0 mmol/L (normal range: 3.8-4.9 mmol/L); chloride of 109 mmol/L (normal range: 101-107 mmol/L); HCO3 of 9 mmol/L (normal range: 17-26 mmol/L); anion gap of 24 mmol/L (normal range: 10-14 mmol/L); blood urea nitrogen of 17 mg/dL (normal range: 7.3-21 mg/dL); creatinine of 1.1 mg/dL (normal range: 0.31-0.61 mg/dL); venous pH of 7.3 (normal range: 7.35-7.45); base excess of -15.2 mmol/L [normal range: (-2)-(+2) mmol/L]; and glucose of 49 mg/dL (normal range: 70-100 mg/dL). Further laboratory studies showed increased serum lactate (3.7 mmol/L; normal range: 1-2 mmol/L), serum ketone (2.7 mmol/L; normal range: 0-1 mmol/L), uric acid (12.5 mg/dL; normal range: 3.4-7.0 mg/dL), and triglycerides (354 mg/dL; normal range: 0-200 mg/dL), while serum cortisol and blood ammonia were normal (20.9 µg/dL and 50 µmol/L, respectively).
Based on the patient’s clinical signs and symptoms, wide anion gap metabolic acidosis with ketotic hypoglycemia, IEM especially gluconeogenesis defects, glycogen storage disorders, and organic acidemia were considered. Subsequently, a specific test for plasma amino acids was performed, but only a nonspecific increase in cystathionine of 8.1 nmol/mL (normal range: 0-3 nmol/mL) and β-aminoisobutyric acid of 598.0 nmol/mL (normal range: 0-2 nmol/mL), which is a product of pyrimidine metabolism, was demonstrated. Urine organic acid testing showed an increased excretion of lactic acid and 4-hydroxyphenylactic acid. These findings explained that lactic acidosis was the cause of the wide anion gap metabolic acidosis without any supporting evidence of those IEM.
During admission, the patient developed hypertension and seizure. Computed tomography scan of the brain was performed, which revealed posterior reversible encephalopathy syndrome. The results of the metabolic workup showed that he had hyperuricemia, hyperphosphatemia, hypocalcemia, and acute kidney injury, along with markedly elevated levels of lactate dehydrogenase. These abnormal findings were consistent with tumor lysis syndrome.
Further computed tomography scan of the chest and abdomen was performed to evaluate the cause of hypertension and metabolic derangement, which demonstrated a posterior gastric wall thickening, and infiltrative soft tissue thickening of peritoneum and omentum without hepatic involvement. In addition, lobulated multifocal hypo-enhancing lesions were found scattered throughout the bilateral enlarged renal parenchyma. The patient underwent esophagogastroduodenoscopy for gastric tissue biopsy, and histological pathology revealed a monotonous, intermediate-size lymphoid cells with starry sky appearance. These cells showed round nuclei with finely clumped chromatin and several paracentral nucleoli. Tingible body macrophages phagocyting apoptotic debris were also observed. The immunohistochemistry testing was positive for CD20, CD79a, CD10, c-MYC, and Ki67 (> 95%), leading to the definitive diagnosis of Burkitt lymphoma.
After the procedure, the patient experienced disease progression characterized by progressive cytopenia with abnormal cells observed on peripheral blood smear. As part of disease staging, a bone marrow examination was performed, which revealed a lymphomatous involvement of 30%. The final diagnosis was Burkitt leukemia. A combination of rituximab and multiagent chemotherapy was administered following the St. Jude Mature B-Cell lymphoma and leukemia study III group c protocol. The patient responded well to the treatment, and the metabolic derangement was rapidly resolved. As of June 2023, the patient has been in complete remission for 1 year and 3 mo.
Three authors (Permtawee K, Choed-Amphai C, and Chanthong S) independently conducted searches of the PubMed, Scopus, and Cochrane databases without any time restrictions. The following keywords were used for the search: “lactic acidosis”; “Warburg”; “pediatric”; “child”; “leukemia”; and “lymphoma”. For PubMed, the specific search-term strategy was: [“acidosis, lactic” (MeSH Terms) or “acidosis” (All Fields) and “lactic” (All Fields) or “lactic acidosis” (All Fields) or “lactic” (All Fields) and “acidosis” (All Fields) or “warburg” (All Fields) or “warburg’s” (All Fields)] and [“paediatrics” (All Fields) or “pediatrics” (MeSH Terms) or “pediatrics” (All Fields) or “paediatric” (All Fields) or “pediatric” (All Fields) or “child” (MeSH Terms) or “child” (All Fields) or “children” (All Fields) or “child’s” (All Fields) or “children’s” (All Fields)] and [“leukaemia” (All Fields) or “leukemia” (MeSH Terms) or “leukemia” (All Fields) or “leukaemias” (All Fields) or “leukemias” (All Fields) or “leukemia’s” (All Fields) or [“lymphoma” (MeSH Terms) or “lymphoma” (All Fields) or “lymphomas” (All Fields) or “lymphoma’s” (All Fields)] or [“haematologic malignancy” (All Fields) or “hematologic neoplasms” (MeSH Terms) or “hematologic” (All Fields) and “neoplasms” (All Fields) or “hematologic neoplasms” (All Fields) or “hematologic” (All Fields) and “malignancy” (All Fields) or “hematologic malignancy” (All Fields)]. Only articles published in English language were considered for selection.
Inclusion criteria were as follows: Type B lactic acidosis in children with hematologic malignancies (including leukemia and lymphoma); and presence of data on clinical course, treatment strategies, and outcomes. Articles which did not meet these criteria were excluded. Summarization of the inclusion and exclusion criteria for this systematic review is presented in Table 1. The study followed the preferred reporting items for systematic reviews and meta-analysis 2020 guideline[7].
| Criteria |
| Inclusion |
| Children, 0 to 18 yr |
| Diagnosis of hematologic malignancy, including leukemia and lymphoma |
| Presence of lactic acidosis |
| Availability of clinical course, treatment, and outcome data |
| Exclusion |
| Adults, > 18 yr |
| Diagnosis of other solid tumors |
| Presence of other causes of metabolic acidosis without lactic acidosis |
| Incomplete information regarding clinical courses, treatment, and outcomes |
Extracted data included demographic and disease characteristics, laboratory parameters, clinical course, treatment strategies, and outcomes.
A qualitative systematic analysis was conducted using descriptive statistics. Due to differences among individual cases and small sample sizes, a meta-analysis could not be performed.
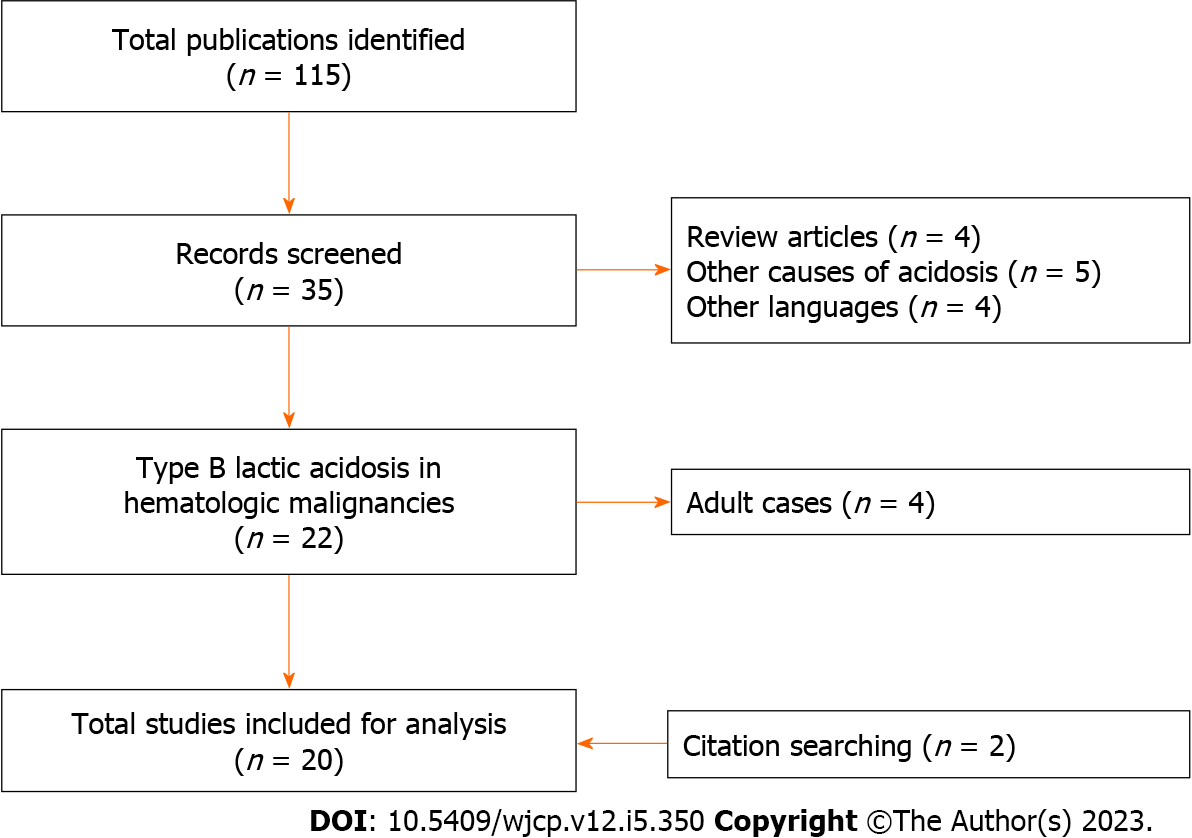
A total of 115 publications were obtained through the search. Thirty-five abstracts were screened. The publications were limited to English language articles that provided detailed information about the clinical courses of type B lactic acidosis in children with leukemia or lymphoma resulting in the inclusion of 18 articles. A further two publications were discovered from manually searching the references of prior articles (Figure 1). The 20 included publications were case reports and case series, and there was no higher quality evidence available for this rare complication. These could be categorized into three groups according to cause of type B lactic acidosis, including Warburg effect, thiamine deficiency, and medications. Table 2 summarizes the clinical and laboratory parameters, treatments, and outcomes of the previously reported cases[3,8-26].
| Ref. | Primary diagnosis | Age in yr, Sex | Clinical manifestation | Initial lactate in mmol/L | Blood glucose in mg/dL | Hepatic involvement | Renal involvement | Treatment | Clinical course | |
| Lactic acidosis | Outcome | |||||||||
| Type B lactic acidosis-Warburg effect | ||||||||||
| Field et al[8] | Relapsed ALL | 4, M | Bleeding, hepatosplenomegaly | 20.2 | NR | Yes | NR | Bicarbonate, radiotherapy, chemotherapy | Improved but recurred | Death from disease |
| ALL | 8, M | NR | 9.9 | NR | NR | NR | Bicarbonate, chemotherapy | Improved but recurred | Death from infection | |
| Coleman et al[9] | Relapsed T-ALL | 2, M | Lymphadenopathy, splenomegaly, dyspnea | 24.6 | 74 | No | NR | Bicarbonate, thiamine, insulin, methylglyoxal | Improved but recurred | Death from disease |
| Ali et al[10] | Relapsed B-ALL | 12, M | Poor appetite, weight loss, abdominal pain | 12.0 | NR | No | Yes | Bicarbonate, chemotherapy | Improved but recurred | Death from infection |
| Révész et al[11] | Relapsed Burkitt lymphoma | 8, M | Seizure, altered mental status, dyspnea, polydipsia, polyuria | 24.0 | 93 | No | Yes | Bicarbonate, thiamine, chemotherapy, radiotherapy | Improved but recurred | Death from disease |
| Sillos et al[3] | Relapsed T-ALL | 11, F | Altered mental status | 10.8 | 47 | Yes | No | Bicarbonate, CRRT, chemotherapy | Improved but recurred | Death from disease |
| Relapsed T-ALL | 17, M | Altered mental status, dyspnea, edema | 16.0 | 132 | No | No | Bicarbonate | Worsened | Death from disease | |
| T-NHL | 18, F | Fever, weight loss, lymphadenopathy, splenomegaly, dyspnea | 15.4 | 44 | Yes | No | Bicarbonate, chemotherapy | Resolved but recurred | Death from disease | |
| Hayek and Srinivasan[12] | B-ALL | 7, M | Anemia, lymphadenopathy, hepatosplenomegaly | 8.4 | 96 | Yes | Yes | Bicarbonate, PD, chemotherapy | Resolved after chemotherapy | CR |
| Rastogi et al[13] | Burkitt lymphoma, HIV | 11, M | Fever, hepatosplenomegaly, periorbital and pedal edema | 4.2 | 26 | Yes | NR | Bicarbonate, glucagon | Improved | Death from disease |
