Published online Dec 9, 2023. doi: 10.5409/wjcp.v12.i5.319
Peer-review started: July 19, 2023
First decision: August 31, 2023
Revised: September 7, 2023
Accepted: September 25, 2023
Article in press: September 25, 2023
Published online: December 9, 2023
Processing time: 141 Days and 12.9 Hours
Rotavirus is still a significant contributing morbidity and mortality in pediatric patients.
To look at clinical signs and symptoms and laboratory findings that can predict rotavirus gastroenteritis compared to non-rotavirus gastroenteritis.
This was a cross-sectional study with medical records obtained from December 2015 to December 2019. Inclusion criteria for this study include all hospitalised pediatric patients (0-18 years old) diagnosed with suspected rotavirus diarrhea. The receiver operating curve and Hosmer-Lemeshow test would be used to assess the final prediction findings' calibration (goodness of fit) and discrimination performance.
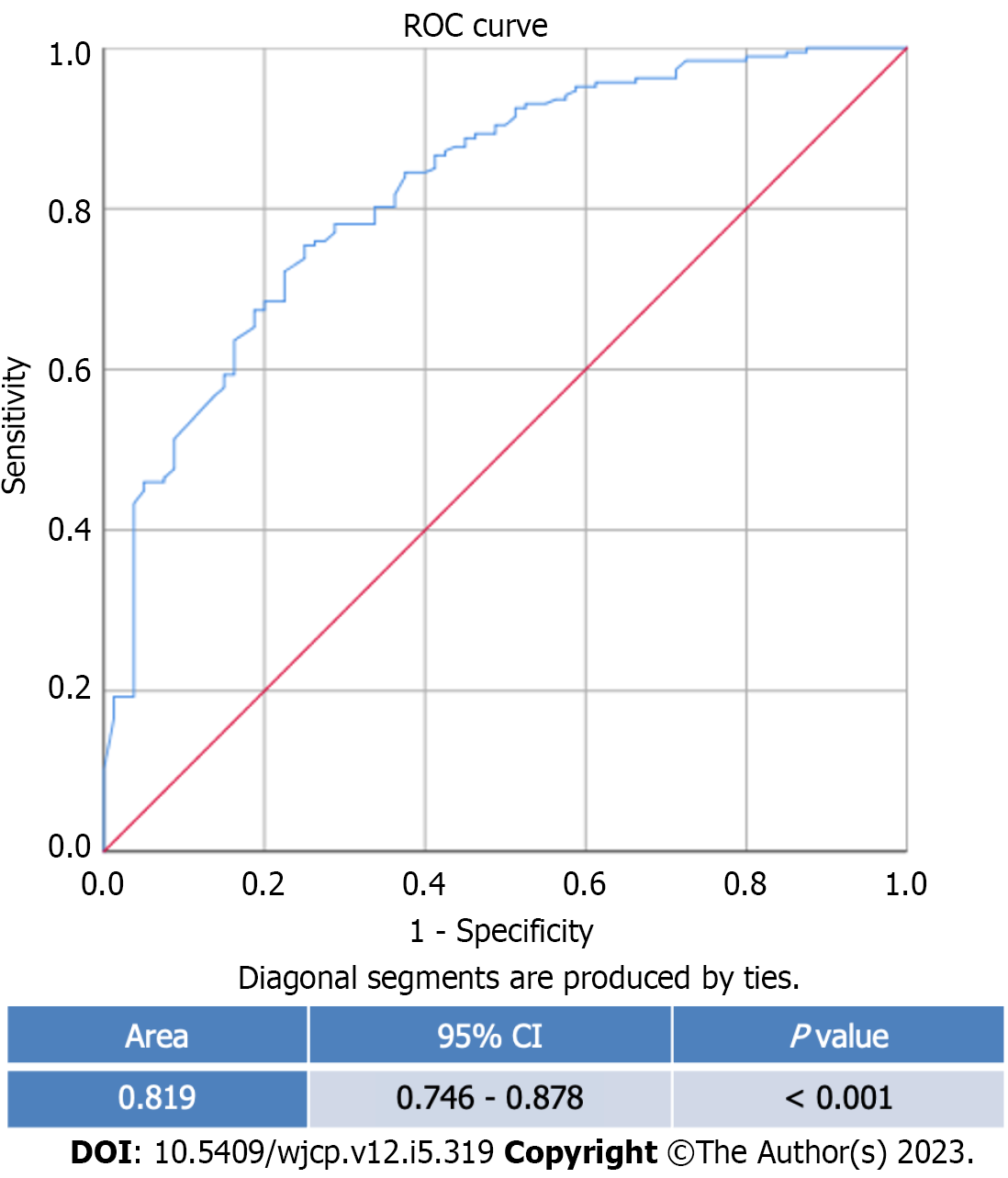
This study included 267 participants with 187 (70%) rotavirus-diarrhea cases. The patients were primarily male in both rotavirus (65.2%) and non-rotavirus (62.5%) groups. The median age is 1.33 years old (0.08-17.67 years old). Multivariate analysis shows that wet season (ORadj = 2.5; 95%CI: 1.3-4.8, Padj = 0.006), length of stay (LOS) ≥ 3 days (ORadj = 5.1; 95%CI: 1.4-4.8, Padj = 0.015), presence of abdominal pain (ORadj = 3.0; 95%CI: 1.3-6.8, Padj = 0.007), severe dehydration (ORadj = 2.9; 95%CI: 1.1-7.9, Padj = 0.034), abnormal white blood cell counts (ORadj = 2.8; 95%CI: 1.3-6.0, Padj = 0.006), abnormal random blood glucose (ORadj = 2.3; 95%CI: 1.2-4.4, Padj = 0.018) and presence of fecal leukocytes (ORadj = 4.1, 95%CI: 1.7-9.5, Padj = 0.001) are predictors of rotavirus diarrhea. The area under the curve for this model is 0.819 (95%CI: 0.746-0.878, P value < 0.001), which shows that this model has good discrimination.
Wet season, LOS ≥ 3 d, presence of abdominal pain, severe dehydration, abnormal white blood cell counts, abnormal random blood glucose, and presence of fecal leukocytes predict rotavirus diarrhea.
Core Tip: Rotavirus gastroenteritis accounted for 19.11% of diarrheal deaths worldwide in 2019 and is still a leading cause of morbidity and mortality, especially in children under five. This cross-sectional study involving 267 children found that wet season (ORadj = 2.5; 95%CI: 1.3-4.8, Padj = 0.006), length of stay ≥ 3 d (ORadj = 5.1; 95%CI: 1.4-4.8, Padj = 0.015), presence of abdominal pain (ORadj = 3.0; 95%CI: 1.3-6.8, Padj = 0.007), severe dehydration (ORadj = 2.9; 95%CI: 1.1-7.9, Padj = 0.034), abnormal white blood cell counts (ORadj = 2.8; 95%CI: 1.3-6.0, Padj = 0.006), abnormal random blood glucose (ORadj = 2.3; 95%CI: 1.2-4.4, Padj = 0.018) and presence of fecal leukocytes (ORadj = 4.1, 95%CI: 1.7-9.5, Padj = 0.001) are predictors of rotavirus diarrhea.
- Citation: Indrawan M, Chendana J, Handoko TGH, Widjaja M, Octavius GS. Clinical factors predicting rotavirus diarrhea in children: A cross-sectional study from two hospitals. World J Clin Pediatr 2023; 12(5): 319-330
- URL: https://www.wjgnet.com/2219-2808/full/v12/i5/319.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v12.i5.319
Rotavirus gastroenteritis accounted for 19.11% of diarrheal deaths worldwide in 2019 and is still a leading cause of morbidity and mortality, especially in children under five. Surveillance data from 2008-2018 showed that 40.78% of all diarrheal diseases in children in Southeast Asia were attributable to rotavirus infection[1]. The fecal-oral transmission from person-to-person contact or ingestion of fecally contaminated food and water commonly leads to a rapid spread among communities, especially in developing countries[2].
When diagnosing gastroenteritis, it is necessary to analyse fecal specimens using widely available assays because rotavirus-caused gastroenteritis cannot be clinically distinguished from that caused by other enteric pathogens[3]. However, because the findings do not change clinical management, which primarily depends on effective rehydration therapy, rotavirus is not routinely tested for in patients with gastroenteritis[4]. Despite its worldwide prevalence, detecting rotavirus in stool samples is still a logistical and financial challenge in developing countries[5].
Considering the disease burden, it is essential to know reliable clinical signs and symptoms and other non-confirmatory laboratory tests that clinicians can use to guide their treatment. Hence, this study aims to look at clinical signs and symptoms and laboratory findings that can predict rotavirus gastroenteritis compared to non-rotavirus gastroenteritis.
This study was cross-sectional, with medical records obtained from December 2015 to December 2019 from Siloam General Hospital and Siloam Hospital Lippo Village (SHLV). Patients covered by Indonesia's national health insurance can receive care at Siloam General Hospital, a teaching hospital. SHLV, on the other hand, primarily consists of patients who are self-paying or have private insurance. Inclusion criteria for this study include all hospitalised pediatric patients (0-18 years old) diagnosed with suspected rotavirus diarrhea, defined as the passing of ≥ 3 watery or loose stools each day[6]. Children who have previously experienced significant immunosuppression due to prolonged steroid usage or illnesses like the human immunodeficiency virus or primary immunodeficiency were excluded from this study. Another exclusion criterion was the presence of concurrent infections such as urinary tract infections or pneumonia. The sample size calculation used the following formula:
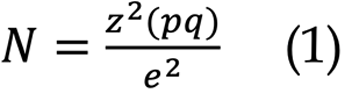
Where N is the sample size, z denotes the standard error, which was 1.96, p was the estimated prevalence in the population, q was 100-p, and e was the acceptable sample error set at 6% in this study. The prevalence of pediatric rotavirus diarrhea in Indonesia is 37.5% to 53.5%[7]. Hence, the minimum sample size required was 265 children.
We collected demographic data such as age, gender, and nutritional status. Clinical signs such as temperature upon arrival, vital signs, clinical manifestations (abdominal pain, respiratory symptoms, dehydration status according to World Health Organization (WHO)[6], duration and frequency of symptoms (diarrhea, vomiting, fever), length of stay (LOS), treatment given during hospitalisation [intravenous (IV) rehydration and any antibiotics], rotavirus vaccination status, as well as the seasons during which the children contracted diarrhea. The need for IV rehydration represented severe dehydration in this study. Meteorology Climatology and Geophysics Council's data in 2017 were used to determine the seasons where October – February was the rainy season, and the rest was the dry season in Tangerang[8]. We adhered to WHO 2006 growth chart for children below five years, while the Centers for Disease Control and Prevention 2000 growth charts were used for children aged 5-18 years old to classify nutritional status[9]. We did not collect any data on zinc used for diarrhea as all children were given zinc for diarrhea as per standard protocol in our hospitals. Lastly, we collected laboratory findings such as complete blood count, serum electrolytes, random blood glucose, erythrocyte sedimentation rate (ESR), and urinary ketone for dehydration markers and fecal leukocytes[10]. We present laboratory values dichotomously (normal vs abnormal values) for multivariate analysis and in numerical forms for descriptive purposes. The reference range will be based on our population study's mean or median age group.
The neutrophil-lymphocyte ratio (NLR) is obtained by dividing the total band and segmented neutrophil by the lymphocyte. In contrast, the monocyte-lymphocyte ratio is obtained by dividing the monocyte by the lymphocyte. Absolute neutrophil count is calculated with the formula as follows: (Total WBC × % [PMNs + bands]) ÷ 100.
While the absolute lymphocyte count is calculated with the formula: WBC count × 1000 × % lymphocyte. (NLR were obtained by dividing the total neutrophils by lymphocyte counts, and the same methodology was applied to obtain lymphocyte-to-monocyte ratio (LMR) and platelet-to-lymphocyte ratio (PLR)[11]. One or two ccs of peripheral venous blood samples were collected by antecubital venipuncture into vacutainer tubes (Becton Dickinson, Rutherford, NJ, United States) containing tri potassium ethylenediaminetetraacetic acid. The complete blood count was done within one to two hours after the blood samples were drawn, and the analysis was performed using the Advia 2120i automated analyser (Siemens Healthcare Diagnostics, Deerfield, IL, United States). Erythrocyte sedimentation rate levels were measured by TEST 1 (Alifax, Padova, Italy). The rotavirus analysis was done using immunochromatography (Alcore One-Step Rotavirust Test). Those who tested positive would be categorised as having rotavirus diarrhea, and those who tested negative would be categorised as having non-rotavirus diarrhea. Strict quality control procedures were adopted. All of the independent variables were chosen based on previous studies[7,12,13].
The data was processed using IBM SPSS 26.0 (Statistical Package for the Social Sciences, IBM Corp., Armonk, NY, United States). After a normality test, data with a normal distribution will be presented as mean and standard deviation. If not, the median and range will be applied. The Mann-Whitney-U test is used when the distribution of numerical data is non-normal, while the T-test is used when the distribution of numerical data is normal. Chi-square was used for bivariate analysis, and variables with p-values less than 0.25 would be used in multivariate logistic regression analysis. The receiver operating curve (ROC) and Hosmer-Lemeshow test would be used to assess the final prediction findings' calibration (goodness of fit) and discrimination performance. The area under the curve (AUC) is equal to 0.5 when the ROC curve represents chance, and it is equal to 1.0 when the ROC curve represents accuracy. A good calibration would be indicated by a P value > 0.05. The sensitivity and specificity of the various predictor variables in identifying non-severe and severe pneumonia were then calculated using the area under the curve. We also analysed casewise diagnostics to identify any outliers.
This study protocol was approved by the Committee on Ethics at the University of Pelita Harapan, Tangerang, Indonesia, with Code Ethic No. 430/FK-UPH/Ext./V/2019. The ethical board exempted informed consent due to the retrospective nature of our study. Identities were removed entirely, and data were analysed anonymously.
This study included 267 participants with 187 (70%) rotavirus-diarrhea cases (Table 1). The patients were primarily male in both rotavirus (65.2%) and non-rotavirus (62.5%) groups. The median age is 1.33 years old (0.08-17.67 years old), with the majority belonging to the 0-11 mo old category (34.5%). Most patients had good nutritional status in the non-rotavirus group (51.3%) and rotavirus group (53.5%). Most rotavirus cases occurred during the dry season (77.1%), while non-rotavirus cases occurred mainly in the wet seasons (37.8%) with an odds ratio (OR) of 0.49 [95% confidence interval (CI) 0.29-0.93, P value 0.01]. The majority of patients had a length of stay of ≥ 3 d in both groups (95% in the non-rotavirus group and 83.4% in the rotavirus group) (OR 0.27; 95%CI: 0.1-0.78, P value 0.02). Only one patient was put in the intensive care unit, belonging to the non-rotavirus group. In both groups, most patients are not vaccinated for rotavirus (86.3% in non-rotavirus diarrhea vs 94.7% in rotavirus diarrhea) with an OR of 0.35 (95%CI: 0.14-0.97, P value 0.04) (Table 1).
| Characteristics | Frequency | OR (95%CI) | P value | |
| Non-rotavirus | Rotavirus | |||
| Gender | ||||
| Male | 50 (29.1) | 122 (70.9) | 0.89 (0.52-1.53) | 0.77 |
| Female | 30 (31.6) | 65 (68.4) | ||
| Age (mo) | ||||
| 0-11 | 21 | 71 | Ref | Ref |
| 12-23 | 24 | 60 | 0.74 (0.38-1.46) | 0.38 |
| 24-59 | 17 | 44 | 0.77 (0.37-1.61) | 0.48 |
| ≥ 60 | 18 | 12 | 0.19 (0.08-0.47) | < 0.001 |
| Nutritional status | ||||
| Severely underweight | 10 | 34 | 1.39 (0.63-3.08) | 0.4 |
| Underweight | 14 | 32 | 0.94 (0.45-1.94) | 0.86 |
| Normoweight | 41 | 100 | Ref | Ref |
| Overweight | 7 | 6 | 0.35 (0.11-1.11) | 0.08 |
| Obese | 8 | 15 | 0.77 (0.30-1.95) | 0.58 |
| Season | ||||
| Dry | 32 (22.9) | 108 (77.1) | 0.49 (0.29-0.83) | 0.01 |
| Wet | 48 (37.8) | 79 (62.2) | ||
| Hospitalisation | ||||
| In-patient | 79 (29.7) | 187 (70.3) | N/A | 0.30 |
| Intensive Care Unit | 1 (100) | 0 (0) | ||
| Length of stay (d) | ||||
| < 3 | 4 (11.4) | 31 (88.6) | 0.27 (0.10-0.78) | 0.02 |
| ≥ 3 | 76 (32.8) | 156 (67.2) | ||
| Clinical manifestations | ||||
| Diarrhea duration (d) | ||||
| < 2 | 2 (22.2) | 7 (77.8) | Ref | Ref |
| 2-4 | 32 (25) | 96 (75) | 0.86 (0.17-4.34) | 1 |
| > 4 | 46 (35.4) | 84 (64.6) | 0.52 (0.10-2.62) | 0.72 |
| Diarrhea frequency (d) | ||||
| < 3 | 4 (66.7) | 2 (33.3) | Ref | Ref |
| 3-4 | 32 (30.2) | 74 (69.8) | 4.63 (0.81-26.54) | 0.08 |
| > 5 | 44 (28.4) | 111 (71.6) | 5.05 (0.90-28.54) | 0.07 |
| Vomiting frequency (d) | ||||
| 0-3 | 50 (36) | 89 (64) | Ref | Ref |
| 4-5 | 9 (20) | 36 (80) | 2.25 (1.00-5.04) | 0.07 |
| > 5 | 21 (25.3) | 62 (74.7) | 1.66 (0.91-3.04) | 0.13 |
| Fever duration (d) | ||||
| < 3 | 35 (25.4) | 103 (74.6) | 0.63 (0.38-1.01) | 0.12 |
| ≥3 | 45 (34.9) | 84 (65.1) | ||
| Abdominal pain | ||||
| Negative | 56 (25) | 168 (75) | 0.26 (0.14-0.52) | < 0.01 |
| Positive | 24 (55.8) | 19 (44.2) | ||
| Respiratory symptoms | ||||
| Negative | 59 (28.8) | 146 (71.2) | 0.79 (0.43-1.45) | 0.54 |
| Positive | 21 (33.9) | 41 (66.1) | ||
| Dehydration | ||||
| No dehydration | 35 (46.7) | 40 (53.3) | Ref | Ref |
| Mild-moderate | 33 (20.8) | 126 (79.2) | 3.34 (1.85-6.10) | < 0.01 |
| Severe | 12 (36.4) | 21 (63.6) | 1.53 (0.66-3.55) | 0.43 |
| Treatment | ||||
| Antibiotic | ||||
| Negative | 3 (1.7) | 176 (98.3) | 0.002 (0.001-0.01) | < 0.01 |
| Positive | 77 (87.5) | 11 (12.5) | ||
| IV rehydration | ||||
| Negative | 19 (32.2) | 40 (67.8) | 1.15 (0.61-2.13) | 0.80 |
| Positive | 61 (29.3) | 147 (70.7) | ||
| Rotavirus vaccination | ||||
| Negative | 69 (28) | 177 (72) | 0.35 (0.14-0.97) | 0.04 |
| Positive | 11 (52.4) | 10 (47.6) | ||
| Laboratory examinations | ||||
| Hemoglobin | ||||
| Normal | 52 (28.1) | 133 (71.9) | 0.75 (0.43-1.32) | 0.40 |
| Abnormal | 28 (34.1) | 54 (65.9) | ||
| White blood cell count | ||||
| Normal | 50 (24.6) | 153 (75.4) | 0.37 (0.21-0.67) | < 0.01 |
| Abnormal | 30 (46.9) | 34 (53.1) | ||
| Basophils | ||||
| Normal | 63 (31.5) | 137 (68.5) | 1.35 (0.72-2.53) | 0.43 |
| Abnormal | 17 (25.4) | 50 (74.6) | ||
| Eosinophils | ||||
| Normal | 23 (26.1) | 65 (73.9) | 0.76 (0.43-1.34) | 0.42 |
| Abnormal | 57 (31.8) | 122 (68.2) | ||
| Band neutrophils | ||||
| Normal | 66 (30.7) | 149 (69.3) | 1.20 (0.61-2.37) | 0.72 |
| Abnormal | 14 (26.9) | 38 (73.1) | ||
| Segment neutrophils | ||||
| Normal | 9 (18.8) | 39 (81.3) | 0.48 (0.22-1.05) | 0.09 |
| Abnormal | 71 (32.4) | 148 (67.6) | ||
| Total neutrophils | ||||
| Normal | 10 (20.4) | 39 (79.6) | 0.54 (0.26-1.15) | 0.15 |
| Abnormal | 70 (32.1) | 148 (67.9) | ||
| Lymphocytes | ||||
| Normal | 13 (26) | 37 (74) | 0.79 (0.40-1.58) | 0.61 |
| Abnormal | 67 (30.9) | 150 (69.1) | ||
| Monocytes | ||||
| Normal | 44 (32.1) | 93 (67.9) | 1.24 (0.73-2.10) | 0.51 |
| Abnormal | 36 (27.7) | 94 (72.3) | ||
| Absolute lymphocytes count | ||||
| Normal | 28 (23.5) | 91 (76.5) | 0.57 (0.33-0.98) | 0.05 |
| Abnormal | 52 (35.1) | 96 (64.9) | ||
| Absolute neutrophils count | ||||
| Normal | 40 (23.8) | 128 (76.2) | 0.46 (0.30-0.79) | 0.007 |
| Abnormal | 40 (40.4) | 59 (59.6) | ||
| Neutrophils-to-lymphocyte ratio | ||||
| Normal | 33 (29.5) | 4 (57.1) | 0.96 (0.57-1.63) | 0.99 |
| Abnormal | 77 (29.6) | 183 (70.4) | ||
| Lymphocyte-to-monocyte ratio | ||||
| Normal | 3 (42.9) | 4 (57.1) | 1.79 (0.40-8.15) | 0.74 |
| Abnormal | 77 (29.6) | 183 (70.4) | ||
| Platelet-to-lymphocyte ratio | ||||
| Normal | 6 (22.2) | 21 (77.8) | 0.64 (0.25-1.64) | 0.47 |
| Abnormal | 74 (31) | 165 (69) | ||
| Erythrocyte sedimentation rate | ||||
| Normal | 37 (31.1) | 82 (68.9) | 1.1 (0.65-1.86) | 0.82 |
| Abnormal | 43 (29.1) | 105 (70.9) | ||
| Random blood glucose | ||||
| Normal | 45 (24.6) | 138 (75.4) | 0.46 (0.26-0.80) | 0.007 |
| Abnormal | 35 (41.7) | 49 (58.3) | ||
| Sodium | ||||
| Normal | 42 (27.5) | 111 (72.5) | 0.76 (0.45-1.28) | 0.37 |
| Abnormal | 38 (33.3) | 76 (66.7) | ||
| Potassium | ||||
| Normal | 33 (29.5) | 79 (70.5) | 0.96 (0.56-1.63) | 0.99 |
| Abnormal | 47 (30.3) | 108 (69.7) | ||
| Chloride | ||||
| Normal | 42 (29.6) | 100 (70.4) | 0.96 (0.57-1.63) | 0.99 |
| Abnormal | 38 (30.4) | 87 (69.6) | ||
| Urine and fecal tests | ||||
| Urinary ketone | ||||
| Negative | 74 (29.8) | 174 (70.2) | 0.92 (0.34-2.52) | 1.00 |
| Positive | 6 (31.6) | 13 (68.4) | ||
| Fecal leukocytes | ||||
| Negative | 58 (25.9) | 166 (74.1) | 0.33 (0.17-0.65) | 0.002 |
| Positive | 22 (51.2) | 21 (48.8) | ||
The clinical manifestations vary between the two groups. Most patients presented with mild-moderate dehydration (67.4%) in rotavirus diarrhea, while most in the non-rotavirus group presented with no dehydration (43.8%). Fever mostly lasts less than three days for rotavirus diarrhea (55.1%), while fever lasts mostly ≥ 3 d in the non-rotavirus group (56.3%). The last difference in clinical manifestation between the two groups is in diarrhea duration, where in rotavirus diarrhea it lasts 2-4 d (51.3%) while it mostly lasts > 4 d (57.5%) in the non-diarrhea group. Meanwhile, in both groups, most patients suffer from more than five diarrhea episodes per day, 0-3 vomiting episodes, abdominal pain, and respiratory symptoms (Figure 1).
98.3% of patients that are rotavirus positive did not receive antibiotics. In comparison, almost all (96.3%) patients who suffer from non-rotavirus diarrhea receive antibiotics with an OR of 0.002 (95%CI: 0.001-0.01, P value < 0.01). In both groups, most patients receive IV rehydration with no significant statistical difference (OR 1.15; 95%CI: 0.61-2.13, P value 0.08).
Most laboratory parameters are within normal range except for decreased LMR and PLR in both groups, while ESR is slightly elevated. The potassium level is also slightly below the normal reference range, with a median of 4 mmol/L in both groups. Most urinary ketones are negative in both groups (92.5% in the non-rotavirus vs 93% in the rotavirus group). There is a significant difference in fecal leukocyte findings between both groups with an OR of 0.33 (95%CI: 0.17-0.65, P value 0.002) (Tables 1 and 2).
| Variable | Reference range | Non rotavirus | Rotavirus |
| Hemoglobin (g/dL) | 10.5-14.0 | 13.49 (± 16.0) | 13.0 (± 11.3) |
| White blood cell count (109 L) | 6.0-17.5 | 14.54 (4.21-42.07) | 11.72 (2.86-38.6) |
| Basophils (%) | 0-0.75 | 0 (0-1) | 0 (0-1) |
| Eosinophils (%) | 1-3 | 0 (0-5) | 0 (0-5) |
| Band neutrophils (%) | 3-5 | 3 (2-12) | 3 (1-8) |
| Segment neutrophils (%) | 54-62 | 62 (20-90) | 51 (15-92) |
| Total neutrophils (%) | 58-66 | 65 (23-93) | 54 (18-95) |
| Lymphocytes (%) | 25-33 | 27 (3-69) | 37 (3-75) |
| Monocytes (%) | 3-7 | 7 (3-13) | 7 (1-15) |
| Absolute lymphocyte count | 4000-10500 | 3354.6 (290.1-15356) | 4232.8 (459-24532.2) |
| Absolute neutrophils count | 1500-8500 | 8662 (1768.7-32814.6) | 6100 (972.4-27898) |
| Neutrophil-to-lymphocyte ratio | Male: 1.48-6.37; Female: 1.22-5.59 | 2.5 (0-31) | 2 (0-32) |
| Lymphocyte-to-monocyte ratio | Male: 11.12-26.82; Female: 16.08-28.18 | 4.29 (0.38-16.50) | 5.29 (0.50-54) |
| Platelet-to-lymphocyte ratio | Male: 132.07-178.53; Female: 132.46-181.90 | 96.7 (20.5-722) | 98 (15.89-969) |
| Thrombocyte count (μ/mm3) | 150000-350000 | 350000 (109800-1099000) | 381000 (115000-1094000) |
| Erythrocyte sedimentation rate (mm/hour) | 0-10 | 12 (2-215) | 14 (1-68) |
| Random blood glucose (mg/dL) | 60-100 | 94.5 (48-238) | 85 (15-160) |
| Sodium (mmol/L) | 134-143 | 136 (113-154) | 135 (124-165) |
| Potassium (mmol/L) | 4.1-5.3 | 4 (1.6-5.9) | 4 (1.4-7.1) |
| Chloride (mmol/L) | 98-106 | 100 (34.5-134) | 101 (9.7-141) |
Multivariate logistic regression analysis adjusted for variables with a p-value of < 0.25 is shown in Table 3. Multivariate analysis shows that wet season (ORadj = 2.5; 95%CI: 1.3-4.8, Padj = 0.006), LOS ≥ 3 d (ORadj = 5.1; 95%CI: 1.4-4.8, Padj = 0.015), presence of abdominal pain (ORadj = 3.0; 95%CI: 1.3-6.8, Padj = 0.007), severe dehydration (ORadj = 2.9; 95%CI: 1.1-7.9, Padj = 0.034), abnormal white blood cell counts (ORadj = 2.8; 95%CI: 1.3-6.0, Padj = 0.006), abnormal random blood glucose (ORadj = 2.3; 95%CI: 1.2-4.4, Padj = 0.018) and presence of fecal leukocytes (ORadj = 4.1, 95%CI: 1.7-9.5, Padj = 0.001) are predictors of rotavirus diarrhea. The Hosmer-Lemeshow test shows this model is a good fit with a p-value of 0.361. The AUC for this model is 0.819 (95%CI: = 0.746-0.878, P value < 0.001), which shows that this model has good discrimination (Figure 1).
| Values | ORadj (95%CI) | P valueadj |
| Wet season | 2.5 (1.3-4.8) | 0.006 |
| Length of stay ≥ 3 d | 5.1 (1.4-4.8) | 0.015 |
| Fever lasts ≥ 3 d | 1.9 (0.97-3.5) | 0.06 |
| Abdominal pain | 3.0 (1.3-6.8) | 0.007 |
| Mild-moderate dehydration | 1.2 (0.4-3.5) | 0.75 |
| Severe dehydration | 2.9 (1.1-7.9) | 0.034 |
| Abnormal white blood cell counts | 2.8 (1.3-6.0) | 0.006 |
| Abnormal absolute neutrophil counts | 1.9 (0.9-3.8) | 0.072 |
| Abnormal random blood glucose | 2.3 (1.2-4.4) | 0.018 |
| Presence of fecal leukocytes | 4.1 (1.7-9.5) | 0.001 |
Rotavirus was shown to be the leading cause of morbidity and mortality in children, especially five years old and below[13]. Symptoms tend to be most severe in children between 3-24 mo old. However, in approximately 25% of rotavirus cases, severe disease occurs after two years of age. Serologic evidence of rotavirus infection can be virtually observed in all children aged 4-5[12]. Our results showed that our samples have a median age of 1.3 years old. In multivariate analysis, the wet season showed a significant association with rotavirus infection. A previous study showed that in the tropics, rotavirus infection tends to occur all year round compared to the seasonal pattern of infection in countries with temperate climates. However, factors other than temperature, such as rainfall and humidity, play a significant role in rotavirus incidence in the tropics. Due to the waterborne nature of rotavirus transmission, the outbreak pattern might be altered by precipitation levels[14]. In low-income areas, stagnant water sources and poor access to uncontaminated water and sanitation were hypothesised to pose a higher risk for rotavirus infection[15]. Study showed that monsoon season was significantly correlated with dehydrating rotavirus diarrhea among children aged 0-59 mo in South Asia[16]. Previous meta-analyses have also concluded that every 1'C increase in temperature is associated with a 4%-10% decrease in rotavirus infection incidence in the tropics. However, for every one-centimetre increase in mean monthly rainfall and 1% increase in relative humidity (22%), rotavirus incidence decreased by 1% and 3%, respectively. Based on the evidence, it was previously concluded that rotavirus incidence in the tropics was the highest during colder and drier times of the year[17].
Gastroenteritis is generally more severe in the rotavirus group than in the non-rotavirus sample. Length of stay was shown to be prolonged in children age below two years old with rotavirus gastroenteritis. Prolonged LOS, especially in pediatric patients, promote work absenteeism in 70% of parents and could negatively impact the quality of life[18]. Prolonged LOS ≥ 3 d is significantly associated with rotavirus gastroenteritis in this study.
Rotavirus has a broad spectrum of symptoms after 1 to 3 d of incubation, varying from subclinical illness to severe dehydration, shock and death. Rotavirus infection has a similar but more severe clinical manifestation than other gastrointestinal infections. Diagnosis of rotavirus gastroenteritis is commonly clinical based on the presence of vomiting and low-grade fever, followed by watery, non-bloody diarrhea. Moderate fever (temperature < 39°C) is found in approximately one-third of infected patients. Fever and vomiting frequently cease within 1 to 3 d. This finding explains our findings that prolonged fever and vomiting are not significantly associated with rotavirus gastroenteritis. Other physical findings such as abdominal cramping, fatigue and signs of dehydration might also occur during the 5 to 7 d disease course. Diagnosis can be further established by the absence of atypical features such as high-grade fever, which is more commonly present in bacterial gastroenteritis, projectile vomiting, bilious vomiting, blood or mucus in stool, persistent diarrhea for more than seven days, focal abdominal pain, absent bowel sound, and history of antibiotic use[4,19,20]. Presence of abdominal pain and severe dehydration was associated with rotavirus infection in this study. These findings correlate with findings from the previous study that rotavirus-positive subjects were more likely to present with severe dehydration and tend to require intravenous rehydration therapy than rotavirus-negative subjects[16]. However, the role of abdominal pain as a predictor of rotavirus infection is still controversial. Abdominal pain is hypothesised to be limited in rotavirus infection due to low inflammatory response demonstrated by minimal elevation of C-reactive protein or calprotectin levels as clinical markers of inflammation. Rotavirus replication appears to be limited exclusively in the villous epithelium of small intestines, and the diarrhea was considered malabsorptive secondary to enterocyte destruction. Despite non-specific symptomatology, severe abdominal pain and tenesmus tend to indicate large intestines involvement[21,22]. Previous studies demonstrated that abdominal pain is particularly frequent in rotavirus-positive subjects or co-infection with rotavirus subjects. However, no significant association has been found in statistical analyses[23]. Ambiguous interpretation of abdominal pain by parents of young children during history taking could lead to a bias in pain assessment. This finding might explain the contradictory result regarding abdominal pain in this study.
In this study, rotavirus-positive patients demonstrated abnormal white blood cell counts and abnormal random blood glucose. However, previous studies showed no significant difference in white blood cell count between the rotavirus-positive and rotavirus-negative groups. These studies did not mainly compare rotavirus and the specific etiologic agent of gastroenteritis[24-26]. The wide causative range of non-rotavirus gastroenteritis might explain the different findings. Other more significant variables to be compared with rotavirus are indicators of metabolic acidosis secondary to fluid loss. A previous study showed that rotavirus-positive subjects had lower blood pH, higher base deficit, and lower bicarbonate[25]. Complete blood count examination appears to have minimal value in predicting rotavirus infection.
Several reports have stated that respiratory symptoms might also occur during the rotavirus infection course. However, the mechanism that explains this finding is still controversial. Coincidental infection with respiratory viruses during rotavirus endemic season might manifest as respiratory symptoms. Previous studies have indicated that rotavirus might infect extra-intestinal organs during viremia, but there has not been sufficient evidence to prove its replication ability outside the intestine. Rotavirus antigens or RNA were detected in the spleen, heart, kidneys, testes, bladder, liver, cells or secretions from the respiratory tract, and endothelial cells[20,27]. Our result showed that respiratory symptoms do not predict rotavirus infection in children. Stool analyses in rotavirus-positive subjects are common without blood or white blood cells[20]. This result correlates with our findings that faecal leukocytes are a predictor of rotavirus infection. The presence of fecal leucocytes in rotavirus infection may indicate inflammatory processes in the intestines[28]. No other hematologic findings were significantly correlated with rotavirus infection in this study.
This study has its limitations. Our research is a cross-sectional study to start with. As a result, we could not account for some factors that might affect the severity of rotavirus diarrhea, such as birth weight, source of water, and house-crowding status. Second, the study's focus on just two institutions raises the possibility that this study may not be generalisable to other centres. However, given the nature of the two different hospitals, we were able to include kids from a range of racial and ethnic backgrounds, assuring that both high- and low-income parents were represented in this study. Some variables have insufficient strength for analysis due to missing or unanalysed laboratory data. Third, we could not account for any temporal changes occurring in the four years of the study period. Any seasonality, changes in guidelines and diagnosis of rotavirus diarrhea, as well as immunization update may have altered the results of the study. Lastly, we did not analyse the antigenic properties of the rotavirus due to limited equipment and funding.
In this study, wet season, LOS ≥ 3 d, presence of abdominal pain, severe dehydration, abnormal white blood cell counts, abnormal random blood glucose and presence of fecal leukocytes predict rotavirus diarrhea. Since these parameters have good discrimination, these findings should alert clinicians to the presence of rotavirus diarrhea. Clinicians may use these parameters to further alert them to the possibility of rotavirus diarrhea in children and order tests more prudently as well as prescribing appropriate therapy.
Rotavirus gastroenteritis accounted for 19.11% of diarrheal deaths worldwide in 2019 and is still a leading cause of morbidity and mortality, especially in children under five. Surveillance data from 2008-2018 showed that 40.78% of all diarrheal diseases in children in Southeast Asia were attributable to rotavirus infection.
Rotavirus diarrhea is still a leading cause of mortality among Indonesian children. However, since antigen detection is not affordable amongst many families, other cheap clinical proxies for rotavirus diarrhea must be determined.
This study aims to determine clinical and laboratory values that may serve as an indicator to raise clinicians' awareness about rotavirus diarrhea.
This study was cross-sectional, with medical records obtained from December 2015 to December 2019 from Siloam General Hospital and Siloam Hospital Lippo Village. Inclusion criteria for this study include all hospitalised pediatric patients (0-18 years old) diagnosed with suspected rotavirus diarrhea, defined as the passing of ≥ 3 watery or loose stools each day. We collected demographic data such as age, gender, and nutritional status. Clinical signs such as temperature upon arrival, vital signs, clinical manifestations (abdominal pain, respiratory symptoms, dehydration status according to World Health Organization), duration and frequency of symptoms (diarrhea, vomiting, fever), length of stay (LOS), treatment given during hospitalisation [intravenous (IV) rehydration and any antibiotics], rotavirus vaccination status, as well as the seasons during which the children contracted diarrhea.
This study included 267 participants with 187 (70%) rotavirus-diarrhea cases. The patients were primarily male in both rotavirus (65.2%) and non-rotavirus (62.5%) groups. The median age is 1.33 years old (0.08-17.67 years old). Multivariate analysis shows that wet season (ORadj = 2.5; 95%CI: 1.3-4.8, Padj = 0.006), LOS ≥ 3 d (ORadj = 5.1; 95%CI: 1.4-4.8, Padj = 0.015), presence of abdominal pain (ORadj = 3.0; 95%CI: 1.3-6.8, Padj = 0.007), severe dehydration (ORadj = 2.9; 95%CI: 1.1-7.9, Padj = 0.034), abnormal white blood cell counts (ORadj = 2.8; 95%CI: 1.3-6.0, Padj = 0.006), abnormal random blood glucose (ORadj = 2.3; 95%CI: 1.2-4.4, Padj = 0.018) and presence of fecal leukocytes (ORadj = 4.1, 95%CI: 1.7-9.5, Padj = 0.001) are predictors of rotavirus diarrhea. The area under the curve for this model is 0.819 (95%CI: = 0.746-0.878, P value < 0.001), which shows that this model has good discrimination.
In this study, wet season, LOS ≥ 3 d, presence of abdominal pain, severe dehydration, abnormal white blood cell counts, abnormal random blood glucose and presence of fecal leukocytes predict rotavirus diarrhea. Since these parameters have good discrimination, these findings should alert clinicians to the presence of rotavirus diarrhea. Clinicians may use these parameters to further alert them to the possibility of rotavirus diarrhea in children and order tests more prudently as well as prescribing appropriate therapy.
More bigger and confirmatory studies are needed to confirm our findings.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Indonesia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; Viswanathan VK, United States S-Editor: Liu JH L-Editor: A P-Editor: Zhang XD
| 1. | Du Y, Chen C, Zhang X, Yan D, Jiang D, Liu X, Yang M, Ding C, Lan L, Hecht R, Zhu C, Yang S. Global burden and trends of rotavirus infection-associated deaths from 1990 to 2019: an observational trend study. Virol J. 2022;19:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 2. | Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C; Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1912] [Cited by in RCA: 1890] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 3. | Zuccotti G, Meneghin F, Dilillo D, Romanò L, Bottone R, Mantegazza C, Giacchino R, Besana R, Ricciardi G, Sterpa A, Altamura N, Andreotti M, Montrasio G, Macchi L, Pavan A, Paladini S, Zanetti A, Radaelli G. Epidemiological and clinical features of rotavirus among children younger than 5 years of age hospitalized with acute gastroenteritis in Northern Italy. BMC Infect Dis. 2010;10:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Parashar UD, Nelson EA, Kang G. Diagnosis, management, and prevention of rotavirus gastroenteritis in children. BMJ. 2013;347:f7204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Justino MCA, Campos EA, Mascarenhas JDP, Soares LS, Guerra SFS, Furlaneto IP, Pavão MJC Jr, Maciel TS, Farias FP, Bezerra OM, Vinente CBG, Barros RJS, Linhares AC. Rotavirus antigenemia as a common event among children hospitalised for severe, acute gastroenteritis in Belém, northern Brazil. BMC Pediatr. 2019;19:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | World Health Organization. The Treatment of diarrhoea: a manual for physicians and other senior health workers. 5th April, 2023. Available from: https://apps.who.int/iris/bitstream/handle/10665/43209/9241593180.pdf?sequence=1. |
| 7. | Prasetyo D, Ermaya YS, Sabaroedin IM, Widhiastuti D, Bachtiar NS, Kartasasmita CB. Genotype Profiles of Rotavirus Strains in Children under 5-year-old Outpatients with Diarrhea in Bandung, West Java, Indonesia. J Glob Infect Dis. 2022;14:142-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Badan Meteorologi K, dan Geofisika. Prakiraan Musim Hujan 2016/2017 Di Indonesia. 5th April, 2023. Available from: https://www.bmkg.go.id/iklim/prakiraan-musim.bmkg?p=prakiraan-musim-hujan-20162017-di-indonesia&tag=prakiraan-musim&lang=ID. |
| 9. | Sjarif DR, Nasar SS, Devaera Y, Tanjung CF. Asuhan Nutrisi Pediatrik (Pediatric Nutrition Care). 1st ed. Unit Kerja Koordinasi Nutrisi dan Penyakit Metabolik Ikatan Dokter Anak Indonesia; 2011. |
| 10. | Steiner MJ, Nager AL, Wang VJ. Urine specific gravity and other urinary indices: inaccurate tests for dehydration. Pediatr Emerg Care. 2007;23:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Moosmann J, Krusemark A, Dittrich S, Ammer T, Rauh M, Woelfle J, Metzler M, Zierk J. Age- and sex-specific pediatric reference intervals for neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and platelet-to-lymphocyte ratio. Int J Lab Hematol. 2022;44:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Tate JE, Burton AH, Boschi-Pinto C, Parashar UD; World Health Organization–Coordinated Global Rotavirus Surveillance Network. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000-2013. Clin Infect Dis. 2016;62 Suppl 2:S96-S105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 828] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 13. | Hallowell BD, Chavers T, Parashar U, Tate JE. Global Estimates of Rotavirus Hospitalizations Among Children Below 5 Years in 2019 and Current and Projected Impacts of Rotavirus Vaccination. J Pediatric Infect Dis Soc. 2022;11:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Kraay ANM, Brouwer AF, Lin N, Collender PA, Remais JV, Eisenberg JNS. Modeling environmentally mediated rotavirus transmission: The role of temperature and hydrologic factors. Proc Natl Acad Sci USA. 2018;115:E2782-E2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Ghoshal V, Das RR, Nayak MK, Singh S, Das P, Mohakud NK. Climatic Parameters and Rotavirus Diarrhea Among Hospitalized Children: A Study of Eastern India. Front Pediatr. 2020;8:573448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Yeasmin S, Hasan SMT, Chisti MJ, Khan MA, Faruque ASG, Ahmed T. Factors associated with dehydrating rotavirus diarrhea in children under five in Bangladesh: An urban-rural comparison. PLoS One. 2022;17:e0273862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38:1487-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 201] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Albano F, Bruzzese E, Bella A, Cascio A, Titone L, Arista S, Izzi G, Virdis R, Pecco P, Principi N, Fontana M, Guarino A. Rotavirus and not age determines gastroenteritis severity in children: a hospital-based study. Eur J Pediatr. 2007;166:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Bernstein DI. Rotavirus overview. Pediatr Infect Dis J. 2009;28:S50-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA, Greenberg HB, O'Ryan M, Kang G, Desselberger U, Estes MK. Rotavirus infection. Nat Rev Dis Primers. 2017;3:17083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 429] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 21. | Greenberg HB, Estes MK. Rotaviruses: from pathogenesis to vaccination. Gastroenterology. 2009;136:1939-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 287] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 22. | Graves NS. Acute gastroenteritis. Prim Care. 2013;40:727-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Borade A, Bais AS, Bapat V, Dhongade R. Characteristics of rotavirus gastroenteritis in hospitalized children in Pune. Indian J Med Sci. 2010;64:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Greenberg DE, Wilimas JA, Buckingham SC. Hematologic findings in children with rotavirus-positive and -negative diarrhea. Pediatr Hematol Oncol. 2003;20:453-456. [PubMed] |
| 25. | Karampatsas K, Osborne L, Seah ML, Tong CYW, Prendergast AJ. Clinical characteristics and complications of rotavirus gastroenteritis in children in east London: A retrospective case-control study. PLoS One. 2018;13:e0194009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Zaraket R, Salami A, Bahmad M, El Roz A, Khalaf B, Ghssein G, Bahmad HF. Prevalence, risk factors, and clinical characteristics of rotavirus and adenovirus among Lebanese hospitalized children with acute gastroenteritis. Heliyon. 2020;6:e04248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Candy DC. Rotavirus infection: a systemic illness? PLoS Med. 2007;4:e117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Chen CC, Chang CJ, Lin TY, Lai MW, Chao HC, Kong MS. Usefulness of fecal lactoferrin in predicting and monitoring the clinical severity of infectious diarrhea. World J Gastroenterol. 2011;17:4218-4224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |