Published online Dec 9, 2023. doi: 10.5409/wjcp.v12.i5.310
Peer-review started: July 17, 2023
First decision: August 31, 2023
Revised: September 18, 2023
Accepted: September 28, 2023
Article in press: September 28, 2023
Published online: December 9, 2023
Processing time: 144 Days and 3.1 Hours
Down syndrome (DS) is one of the most common causes of intellectual disability. Children with DS have varying intelligence quotient (IQ) that can predict their learning abilities.
To assess the brain metabolic profiles of children with DS and compare them to standard controls, using magnetic resonance spectroscopy (MRS) and correlating the results with IQ.
This case-control study included 40 children with DS aged 6-15 years and 40 age and sex-matched healthy children as controls. MRS was used to evaluate ratios of choline/creatine (Cho/Cr), N-acetyl aspartic acid/creatine (NAA/Cr), and myoinositol/creatine (MI/Cr (in the frontal, temporal, and occipital lobes and basal ganglia and compared to controls and correlated with IQ.
Children with DS showed significant reductions in NAA/Cr and MI/Cr and a non-significant reduction in Cho/Cr in frontal lobes compared to controls. Additionally, we observed significant decreases in NAA/Cr, MI/Cr, and Cho/Cr in the temporal and occipital lobes and basal ganglia in children with DS compared to controls. Furthermore, there was a significant correlation between IQ and metabolic ratios in the brains of children with DS.
Brain metabolic profile could be a good predictor of IQ in children with DS.
Core Tip: This study compared the brain metabolic profiles of children with Down syndrome (DS) using magnetic resonance spectroscopy to healthy controls. The results showed significant reductions in specific metabolic ratios (N-acetyl aspartic acid/creatine and myoinositol/creatine) in the frontal lobes of children with DS compared to controls, as well as decreases in these ratios in the temporal and occipital lobes and basal ganglia. The study also found a significant correlation between intelligence quotient (IQ) and metabolic ratios in children with DS. These findings suggest that brain metabolic profiling could be a valuable predictor of IQ in children with DS.
- Citation: El Feil NS, Elmahdy HS, Elmahdy RA, Aboelezz AAE, Dawoud HS, Al-Beltagi M. Brain metabolic profile assessed by magnetic resonance spectroscopy in children with Down syndrome: Relation to intelligence quotient. World J Clin Pediatr 2023; 12(5): 310-318
- URL: https://www.wjgnet.com/2219-2808/full/v12/i5/310.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v12.i5.310
Down syndrome (DS) is the most prevalent chromosomal disorder in humans and one of the leading causes of intellectual disability. It arises from the presence of an additional chromosome 21[1]. Children with DS exhibit various physical dysmorphic features affecting nearly every part of their bodies[2]. DS is a systemic disorder that impacts all body systems, particularly the central nervous system. Individuals with DS experience cognitive impairment and a range of conditions related to speech, language, and motor functions. They also face an elevated risk of early-onset dementia and Alzheimer's disease (AD)[3]. Two hypotheses have been proposed to elucidate the pathogenesis of DS: Developmental instability resulting from chromosomal imbalance loss and the gene-dosage effect[4]. According to the gene-dosage effect hypothesis, the genes located on chromosome 21 are overexpressed in the cells and tissues of DS patients, contributing to various phenotypic abnormalities[5].
Magnetic resonance (MR) spectroscopy (MRS) is an analytical technique used to quantify metabolites. Unlike conventional MR imaging (MRI), MRS provides physiological and chemical information rather than anatomical details[6]. Proton MRS (1 H-MRS) is widely employed to characterize brain neurochemical changes associated with health and disease. N-acetyl aspartic acid (NAA) is a derivative of aspartic acid primarily synthesized and stored in neurons. Therefore, it is a marker for neuronal density and viability[7]. As a neuronal marker, the concentration of NAA decreases in conditions such as extensive brain lesions, dementia, hypoxia, or multiple sclerosis, where neuronal destruction occurs[8]. Total creatine (Cr) reflects the amount of phosphocreatine and Cr present in neurons and glial cells. It appears as a prominent peak at 3.0 ppm in MR spectra, with an additional peak for Cr possibly visible at 3.94 ppm[9]. Cr remains relatively constant in the brain's energetic cellular metabolism and is often used as a reference metabolite for in vivo MRS. For instance, it is frequently employed in the calculation of metabolite ratios such as choline (Cho)/Cr, NAA/Cr, and myoinositol (MI)/Cr[10].
Cho serves as an indicator of cellular proliferation and cell membrane integrity. Increased choline levels reflect heightened synthesis and degradation of cell membranes[11]. MI functions as a marker primarily synthesized in glial cells. Elevated MI levels are often observed in cerebral diseases associated with significant gliosis[12]. Metabolic abnormalities, specifically elevated MI levels, have been observed throughout development in mouse models of DS and the brains of children and adults with DS. However, there is a scarcity of studies investigating brain metabolism during early childhood[13].
The intelligence quotient (IQ) is critical in assessing the neurological and cognitive development of children with DS. It may also reflect their physical and somatic growth, as documented by Kłosowska et al[14]. Therefore, establishing a connection between the brain's metabolic profiles and IQ in children with DS could provide insights into the underlying reasons for this relationship and aid in developing strategies to improve their IQ. Limited research has been conducted on elucidating metabolic changes in the developing brains of children with DS. Hence, this study aims to evaluate the brain's metabolic profile using MRS in children with DS, comparing it with normal controls and examining the correlation between this metabolic profile and IQ.
This case-control study was conducted between April 2019 and April 2021, involving a patient group of 60 children with DS. Out of these, 40 children completed the study. We also included a control group of 40 healthy children, matched in age and sex. The age range of the participating children was between 6 and 15 years. We recruited the children from the Outpatient Clinic of the Genetics Unit, Pediatric Department, at Tanta University in Egypt. The diagnosis of DS was based on clinical findings and confirmed through karyotyping. A comprehensive medical history was obtained, including demographic data, prenatal, natal, postnatal, family, and developmental history. Thorough clinical and psychiatric evaluations were performed as well. All patients underwent thyroid function tests, echocardiography, and glycated hemoglobin (HbA1c) measurements. We utilized the Stanford-Binet Intelligence Scales, Fifth Edition, to assess the IQ of the study groups. Cases with diseases that could potentially influence the results or pose risks during the MRS procedure were excluded. Exclusion criteria encompassed complex congenital heart disease, congenital neurological abnormalities, a history of perinatal asphyxia, suspected metabolic disease, epilepsy, attention deficit hyperactivity disorder, autistic spectrum disorders, and conditions that precluded MRI scans, such as the presence of metal devices like pacemakers, defibrillators, orthodontic braces, implants, or severe claustrophobia. Patients with known endocrine disorders like hypothyroidism or diabetes mellitus were excluded from the study. Ethical approval for this research was obtained from the Faculty of Medicine Ethics Committee, Tanta University. Written informed consent was acquired from the legal guardians of both patients and controls before the imaging procedure. The study adhered to the principles of the Declaration of Helsinki and good clinical practice. No adverse physiological effects resulting from the main magnetic field are known. Furthermore, no unexpected risks emerged during the research.
The MRS studies were conducted at the Radiology Department of Tanta University in Egypt. A 1.5 Tesla system (Signa; GE Medical Systems, Milwaukee, WI, United States) with a quadrature head coil was used for all MRI and 1H-MRS procedures. Before the examination, the children were sedated using chloral hydrate at 25-100 mg/kg (maximum 2 g).
Initially, a conventional MRI scan was performed, followed by placing the voxel at various positions. Water suppression and the point-resolved spectroscopy methods were then employed, with a Time to Echo of 135 msec and a Repetition Time of 1500 msec. The NAA, Cho, MI, and Cr spectra were analyzed, and ratios for Cho/Cr, NAA/Cr, and MI/Cr were calculated in the frontal, temporal, occipital lobes, and basal ganglia. These ratios were compared between the patients and controls. NAA serves as an indicator of neuronal integrity, with its peak occurring at 2.02 ppm. Cho reflects cell turnover, with its highest point at 3.22 ppm. Cr is involved in cellular metabolism and peaks at three ppm. Normally, no lipid or lactate peak is observed in the brain; even if present, they overlap at 1.33 ppm[15].
Oxygen saturation, heart rate, and temperature were continuously monitored during the scan, and a skilled pediatrician was present for all examinations. The total examination time for each child did not exceed one hour. The child lay supine with their head positioned in the head coil, which was surrounded by a lunar pillow to minimize the sound from the magnetic field. Subsequently, the patient and coil were moved into the magnetic bore.
The study's power level was estimated using The Power and Precision V3 program (http://www.Power-Analysis.com, Englewood, New Jersey). The collected data were organized, tabulated, and subjected to statistical analysis using the SPSS version 19. For parametric variables, the mean and standard deviation were calculated. A comparison of mean values between the cases and controls was conducted using an unpaired Student's t-test, assuming equal variance in the two populations. Nonparametric variables were analyzed by calculating the number and percentage. The chi-square test was employed to compare different observations of categorical variables. Pearson's correlation coefficient was utilized to examine the association between two variables. A P value less than 0.01 was considered statistically significant after applying the Bonferroni correction of multiple comparisons to avoid any spurious significance and to mitigate the risk of Type I errors associated with multiple comparisons.
Table 1 illustrates the study's demographics. Sixty children with DS were initially recruited for the study, but 20 were excluded due to missed follow-up, inability to participate, or refusal to do an MRI. Ultimately, the study included 40 children with DS and 40 healthy children who completed the study. There were no significant differences in age, sex, and mean IQ between the excluded and included children with DS. Table 1 presents the demographic data of the included children. The patient group had an age range of 6-15 years, with a mean of 10.85 ± 2.7, while the control group ranged from 5-17 years, with a mean of 10.70 ± 2.6. Among the children with DS, 28 were males, and 12 were females, resulting in a male-to-female ratio of 1.4:1. The control group consisted of 24 males and 16 females, with a male-to-female ratio of 1.5:1. There were no significant differences in age and sex between the two groups. All included children with DS had the non-disjunction type of DS. The IQ was significantly lower in children with DS (68.45 ± 6.11) compared to the controls (96.73 ± 2.68), with a P value < 0.001.
| DS (n = 40) | Controls (n = 40) | P value | |
| Age (yr, mean ± SD) | 10.85 ± 2.72 | 10.70 ± 2.64 | 0.87 |
| Sex, n (%) | 0.24 | ||
| Male | 28 (70%) | 26 (65%) | |
| Female | 12 (30%) | 14 (35%) | |
| IQ (mean ± SD) | 68.45 ± 6.11 | 96.73 ± 2.68 | 0.0011 |
Table 2 presents the metabolic profile of the studied groups. It revealed a significant reduction in NAA/Cr and MI/Cr ratios (P value < 0.001) and a non-significant reduction in the Cho/Cr ratio in the frontal lobe of children with DS compared to the controls. Furthermore, children with DS exhibited non-significant reductions in NAA/Cr (P < 0.05), Cho/Cr, and MI/Cr ratios in the temporal and occipital lobes compared to the control group. The occipital lobe showed the highest significant reduction in MI/Cr ratio (P value < 0.001). In addition, the basal ganglia exhibited the most substantial reduction in NAA/Cr, Cho/Cr, and MI/Cr ratios in children with DS compared to the control group.
| Brain metabolite ratios (mean ± SD) | DS (n = 40) | Controls (n = 40) | P value |
| Frontal lobe | |||
| NAA/Cr | 1.19 ± 0.44 | 1.788 ± 0.273 | 0.0011 |
| Cho/Cr | 1.03 ± 0.40 | 1.08 ± 0.187 | 0.473 |
| MI/Cr | 0.56 ± 0.21 | 1.50 ± 0.47 | 0.0011 |
| Temporal lobe | |||
| NAA/Cr | 1.26 ± 1.19 | 1.75 ±0.21 | 0.011 |
| Cho/Cr | 0.71 ± 0.30 | 1.07 ±0.16 | 0.0011 |
| MI/Cr | 0.41 ± 0.19 | 1.40 ±0.56 | 0.0011 |
| Basal ganglia | |||
| NAA/Cr | 1.21 ± 0.55 | 1.78 ± 0.19 | 0.0011 |
| Cho/Cr | 0.68 ± 0.33 | 1.06 ± 0.17 | 0.0011 |
| MI/Cr | 0.35 ± 0.17 | 1.50 ± 0.54 | 0.0011 |
| Occipital lobe | |||
| NAA/Cr | 1.72 ± 0.49 | 1.92 ± 0.34 | 0.033 |
| Cho/Cr | 0.83 ± 0.35 | 0.99 ± 0.14 | 0.011 |
| MI/Cr | 0.44 ± 0.17 | 1.48 ± 0.54 | 0.0011 |
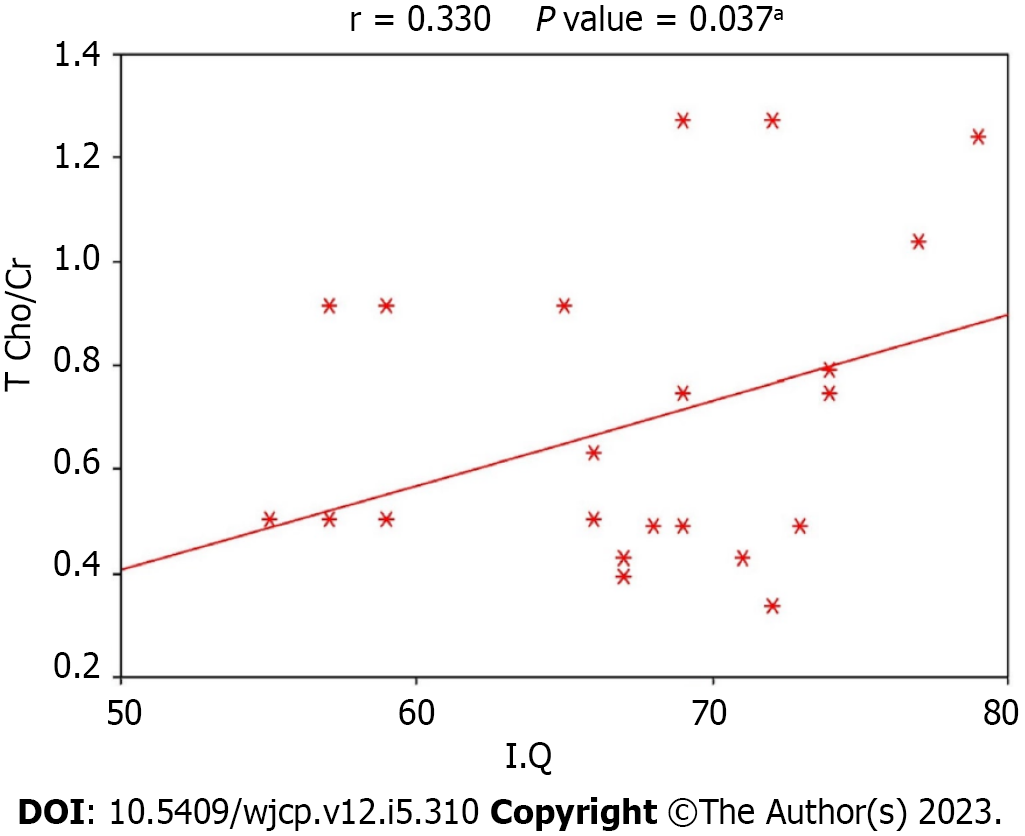
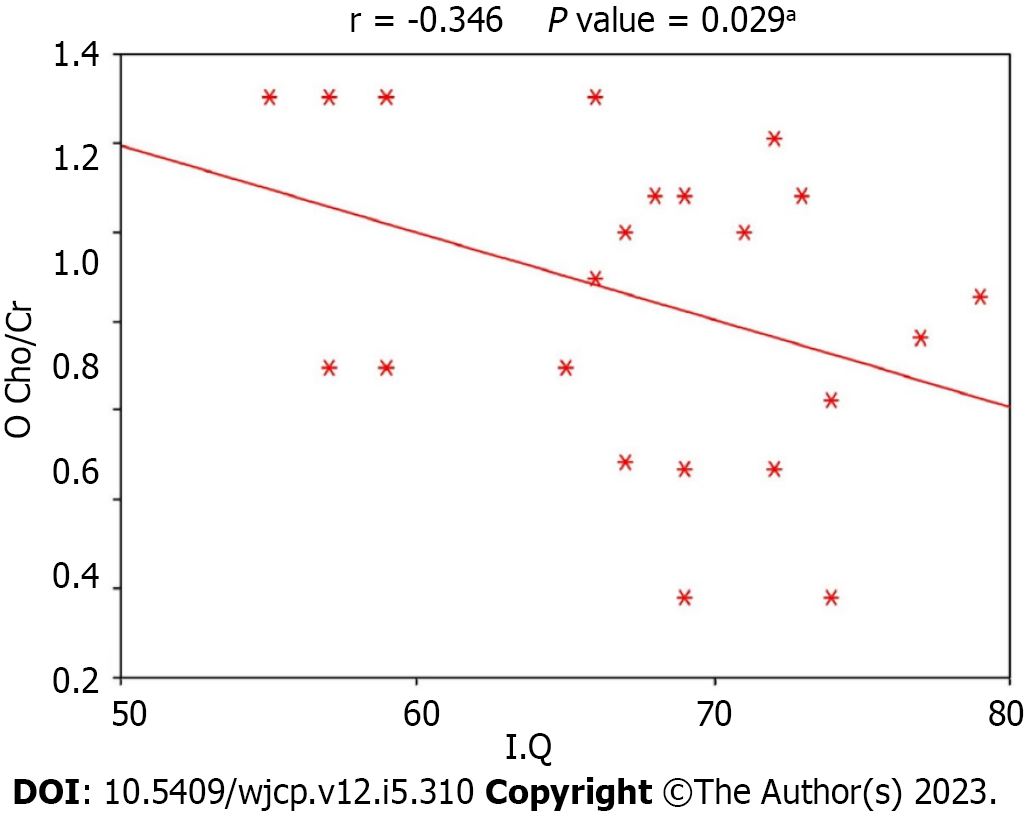
Figures 1-3 display the correlation between IQ and various metabolic ratios. There was a statistically significant positive correlation between IQ and NAA/Cr ratio in the frontal lobe and Cho/Cr ratio in the temporal lobe among children with DS. Conversely, a statistically significant negative correlation existed between IQ and Cho/Cr ratio in the occipital lobe among children with DS, as shown in Table 3. The IQ was primarily influenced by NAA/Cr in the frontal lobe, Cho/Cr in the temporal lobe, and Cho/Cr in the occipital lobe in order of significance. Regression analysis for frontal NAA/Cr and IQ demonstrated a P value of < 0.001. However, the multivariate analysis presented in Table 4 showed that frontal Naa/Cr and temporal Cho/Cr had the most significant correlation with IQ.
| Correlations with IQ | r | P value |
| F NAA/Cr | 0.533 | < 0.0011 |
| F Cho/Cr | -0.210 | 0.193 |
| F MI/Cr | -0.259 | 0.107 |
| T NAA/Cr | 0.215 | 0.182 |
| T Cho/Cr | 0.330 | 0.037 |
| T MI/Cr | -0.018 | 0.913 |
| BG NAA/Cr | 0.100 | 0.541 |
| BG Cho/Cr | 0.105 | 0.518 |
| BG MI/Cr | 0.008 | 0.961 |
| O NAA/Cr | 0.091 | 0.578 |
| O Cho/Cr | -0.346 | 0.029 |
| O MI/Cr | 0.215 | 0.184 |
The current study demonstrated a significant reduction in IQ among children with DS (55-75) compared to normal children. This is consistent with previous research, including Rachidi and Lopes’s[3] study, which indicated that cognitive impairment is consistently observed in all individuals with DS. Most children and adults with DS exhibit mild to moderate intellectual disability, with an average IQ score of around 50 and individual values ranging from 30 to 70. Approximately 10% of individuals with DS fall within the low average borderline range of intellectual disability, while a minority have severe intellectual disability. The exact mechanisms underlying brain development in DS remain poorly understood. Still, multiple volumetric MRI studies have been conducted to gain further insights into the brain characteristics of individuals with DS. Several neuroimaging studies have reported reductions in total brain volume, as demonstrated by Beacher et al[16] in 2010, who observed volume reductions in the cerebrum, cerebellum, hippocampus, brain stem, and frontal lobes.
Our study calculated ratios for Cho/Cr, NAA/Cr, and MI/Cr in the frontal, temporal, occipital lobes, and basal ganglia, comparing the patient and control groups. We found a significant reduction in NAA/Cr and MI/Cr ratios in the frontal lobe, with a non-significant reduction in Cho/Cr among children with DS compared to the control group. This finding aligns with the study by Smigielska-Kuzia and Sobaniec[15], who investigated 34 children (14 with DS and 20 healthy children) and reported decreased Glx/Cr, NAA/Cr, Cho/Cr, and MI/Cr in patients with DS, with the first two markers showing a statistically significant difference. Lamar et al[17] also found a reduction in the absolute concentration of NAA in the frontal, temporal, and occipital lobes in patients with DS and AD. However, Shonk et al[18] detected elevated MI in adult patients with DS and AD compared to DS patients without AD. This difference could be attributed to our study’s age range (6-15 years), as AD development may be associated with aging in DS.
Additionally, we observed a significant reduction in NAA/Cr, Cho/Cr, and MI/Cr ratios in the temporal lobe of children with DS compared to the control group. This finding agrees with Śmigielska-Kuzia et al[19] in 2010, who conducted a study on 40 children (20 with DS and 20 healthy children) and reported a statistically significant decrease in NAA/Cr, Cho/Cr, MI/Cr, and GABA/Cr.
Furthermore, a significant reduction in NAA/Cr, Cho/Cr, and MI/Cr ratios in the basal ganglia was observed in children with DS relative to the control group. This finding is consistent with a previous report by Fruen and Lester[20] in 1990, which described an increased MI/Cr ratio in adults with DS. The increased MI levels in brain tissue may be attributed to an extra SLC5A3 gene in trisomic 21 cells, responsible for inositol transporter synthesis. These co-transporters regulate MI concentration in DS cortical cells. The results of the Fruen and Lester’s[20] study and our study suggest that individuals with DS, whether demented or not, exhibit altered neuron-glial metabolism (reduced NAA and increased MI). Lin et al[21] found that this metabolic shift was more pronounced in DS individuals with dementia compared to those without dementia.
In the occipital lobe, we found a significant reduction in NAA/Cr, Cho/Cr, and MI/Cr ratios among children with DS compared to the control group. This is consistent with a case-control study by Shonk and Ross[22] in 1995, which included 23 young DS patients without dementia, revealing elevated MI levels. They also reported one patient with DS and dementia who exhibited high MI but decreased NAA in the occipital cortex and parietal white matter. The decreased NAA findings align with our study, although our results differ from theirs regarding MI. This discrepancy could be attributed to age differences. NAA indicates brain maturation, dendritic and synaptic development, and oligodendroglial proliferation and differentiation (Kato et al[23], 1997). Previous studies in adults with DS have reported lower NAA ratios compared to healthy controls[18,21]. The observed decrease in NAA/Cr ratios in different brain regions among children with DS in our study suggests reduced brain maturation in these individuals.
Our research demonstrated that frontal NAA/Cr had the most significant correlation with IQ, followed by T Cho/Cr, while O Cho/Cr had the least effect. To the best of our knowledge, only one previous study utilized MRS to evaluate brain metabolites in children with DS. Due to the scarcity of studies in the literature, we could not find extensive data for comparison.
The study is subject to certain limitations, including a relatively small sample size and being conducted at a single center. Our study is mainly exploratory and should be followed by confirmatory studies to validate the observed associations. It would also be valuable to examine the correlation between the metabolic profile and the patient’s medical conditions, parents' educational levels, and developmental milestones. Assessing any changes in the metabolic profile resulting from occupational and behavioral therapy could provide insights into improving IQ and promoting self-independence in children with DS. It is recommended to conduct future studies with larger sample sizes to better understand the DS brain's intricacies. Moreover, longitudinal studies involving adolescents and adults with DS, both with and without dementia, would be beneficial in monitoring metabolite changes and their impact on the mental health of patients with DS, particularly those who develop early-onset dementia. In addition, we would like to emphasize that the correlation of IQ to metabolic ratios in the brain does not imply causality. Correlations only establish relationships. We also acknowledge that our study design did not include a separate group of low-functioning children without DS for direct comparison. We will consider this point in the coming research. Adding such a group would have provided a valuable reference point for evaluating whether the observed differences in brain metabolic profiles and their correlation with IQ are specific to DS or could apply to low-functioning children in general.
The brain's metabolic profile in children with DS exhibits differences compared to typically developing children. Additionally, specific brain areas show positive and negative correlations between IQ and specific metabolic ratios. Thus, the brain's metabolic profile could serve as a predictor of IQ in children with DS.
Down syndrome (DS) is the most prevalent chromosomal disorder in humans and one of the leading causes of intellectual disability. Magnetic resonance (MR) spectroscopy (MRS) is an analytical technique used to quantify metabolites. Proton MRS (1 H-MRS) is widely employed to characterize brain neurochemical changes associated with health and disease. N-acetyl aspartic acid (NAA) is a derivative of aspartic acid primarily synthesized and stored in neurons. Therefore, it is a marker for neuronal density and viability.
Establishing a connection between the brain's metabolic profiles and intelligence quotient (IQ) in children with DS could provide insights into the underlying reasons for this relationship and aid in developing strategies to improve their IQ.
We aimed to evaluate the brain's metabolic profile using MRS in children with DS, comparing it with that of normal controls and examining the correlation between this metabolic profile and IQ.
The study was a case-control study that included sixty children with DS and forty healthy controls. IQ was assessed using Stanford-Binet Intelligence Scales, Fifth Edition. A conventional MR imaging scan followed by point-resolved spectroscopy was performed for all the participants.
Children with DS showed significant reductions in NAA/creatine (Cr) and myoinositol (MI)/Cr and a non-significant reduction in choline (Cho)/Cr in frontal lobes compared to controls. Additionally, we observed significant decreases in NAA/Cr, MI/Cr, and Cho/Cr in the temporal and occipital lobes and basal ganglia in children with DS compared to controls. Furthermore, there was a significant correlation between IQ and metabolic ratios in the brains of children with DS.
Brain metabolic profile could be a good predictor of IQ in children with DS.
To generalize our results, the authors must include a larger sample size and perform a multicentre study. We also need to include another group with low IQ for different reasons to investigate the unique features of brain metabolic profiles in children with DS.
We thank the parents and children who participated in this study. We also thank the editors and the reviewers for their efforts to improve the quality of the study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kaufman AS, United States S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | Karmiloff-Smith A, Al-Janabi T, D'Souza H, Groet J, Massand E, Mok K, Startin C, Fisher E, Hardy J, Nizetic D, Tybulewicz V, Strydom A. The importance of understanding individual differences in Down syndrome. F1000Res. 2016;5. [PubMed] [DOI] [Full Text] |
| 2. | Stoll C, Dott B, Alembik Y, Roth MP. Associated congenital anomalies among cases with Down syndrome. Eur J Med Genet. 2015;58:674-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 3. | Rachidi M, Lopes C. Mental retardation in Down syndrome: from gene dosage imbalance to molecular and cellular mechanisms. Neurosci Res. 2007;59:349-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Reeves RH, Baxter LL, Richtsmeier JT. Too much of a good thing: mechanisms of gene action in Down syndrome. Trends Genet. 2001;17:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Cheon MS, Bajo M, Kim SH, Claudio JO, Stewart AK, Patterson D, Kruger WD, Kondoh H, Lubec G. Protein levels of genes encoded on chromosome 21 in fetal Down syndrome brain: challenging the gene dosage effect hypothesis (Part II). Amino Acids. 2003;24:119-125. [PubMed] [DOI] [Full Text] |
| 6. | Bertholdo D, Watcharakorn A, Castillo M. Brain proton magnetic resonance spectroscopy: introduction and overview. Neuroimaging Clin N Am. 2013;23:359-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Smith JK, Castillo M, Kwock L. MR spectroscopy of brain tumors. Magn Reson Imaging Clin N Am. 2003;11:415-429, v. [PubMed] [DOI] [Full Text] |
| 8. | Gupta RK, Jobanputra KJ, Yadav A. MR spectroscopy in brain infections. Neuroimaging Clin N Am. 2013;23:475-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Bulik M, Jancalek R, Vanicek J, Skoch A, Mechl M. Potential of MR spectroscopy for assessment of glioma grading. Clin Neurol Neurosurg. 2013;115:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Nelson SJ. Multivoxel magnetic resonance spectroscopy of brain tumors. Mol Cancer Ther. 2003;2:497-507. [PubMed] |
| 11. | Brandão LA, Castillo M. Adult Brain Tumors: Clinical Applications of Magnetic Resonance Spectroscopy. Magn Reson Imaging Clin N Am. 2016;24:781-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Patkee PA, Baburamani AA, Kyriakopoulou V, Davidson A, Avini E, Dimitrova R, Allsop J, Hughes E, Kangas J, McAlonan G, Rutherford MA. Early alterations in cortical and cerebellar regional brain growth in Down Syndrome: An in vivo fetal and neonatal MRI assessment. Neuroimage Clin. 2020;25:102139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Alam MS, Sajjad Z, Hafeez S, Akhter W. Magnetic resonance spectroscopy in focal brain lesions. J Pak Med Assoc. 2011;61:540-543. [PubMed] |
| 14. | Kłosowska A, Kuchta A, Ćwiklińska A, Sałaga-Zaleska K, Jankowski M, Kłosowski P, Mański A, Zwiefka M, Anikiej-Wiczenbach P, Wierzba J. Relationship between growth and intelligence quotient in children with Down syndrome. Transl Pediatr. 2022;11:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Smigielska-Kuzia J, Sobaniec W. Brain metabolic profile obtained by proton magnetic resonance spectroscopy HMRS in children with Down syndrome. Adv Med Sci. 2007;52 Suppl 1:183-187. [PubMed] |
| 16. | Beacher F, Daly E, Simmons A, Prasher V, Morris R, Robinson C, Lovestone S, Murphy K, Murphy DG. Brain anatomy and ageing in non-demented adults with Down's syndrome: an in vivo MRI study. Psychol Med. 2010;40:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Lamar M, Foy CML, Beacher F, Daly E, Poppe M, Archer N, Prasher V, Murphy KC, Morris RG, Simmons A, Lovestone S, Murphy DGM. Down syndrome with and without dementia: an in vivo proton Magnetic Resonance Spectroscopy study with implications for Alzheimer's disease. Neuroimage. 2011;57:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Shonk TK, Moats RA, Gifford P, Michaelis T, Mandigo JC, Izumi J, Ross BD. Probable Alzheimer disease: diagnosis with proton MR spectroscopy. Radiology. 1995;195:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 185] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Śmigielska-Kuzia J, Boćkowski L, Sobaniec W, Kułak W, Sendrowski K. Amino acid metabolic processes in the temporal lobes assessed by proton magnetic resonance spectroscopy (1H MRS) in children with Down syndrome. Pharmacol Rep. 2010;62:1070-1077. [PubMed] [DOI] [Full Text] |
| 20. | Fruen BR, Lester BR. Down's syndrome fibroblasts exhibit enhanced inositol uptake. Biochem J. 1990;270:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Lin AL, Powell D, Caban-Holt A, Jicha G, Robertson W, Gold BT, Davis R, Abner E, Wilcock DM, Schmitt FA, Head E. (1)H-MRS metabolites in adults with Down syndrome: Effects of dementia. Neuroimage Clin. 2016;11:728-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Shonk T, Ross BD. Role of increased cerebral myo-inositol in the dementia of Down syndrome. Magn Reson Med. 1995;33:858-861. [PubMed] [DOI] [Full Text] |