| Luscri et al[14] | Relapsed B-ALL | 7, M | Fever, ascites, dyspnea | 5.5 | 33 | Yes | NR | Bicarbonate, CRRT | Worsened | Death from LA, disease |
| Kulkarni et al[15] | Burkitt lymphoma | 12, M | Dyspnea, hepatomegaly, abdominal pain | 21.0 | Normal | Yes | Yes | Bicarbonate, chemotherapy | Resolved after chemotherapy | CR |
| Terpe et al[16] | B-ALL | 11, M | Nausea, dyspnea, lymphadenopathy, abdominal pain, pancytopenia | 21.0 | 48 | Yes | Yes | Bicarbonate, chemotherapy | Worsened | Death from LA, ACS |
| Gökçe et al[17] | B-ALL | 13, M | Weight loss, nausea, bone pain | 62.0 | 97 | Yes | Yes | Bicarbonate, chemotherapy | Resolved after chemotherapy | CR |
| Schuh et al[18] | Relapsed B-ALL | 12, M | Fever, abdominal pain, bicytopenia | 14.8 | 80 | Yes | Yes | Chemotherapy | Worsened | Death from disease |
| Narayani et al[19] | ALL | 2, M | Fever, pancytopenia | 13.5 | 96 | NR | No | Bicarbonate, chemotherapy | Resolved after chemotherapy | CR |
| Khera et al[20] | T-ALL | 11, F | Poor appetite, weight loss, nausea, dyspnea, bicytopenia | 20.0 | 104 | No | No | Bicarbonate, chemotherapy | Resolved after chemotherapy | CR |
| O'Rourke et al[21] | Burkitt leukemia | 13, M | NR | NR | Low | NR | NR | Chemotherapy | Worsened | Death from LA |
| Hui et al[22] | Relapsed B-ALL | 17, F | Poor appetite, weight loss, bicytopenia | 15.0 | NR | NR | No | Bicarbonate CRRT | Improved | NR |
| This study | Burkitt leukemia | 10, M | Poor appetite, nausea, vomiting | 3.7 | 49 | No | Yes | Rituximab, chemotherapy | Resolved after chemotherapy | CR |
| Type B lactic acidosis-thiamine deficiency | ||||||||||
| Oriot et al[23] | Relapsed ALL, on TPN without vitamin | 3, M | Altered mental status, fever, dyspnea, hepatomegaly | 30.0 | 43 | Yes | No | Bicarbonate, PD, thiamine | Resolved | CR |
| Svahn et al[24] | B-ALL, on TPN without vitamin | 0.9, F | Altered mental status | 18.6 | NR | NR | NR | Bicarbonate, multivitamin including thiamine | Resolved | CR |
| Didisheim et al[25] | ALL, post-sepsis | 13, M | Fever, abdominal pain, hypotension | 19.6 | NR | NR | NR | ECMO, thiamine | Resolved | CR |
| Type B lactic acidosis-medication (s) | ||||||||||
| Smolka et al[26] | Relapsed B-ALL, pneumonia (antibiotics including linezolid) | 9, F | Altered mental status, dyspnea | 19.0 | NR | Yes | NR | Bicarbonate, withdraw linezolid | Improved | CR |
Among the pediatric cases with hematologic malignancies and the Warburg effect, there were 14 of acute lymphoblastic leukemia (ALL), including 7 with B-ALL, 4 with T-ALL, and 3 with unknown cell type, as well as 6 cases of non-Hodgkin’s lymphoma (NHL), which consisted of 5 of Burkitt leukemia/lymphoma and 1 of T-NHL. Lactic acidosis occurred in 11 cases with newly diagnosed leukemia/lymphoma (55%), while the remaining cases had relapsed disease (45%). The mean age of the patients was 10.3 ± 4.5 years, and 80% of the cases were male.
The most common clinical manifestations were dyspnea (44.4%), symptoms related to cytopenia (38.9%), fever (27.8%), weight loss (27.8%), and splenomegaly (27.8%). Hepatic involvement, which included patients with hepatomegaly, impaired hepatic synthetic function, cholestasis, or imaging-proven infiltrative disease, was found in 62.5% of cases (10 out of 16 patients). Renal involvement, which was defined as patients with imaging-proven infiltrative disease, occurred in about half of the cases (8 out of 14 patients). Patients with renal failure that might have resulted from other specific causes, such as tumor lysis syndrome, were excluded. The mean serum lactate was 16.9 ± 12.6 mmol/L, and 43.8% of the cases had concomitant hypoglycemia.
Almost all cases received sodium bicarbonate infusion (85%) in addition to general management protocols such as delivery of intravenous fluids and glucose. Renal replacement therapies, including continuous renal replacement therapy and peritoneal dialysis, were provided in only 20% of cases. Chemotherapy was given to three-quarters of the patients as a specific treatment.
Lactic acidosis initially subsided in about 80% of patients receiving chemotherapy compared to 60% in the contrast group. Furthermore, patients treated with chemotherapy had a more favorable response (complete remission of 40% vs 0%, respectively). The disease status may also affect the long-term outcome. The mortality rate of patients with newly diagnosed hematologic malignancies and lactic acidosis was 45.5%, while the relapsed patients had a 100% mortality rate. Warburg effect accounted for 23.1% of the causes of death. This review also highlighted that patients in the last two decades have experienced better outcomes than those in the previous review. Prior to 2001, patients diagnosed with leukemia or lymphoma-associated lactic acidosis had extremely poor outcomes. Six out of eight cases died from the disease-related conditions, while the other two cases experienced uncontrolled infections. However, publications between 2003 and 2023 revealed that 54.5% of cases achieved complete remission. In the minority of cases, patients with type B lactic acidosis from thiamine deficiency and linezolid treatment had excellent outcomes, with a 100% complete remission rate.
In the pediatric population, especially young children, an IEM disorder should be considered as a potential differential diagnosis in patients with a history of fasting or poor intake followed by hypoglycemia, and lactic acidosis with or without ketosis. The specific disorders of carbohydrate metabolism that can present with hyperlactatemia include glycogen storage diseases, gluconeogenesis defects, and organic acidemia. Findings from personal history taking, family history taking, and physical examination should be evaluated. Another crucial investigation in these diseases is a critical blood sampling taken during hypoglycemia episodes and including analysis of lactate, ammonia, blood gas analysis, insulin, cortisol, and ketones. Additional plasma amino acid and urine organic acid tests should be performed depending on the clinical suspicion[27,28]. After a comprehensive evaluation in our case, there was no evidence to support these diseases. However, the patient later developed clinical symptoms of hypertensive emergency, tumor lysis syndrome and cytopenia, which led to the final diagnosis of Burkitt leukemia.
Type B lactic acidosis is an uncommon complication in patients with cancer, although there are several precipitating causes such as thiamine deficiency and medications. Cancer itself can lead to this form of metabolic acidosis. The proposed pathophysiology of this condition was first described by Warburg[29] in 1925. Since then, several studies have explored cancer metabolism and Warburg effect, which can be summarized into four main functions, as follows: Cell signaling; rapid adenosine triphosphate synthesis; biosynthesis; and tumor microenvironment[5]. Contrary to the publications on adult cases, the most recent review of lactic acidosis in pediatric leukemia and lymphoma was published in 2001[3]. Sillos et al[3] summarized a total of 9 pediatric cases of hematologic malignancies with lactic acidosis, comprising 2 with lymphomas (1 Burkitt lymphoma and 1 T cell-non-Hodgkin’s lymphoma) and 7 with ALLs. All cases experienced worsening or recurrence of lactic acidosis, and the outcome was extremely poor, with a 100% mortality rate. However, this updated review demonstrated that outcomes have improved in the last two decades, possibly due to increased understanding of disease pathophysiology, advances in treatment, and improvements in supportive care. Patients with newly diagnosed leukemia/lymphoma and those who received a specific treatment with chemotherapy appeared to have a better outcome. These findings also differ from those in the adult population. In the past decade, adults with hematologic malignancies who experienced lactic acidosis still had a mortality rate of more than 80%[30,31]. Despite the same pathophysiology of this complication in the pediatric and adult populations, disease status and response to treatment may explain the dismal outcome in adults. According to the United States’ surveillance, epidemiology, and end results program database, survival rates according to age of diagnosis (all patients) at 17 years, 20 years, and 70 years were 75%, 48%, and 15%, respectively. Different treatment regimens and responses were determined to have played significant roles in this survival cliff drop-off[32]. This systematic review is limited by the rarity of this complication, with only a few case reports and case series available.
When compared to the other pediatric reports, our case presented with only nonspecific symptoms (a less common clinical manifestation). The patient had hypoglycemia with slightly elevated serum lactate levels, which is usually described as hyperlactatemia. However, further comprehensive investigations were performed and demonstrated that hyperlactatemia was the only remaining cause of the wide anion gap metabolic acidosis in this patient. One of the different management approaches used for this patient was administration of rituximab, a monoclonal antibody to CD20, which elicited a rapid response when combined with conventional chemotherapy. The lactic acidosis was abruptly resolved, and the patient has remained in complete remission.
Historically, Warburg effect in childhood hematologic malignancies has led to absolute fatal outcome, but over the past two decades about half of the cases have achieved complete remission. Specific treatment should be promptly initiated in this context. Furthermore, our case illustrates this uncommon metabolic derangement in childhood Burkitt leukemia. While IEM are one of the causes of metabolic acidosis in young children, Warburg effect in hematologic malignancies should also be considered in older children.
Type B lactic acidosis is a rare metabolic complication in children with hematologic malignancies which can mimic inborn errors of metabolism (IEM). There have been few pediatric case series and case reports published on this specific condition. Moreover, the most recent review was conducted over two decades ago, in 2001.
The illustrative case of a 10-year-old boy with Burkitt leukemia who exhibited Warburg effect mimicking IEM was the basis of our systematic review. The previous review of this metabolic complication showed an extremely poor outcome. In recent years, however, advancements in cancer treatments may have led to the overall improvement in the clinical course.
To identify the clinical course, treatment strategies, and outcomes of childhood hematologic malignancies with type B lactic acidosis.
We performed a comprehensive search of the PubMed, Scopus, and Cochrane databases without any time restrictions to identify children with leukemia/lymphoma and type B lactic acidosis. The publications considered for inclusion were limited to English language articles.
Lactic acidosis initially subsided in 80% of patients receiving chemotherapy compared to 60% in the contrast group. The mortality rate of newly diagnosed cases was 45.5%, while the relapsed cases had a 100% mortality rate. All 8 cases reported before 2001 died from disease-related complications, while cases reported between 2003 and 2023 showed a 54.5% rate of complete remission.
Historically, this complication has led to fatal outcome; however, patients who received chemotherapy showed a more favorable response. Therefore, it is crucial to promptly initiate specific treatment in this context.
This systematic review has revealed an improvement in the clinical course and outcomes compared to the past. Future studies in this context might include a larger scale of cases involving multicenter research. Retrospective study on prognostic factors or therapeutic research in the era of immunotherapy and targeted therapy could also be performed in this population.
The authors would like to express their gratitude to Ms. Somjai Sittiprechachan for her contribution to the care of our patient.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeong KY, South Korea; Sultana N, Bangladesh S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY
| 1. | Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992-2004). Cancer. 2008;112:416-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 437] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 2. | Prusakowski MK, Cannone D. Pediatric Oncologic Emergencies. Hematol Oncol Clin North Am. 2017;31:959-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Sillos EM, Shenep JL, Burghen GA, Pui CH, Behm FG, Sandlund JT. Lactic acidosis: a metabolic complication of hematologic malignancies: case report and review of the literature. Cancer. 2001;92:2237-2246. [PubMed] [DOI] [Full Text] |
| 4. | Seheult J, Fitzpatrick G, Boran G. Lactic acidosis: an update. Clin Chem Lab Med. 2017;55:322-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Padda J, Khalid K, Kakani V, Cooper AC, Jean-Charles G. Metabolic Acidosis in Leukemia. Cureus. 2021;13:e17732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Vaupel P, Multhoff G. Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol. 2021;599:1745-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 500] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 7. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40322] [Article Influence: 10080.5] [Reference Citation Analysis (2)] |
| 8. | Field M, Block JB, Levin R, Rall D. Significance of blood lactate elevations among patients with acute leukemia and other neoplastic proliferative disorders. The American Journal of Medicine. 1966;40:528-47. [DOI] [Full Text] |
| 9. | Coleman RA, Sommerville HM, Friedman HS, Falletta JM, Kinney TR. Insulin therapy for ketolactic acidosis complicating malignancy. J Pediatr. 1982;100:584-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Ali AA, Flombaum CD, Brochstein JA, Gillio AP, Bussel JB, Boulad F. Lactic acidosis and renal enlargement at diagnosis and relapse of acute lymphoblastic leukemia. J Pediatr. 1994;125:584-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Révész T, Obeid K, Mpofu C. Severe lactic acidosis and renal involvement in a patient with relapsed Burkitt's lymphoma. Pediatr Hematol Oncol. 1995;12:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Hayek M, Srinivasan A. Acute lymphoblastic leukemia presenting with lactic acidosis and renal tubular dysfunction. J Pediatr Hematol Oncol. 2003;25:488-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Rastogi MV, Desai N, Quintos JB. Non-islet-cell tumor hypoglycemia and lactic acidosis in a child with congenital HIV and Burkitt's lymphoma. J Pediatr Endocrinol Metab. 2008;21:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Luscri N, Mauer M, Sarafoglou K, Moran A, Tolar J. Lactic acidosis and hypoglycemia with ALL relapse following engrafted bone marrow transplant. Pediatr Blood Cancer. 2009;53:223-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Kulkarni K, Kaur S, Sibal A, Jerath N, Arya LS. Severe lactic acidosis, hypertriglyceridemia, and extensive axial skeleton involvement in a case of disseminated Burkitt's lymphoma. Int J Hematol. 2010;91:546-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Terpe F, Siekmeyer M, Bierbach U, Siekmeyer W, Kratzsch J, Till H, Wittekind C, Kiess W. Fulminant and fatal course of acute lymphoblastic leukemia due to lactic acidosis and suspected abdominal compartment syndrome. J Pediatr Hematol Oncol. 2012;34:e80-e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Gökçe M, Unal S, Gülşen H, Başaran O, Cetin M, Gümrük F, Beşbaş N, Gürgey A. A rare metabolic complication of acute lymphoblastic leukemia in childhood: lactic acidosis. Turk J Pediatr. 2012;54:61-63. [PubMed] |
| 18. | Schuh AM, Leger KJ, Summers C, Uspal NG. Lactic Acidosis in a Critically Ill Patient: Not Always Sepsis. Pediatr Emerg Care. 2018;34:e165-e167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Narayani S, Balu P, Santhanam R, Scott JX. Type B Lactic Acidosis: A Rare Initial Presentation of Childhood Acute Lymphoblastic Leukemia. Indian J Nephrol. 2019;29:71-73. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Khera S, Pramanik SK, Kalra S, Dwivedi A. Type B lactic acidosis due to Warburg effect in a child presenting with T cell acute lymphoblastic leukaemia: a milder phenotype. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | O'Rourke E, Malone A, O'Marcaigh A, Storey L, Betts D, McDermott M, Smith OP. Burkitt Lymphoma/Leukaemia in Children & Young Adolescents. Ir Med J. 2020;113:6. [PubMed] |
| 22. | Hui WF, Hon KL, Leung AKC, Leung KKY, Ku SW, Cheng FWT. Continuous Renal Replacement Therapy (CRRT) for Nonrenal Indications among Critically Ill Children with Malignancy. Case Rep Pediatr. 2021;2021:6660466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Oriot D, Wood C, Gottesman R, Huault G. Severe lactic acidosis related to acute thiamine deficiency. JPEN J Parenter Enteral Nutr. 1991;15:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Svahn J, Schiaffino MC, Caruso U, Calvillo M, Minniti G, Dufour C. Severe lactic acidosis due to thiamine deficiency in a patient with B-cell leukemia/lymphoma on total parenteral nutrition during high-dose methotrexate therapy. J Pediatr Hematol Oncol. 2003;25:965-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Didisheim C, Ballhausen D, Choucair ML, Longchamp D, Natterer J, Ferry T, Perez MH, Amiet V. Severe Lactic Acidosis in a Critically Ill Child: Think About Thiamine! A Case Report. J Pediatr Intensive Care. 2021;10:307-310. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Smolka V, Rohanova M, Ludikova B, Novak Z, Zapalka M, Pospisilova D, Volejnikova J. Severe linezolid-induced lactic acidosis in a child with acute lymphoblastic leukemia: A case report. J Infect Chemother. 2020;26:1316-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Saudubray JM, Garcia-Cazorla À. Inborn Errors of Metabolism Overview: Pathophysiology, Manifestations, Evaluation, and Management. Pediatr Clin North Am. 2018;65:179-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Weinstein DA, Steuerwald U, De Souza CFM, Derks TGJ. Inborn Errors of Metabolism with Hypoglycemia: Glycogen Storage Diseases and Inherited Disorders of Gluconeogenesis. Pediatr Clin North Am. 2018;65:247-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Warburg O. The Metabolism of Carcinoma Cells1. The Journal of Cancer Research. 1925;9:148-63. [RCA] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 542] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | De Raes EA, Benoit DD, Depuydt PO, Offner F, Nollet J, Vantilborgh AK, Steel E, Noens LA, Decruyenaere JM. Early recognition of malignant lactic acidosis in clinical practice: report on 6 patients with haematological malignancies. Acta Clin Belg. 2012;67:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 31. | Brault C, Zerbib Y, Delette C, Marc J, Gruson B, Marolleau JP, Maizel J. The Warburg Effect as a Type B Lactic Acidosis in a Patient With Acute Myeloid Leukemia: A Diagnostic Challenge for Clinicians. Front Oncol. 2018;8:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Siegel SE, Stock W, Johnson RH, Advani A, Muffly L, Douer D, Reed D, Lewis M, Freyer DR, Shah B, Luger S, Hayes-Lattin B, Jaboin JJ, Coccia PF, DeAngelo DJ, Seibel N, Bleyer A. Pediatric-Inspired Treatment Regimens for Adolescents and Young Adults With Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia: A Review. JAMA Oncol. 2018;4:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |