Published online Sep 9, 2023. doi: 10.5409/wjcp.v12.i4.220
Peer-review started: May 5, 2023
First decision: June 19, 2023
Revised: June 26, 2023
Accepted: July 7, 2023
Article in press: July 7, 2023
Published online: September 9, 2023
Processing time: 123 Days and 16.3 Hours
Intravitreal anti-vascular endothelial growth factor (IVA) injection is known to cause contraction of fibrovascular proliferation (FVP), when present in severe retinopathy of prematurity (ROP).
To assess the structural outcomes of IVA injection in the treatment of severe posterior ROP with significant FVP.
It was a retrospective study in which 36 eyes of 18 preterm babies who developed > 4 clock hours of FVP in zone I or posterior zone II, were treated with either intravitreal 0.625 mg bevacizumab or intravitreal 0.2 mg of ranibizumab. Favorable structural outcome included resolution of plus disease and FVP without the development of tractional retinal detachment. Secondary outcome measure included either full retinal maturation at follow-up or development of recurrent disease requiring additional treatment. Adverse outcomes included progression to retinal detachment.
The mean gestational age of the 18 preterm babies was 30 wk (range 27-36), and mean birth weight was 1319 g (range 650-1980 g). Mean post-menstrual age (PMA) at the time of primary treatment was 35.5 wk (range 31-41 wk). All eyes showed regression of plus disease and FVP. 5 eyes of 3 babies showed reac
There was good resolution of severe posterior ROP with FVP with IVA, with retinal maturity of 44% at 5 year follow-up and a reactivation rate of 13.8%. When the IVA injection is given prior to 37 wk PMA, while disease is in phase 2, it is less likely to cause contracture of pre-existing FVP.
Core Tip: This is a retrospective study to evaluate if anti-vascular endothelial growth factor injection could cause contraction of preexisting fibrovascular proliferation when present in severe retinopathy of prematurity.
- Citation: Maitra P, Prema S, Narendran V, Shah PK. Safety and efficacy of intravitreal anti vascular endothelial growth factor for severe posterior retinopathy of prematurity with flat fibrovascular proliferation. World J Clin Pediatr 2023; 12(4): 220-229
- URL: https://www.wjgnet.com/2219-2808/full/v12/i4/220.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v12.i4.220
Since the first description of retinopathy of prematurity (ROP) by Terry in 1942, the understanding of the disease has evolved vastly, especially over that last decade with wider armamentarium of treatment options[1,2]. There has been an increase in the popularity of intravitreal anti-vascular endothelial growth factor (IVA) injection as a treatment modality especially with the landmarks trails such as Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity (BEAT–ROP) and Ranibizumab vs laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW), proving their safety and efficacy[3,4]. IVA injection has become the treatment of choice in aggressive and posterior diseases. Although dramatic effects are often noted after IVA injections, minimizing need for laser and related effects such as high myopia and peripheral field defect, reactivations are not uncommon and many of them may need re-treatment[5]. Therefore a through close follow up for these babies is mandatory. In cases associated with flat fibrovascular proliferation (FVP), there is often a risk of development or worsening of anteroposterior traction- also known as crunch phenomemon, especially in babies with higher post menstrual age[6,7]. The current study aims to show the safety and effectiveness of IVA injection in the treatment of severe type 1 ROP with more than 4 clock hours of flat FVP in zone I and posterior zone II.
It is a retrospective study of babies with severe type 1 ROP as defined by the early treatment for retinopathy of prematurity study[8] with significant flat FVP in zone 1 or posterior zone II, who were treated with IVA injections between December 2013 to December 2016 and followed up for five years. The study was approved by the Ethics committee and the Institutional Review Board and adhered to the tenets of declaration of Helsinki. Significant FVP was defined as FVP spanning more than 4 clock hours. Relevant data of the baseline characteristics like gestational age, birth weight, post conceptional age at treatment, date of injection were collected from the electronic medical records. Zone of the disease and stage of the disease was defined as per the latest ICROP guidelines[9]. Informed consent was obtained from all the parents prior to the IVA, and the parents of the babies were offered to choose either Bevacizumab (Avastin; Genentech Inc, South San Francisco, CA, United States) or Ranibizumab (Accentrix; Novartis pharma Stein AG) after they were explained the possible side- effects, need for long follow up, and off label use. Dosage administered was 0.625mg of bevacizumab or 0.2 mg of ranibizumab given under aseptic precaution in operation theatre under topical anesthesia using 30 gauge needle about 1.5 mm from limbus. Two ophthalmologist reviewed fundus images pre and post injection. Documentation was done by taking images using 130 degrees lens using the Retcam 3 (Clarity Medical Systems, Pleasanton CA, United States).
All the babies were treated within 2 days of presentation. Concurrent bilateral injection were given under aseptic precautions for each eye separately, using drugs with different batch numbers for the two eyes. Patients were followed up weekly for two months, two weekly for next two months, monthly till 6 months, two monthly till 12 months and six monthly till 5 years of age.
Patients who demonstrated peripheral avascular retina (PAR), were followed up as per schedule and examination under anesthesia was performed wherever necessary. Reactivation was treated with either repeat IVA injection or peripheral laser photocoagulation depending on the location of the disease and family’s preference. Rescue vitrectomy was planned in case of development of tractional retinal detachment/crunch phenomemon.
Primary outcome was measured in terms of regression of plus disease and absence of FVP. Secondary outcome was measured in terms of absence of reactivation of disease and vessels reaching maturity at the end of 1 year, which was considered as the minimum follow up period. Safety profile was assessed by lack of development of anteroposterior traction or crunch phenomenon. The results were tabulated in Microsoft excel version 2206, in which mean and percentages were calculated.
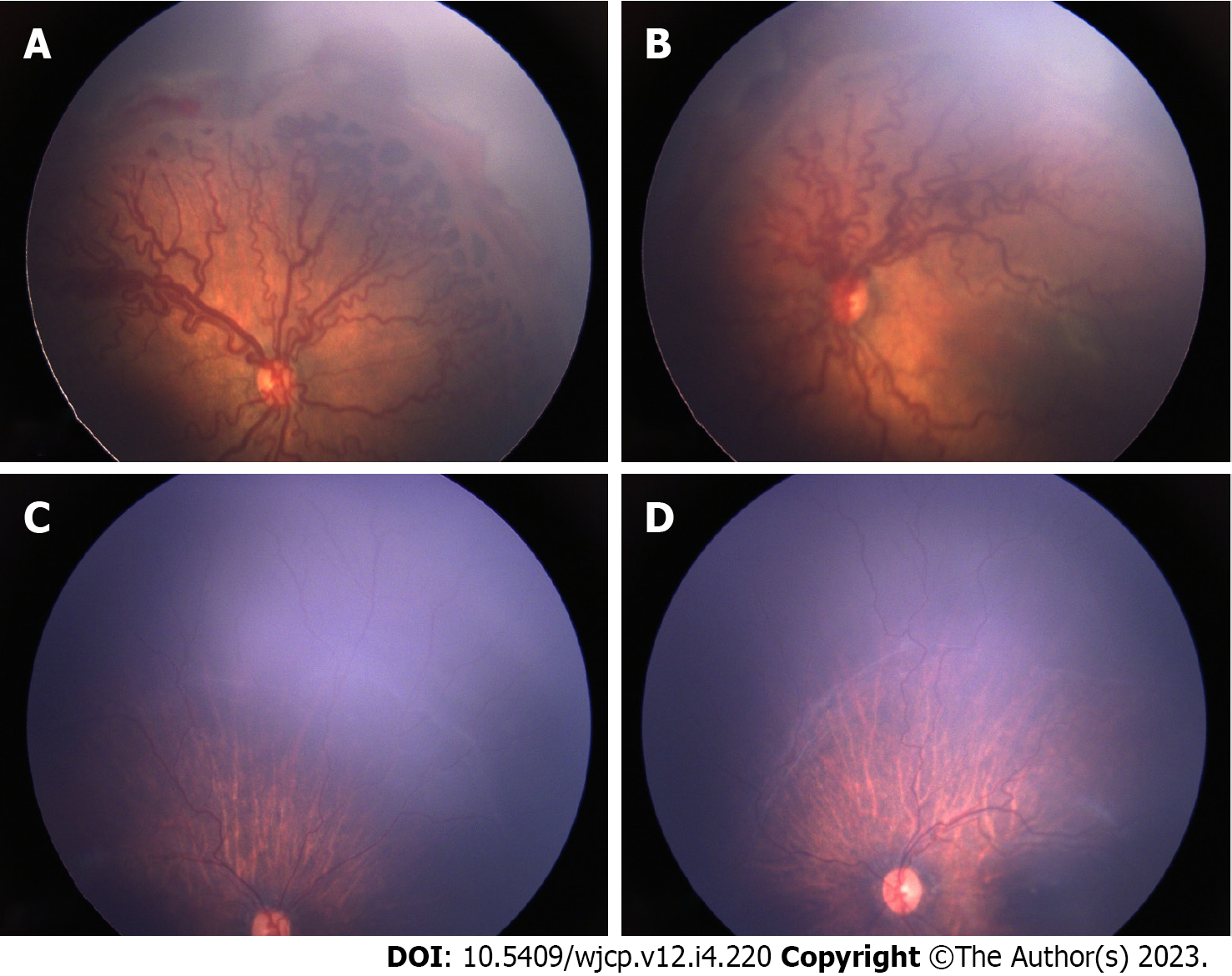
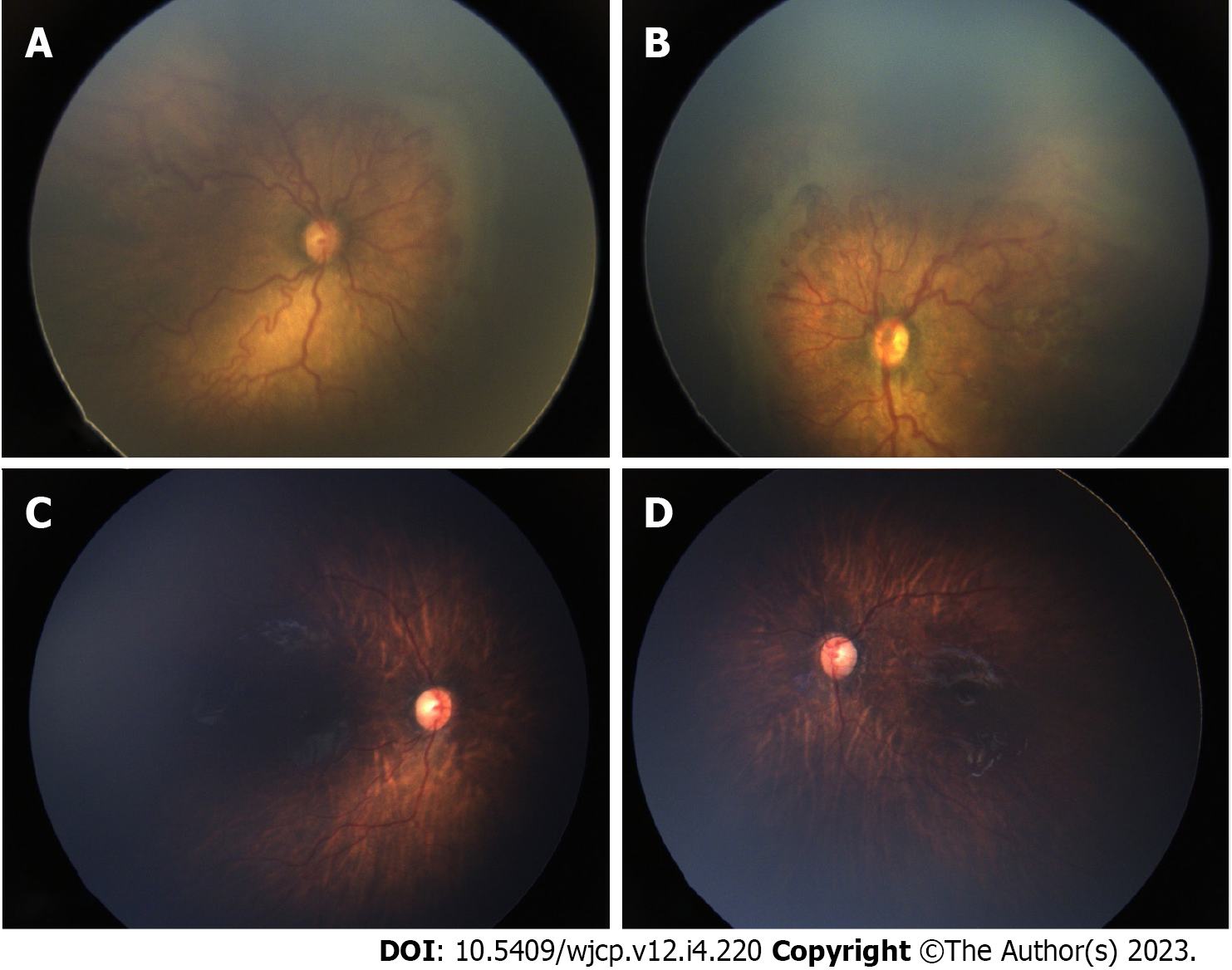
Thirty-six eyes of 18 infants were included in the study. The mean gestational age of the 18 preterm babies was 30 wk (range 27-36), and mean birth weight was 1319 g (range 650-1980g). Mean post-menstrual age (PMA) at the time of IVA was 35 wk (range 31-41 wk). 22 eyes (61.1%) had aggressive ROP (AROP) in zone I, 6 eyes (16.66%) had AROP in posterior zone II. Two eyes had stage 3 in zone I and 6 eyes has stage 3 in posterior zone II. Thirty two eyes (89%) received 0.625 mg of intravitreal bevacizumab and 4 eyes (11%) received 0.2 mg of intravitreal ranibizumab (0.2 mg). All eyes showed regression of plus disease and FVP. In 10 eyes (27%) this regression was within a week of IVA and in additional 25 (69%) eyes it occurred within a month and only in 1 eye regression occurred by 2 mo post IVA. Figures 1 and 2 show pre injection (Figure 1A and B) and post IVA regression of plus disease and FVP (Figure 1C and D). 2 eyes of 2 patients (5.5%) developed vitreous hemorrhage within one week post IVA, which showed resolution within a month. The neovascularization of iris (in 4 eyes) regressed within a week of injection. The regression of disease activity after IVA for each case is shown in Table 1.
| S. No | Eye | GA | BW | PMA | Features | Follow up after IVA | ||||||||
| 1 wk | 1 mo | 2 mo | 3-4 mo | 6 mo | 8 mo | 12 mo | 3 yr | 5 yr | ||||||
| 1 | OD | 30 | 1200 | 35 | Zone 1 AROP | Regressing FVP | Complete FVP regression | PAR | Zone II stage 3 – Lasered | Stable | Stable | Stable | Stable | Stable (lasered) |
| OS | Zone 1 AROP | Regressing FVP | Complete FVP regression | PAR | Zone II stage 3 – Lasered | Stable | Stable | Stable | Stable | Stable (lasered) | ||||
| 2 | OD | 32 | 1200 | 38 | Zone 1 AROP | Regressing FVP | Complete FVP regression | PAR | PAR | PAR | Mature | Mature | Mature | Stable |
| OS | Zone 1 AROP | Regressing FVP | Complete FVP regression | PAR | PAR | PAR | Mature | Mature | Mature | Stable | ||||
| 3 | OD | 30 | 950 | 35 | Zone 1 AROP | Complete FVP regression | PAR | PAR | PAR | Mature | Mature | Mature | Mature | Stable |
| OS | Zone 1 AROP | Complete FVP regression | PAR | PAR | PAR | Mature | Mature | Mature | Mature | Stable | ||||
| 4 | OD | 28 | 1235 | 36 | Zone 1 AROP | Regressing FVP | Complete FVP regression | Zone II stage 3- repeat -R | PAR | PAR | PAR | Mature | Mature | Stable, disc pallor |
| OS | Zone 1 AROP | Regressing FVP | Complete FVP regression | Zone II stage 3- repeat -R | PAR | PAR | PAR | Mature | Mature | Stable, disc pallor | ||||
| 5 | OD | 36 | 1800 | 41 | Zone II P AROP | Regressing FVP | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | FU elsewhere | FU elsewhere |
| OS | Zone II P AROP | Regressing FVP | Complete FVP regression | PAR | Zone II stage 3 – lasered | PAR | PAR | Stable (Lasered) | FU elsewhere | FU elsewhere | ||||
| 6 | OD | 35 | 1700 | 41 | Zone II P AROP | Regressing FVP | Complete FVP regression | PAR | PAR | Mature | Stable | Stable | stable | FU elsewhere |
| OS | Zone II P AROP | Regressing FVP | Complete FVP regression | PAR | PAR | Mature | Stable | Stable | Stable | FU elsewhere | ||||
| 7 | OD | 28 | 1200 | 34 | Zone II stage 3 P + | Regressing FVP | Complete FVP regression | PAR | Mature | Stable | Stable | Stable | Stable | Stable |
| OS | Zone II stage 3 P + | Regressing FVP | Complete FVP regression | PAR | Mature | Stable | Stable | Stable | Stable | Stable | ||||
| 8 | OD | 32 | 1980 | 36 | Zone 1 AROP | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | PAR | FU elsewhere | FU elsewhere |
| OS | Zone 1 AROP | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | PAR | FU elsewhere | FU elsewhere | ||||
| 9 | OD | 29 | 2050 | 35 | Zone 1 AROP | Regressing FVP | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | FU elsewhere | FU elsewhere |
| OS | Zone 1 AROP | Regressing FVP | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | FU elsewhere | FU elsewhere | ||||
| 10 | OD | 28 | 1300 | 34 | Zone 1 AROP (hge) | Regressing FVP | Complete FVP regression | PAR | PAR | PAR | PAR | Mature | Mature | Mature |
| OS | Zone 1 AROP (hge) | Regressing FVP | Complete FVP regression | PAR | PAR | PAR | PAR | Mature | Mature | Mature | ||||
| 11 | OD | 29 | 1265 | 34 | Zone II stage 3 P+ | Regressing FVP | Complete FVP regression | PAR | PAR | Mature | Mature | Mature | Mature | Mature |
| OS | Zone II stage 3 P+ | Regressing FVP | Complete FVP regression | PAR | PAR | Mature | Mature | Mature | Mature | Mature | ||||
| 12 | OD | 29 | 1750 | 33 | Zone 1 AROP | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | PAR | PAR, lattice, WWOP | PAR, lattice, WWOP |
| OS | Zone 1 AROP | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | PAR | PAR, lattice, WWOP | PAR, lattice, WWOP | ||||
| 13 | OD | 27 | 650 | 33 | NVI, Zone 1 Stage 3 + | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | PAR | PAR, WWOP | PAR, WWOP |
| OS | NVI, Zone 1 Stage 3 + | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | PAR | PAR WWROP | PAR, WWOP | ||||
| 14 | OD | 30 | 1300 | 35 | Zone 1 AROP | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | PAR | FU elsewhere | FU elsewhere |
| OS | Zone 1 AROP | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | PAR | FU elsewhere | FU elsewhere | ||||
| 15 | OD | 28 | 1500 | 31 | Zone 1 AROP | Regressing | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | FU elsewhere | FU elsewhere |
| OS | Zone 1 AROP | Regressing | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | FU elsewhere | FU elsewhere | ||||
| 16 | OD | 28 | 1060 | 35 | NVI, Zone II P AROP | regressing | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | PAR | PAR |
| OS | NVI, Zone II P AROP | Regressing | Inomplete FVP regression | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | PAR | ||||
| 17 | OD | 29 | 1450 | 33 | Zone II stage 3 P+ | Regressing | Complete FVP regression | PAR | Mature | Mature | Mature | Mature | Mature | Mature |
| OS | Zone II stage 3 P+ | Regressing | Complete FVP regression | PAR | Mature | Mature | Mature | Mature | Mature | Mature | ||||
| 18 | OD | 29 | 1260 | 34 | Zone II AROP | Regressing | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | FU elsewhere | FU elsewhere |
| OS | Zone II AROP | regressing | Complete FVP regression | PAR | PAR | PAR | PAR | PAR | FU elsewhere | FU elsewhere | ||||
In 14 out of 36 (39%) eyes the retinal vessels reached maturity uneventfully without intervention. In 4 eyes (11%) the vessels matured by 4 months, in 6 eyes (33%) by six months and in another 6 eyes (33%) by one year. Among the 20 (55.5%) eyes that did not reach maturation, recurrence of disease was seen in 5 eyes (13.8%). The mean time of recurrence was 10 weeks in our study (6 weeks for ranibizumab eyes and 12 wk for bevacizumab eyes). An additional dose of IVA was given in 2 eyes of 1 patient (post ranibizumab) at one and half months after initial injection. 3 eyes of 2 patients required peripheral laser photocoagulation. In 15 of 36 eyes, although there was no recurrence of ROP, babies were kept under follow-up with immature vessels/peripheral avascular retina in zone II or III. All eyes were followed up for a minimum period of 1 year at our centre. 12 out of 18 babies (24 eyes) (66%) completed 3 years follow up, and 11 out of 18 babies (22 eyes) (61%) completed 5 years.
At final follow up, vascular maturity was noted in 16 (44%) eyes, PAR was noted in 17(47%) eyes and peripheral laser scars in 3 (8%) eyes. Development of disc pallor was noted in 2 eyes. 4 eyes of 2 babies developed white without pressure in the area of PAR and 2 eyes of 1 baby additionally developed lattice degeneration in the PAR at 5 years follow up. Babies who were lost to follow up at our center due to the emergence of coronavirus disease 2019 outbreak, were followed up over telephonic conversation and directed to the closest ophthalmic center for follow up to rule out the possibility of development of any sight threatening ROP.
None of the babies developed anteroposterior traction or tractional retinal detachment or required rescue vitrectomy at any time until the last follow up. None of developed any systemic complications.
In this study we attempted to show the effectiveness of IVA in severe posterior type 1 ROP with over 4 clock hours of FVP. Trials such as the BEAT ROP[3] study has showed the effectiveness of bevacizumab in ROP in posterior disease, and the RAINBOW trial has showed the outcomes of ranibizumab vs laser in ROP[3,4]. However, its effectiveness in presence of flat FVP is not studied. The fate of the retinal vessels following bevacizumab follow certain scalloped pattern described by Chen et al[10] as having one of the following fate; vascular arrest alone with peripheral non perfusion in 43%, vascular arrest with persistent tortuosity in 38%, reactivated ROP in 18%, and full vascular maturation (by 60 week PMA) in 3%.[10] Although in our cohort we did not look into these patterns, 11% of the eyes had vascular maturation within 4 months post IVA, which appears to be greater than reported by them, and finally 39% eyes reached maturation in 1 year. Even in the RAINBOW trial, full peripheral vascularization (which they assessed only by indirect ophthalmoscopy) occurred in 32% by day 169 in both the combined ranibizumab groups[4].
It is known and has been emphasized even in landmark trails that the retinal vessels advance to the point at which the vascular precursors have ceased migration, with differentiation of the underlying retina, in case of IVA injection monotherapy[3]. Therefore a PAR is expected in majority of these cases. The rate of reactivation was 13.8% in our patients, which was low considering that presence of FVP is not the typical cohort of choice for IVA in most of the studies conducted. Infact, before the widespread use of IVA injections, early vitrectomy was recommended for such cases[11].
The timing of recurrence is influenced by the half-life of the drug in the vitreous cavity. For Ranibizumab, which is a 48 kDa Fab antibody fragment, the serum half-life is much lesser than bevacizumab, which is a 149 kDa monoclonal antibody. Therefore, an earlier recurrence is expected in Ranibizumab eyes[4]. The mean time of recurrence was 10 week in our study (6 week for ranibizumab eyes and 12 week for bevacizumab eyes). These results were similar to the recurrence at 16 week for bevacizumab in BEAT-ROP trial[3]. In the RAINBOW trial the median time to recurrence was 8 week for ranibizumab[4]. Reactivation have been reported with bevacizumab occurring upto 69 weeks after injection and there have been reports of very late reactivations, even as late as 2.5 years of age with tractional retinal detachment (TRD) after bevacizumab for AROP[12,13]. TRD in proliferative diseases like diabetic retinopathy[14] can worsen after IVA injection and studies have showed that similar phenomenon can also occur in advanced ROP, described as crunch effect[7].
Crunch effect is the term given to accelerated posterior/prepapillary fibrosis and contraction as one of the characteristic patterns of TRD which may occur following intravitreal injection of anti-vascular endothelial growth factor (VEGF) therapies in acute ROP, with 49% of detachments occurring within 4 week of the treatment, with older infants (by PMA) at higher risk[7].
In this American Academy of Ophthalmology 2019 interview[15], Dr. Capone Jr. describes his experience with crunch detachment where he states an inverse relationship of PMA with pace of developing detachment - older children are at a greater risk of crunch detachments because they typically have pre-existing fibrosis and an increased chance of abrupt contraction. He also described 2 unique types of crunch—arcuate and prepaillary contraction. Thus, the presence of extensive FVP presents a dilemma in treating zone 1 or posterior zone II disease with anti-VEGF injection. All babies in our study had no clinical evidence of antero-posterior traction unlike described by Honda et al[6] where avastin given to baby with stage 4A resulted in acute contracture of proliferative membrane resulting in worsening of the disease. A similar study by Kychenthal et al[16] showed that bevacizumab given in eyes with extensive neovascular tissue showed higher chances of development of fibrosis. However they also recruited cases with stages 4A and B, which already have established antero-posterior traction.
In a study by Wu et al[17], bevacizumab was injected for stages 3, 4A and stage 5 patients. 33% of eyes with 4A regressed after bevacizumab alone without the requirement of vitrectomy. Many studies have shown the outcomes of use of intravitreal bevacizumab prior to vitrectomy, where reduced vascularity not only aided the surgery but also induced contracture of proliferative membrane indicating earlier surgical intervention[18,19].
This phenomenon is likely to be secondary to a shift in the cytokine signalplex, with exacerbation of the unopposed activity of profibrotic cytokines such as transforming growth factor-beta (TGF-β); TGF-β, an antagonist of VEGF, rises in systemic and intravitreal concentration between 36 and 40 wk PMA, the time period during which many of these premature infants are being treated with anti-VEGF[20]. No contracture of FVP was seen in any of our cases, rather in 75% eyes there was disappearance of FVP within a month of injection, earliest being within a week.
In the attempted normal physiological vascularization in a preterm, various risk factors interrupt this process to and assist in the pathogenesis of ROP. In the Phase 1 or vaso-obliterative phase, there is a low level of VEGF as well as declining levels of circulating insulin like growth factor 1 (IGF-1) from the maternal source. With the maturation of the liver of the premature baby, IGF-1 production starts and its levels rise. This, along with the rise in VEGF levels in phase 2 of pathogenesis of ROP, aids in development of treatable ROP[21-24]. A late component of phase 2 can be described as Phase 3, which is the phase of development of fibrosis and TRD[25]. Increased VEGF activates plasminogen activators which converts plasminogen to plasmin which in turn activates transforming growth factor beta 1 (TGF-β1) which downregulates VEGF and promote fibrosis leading to excessive scarring in stages 4 and 5 ROP[26]. As the baby matures, the liver is able to produce the factors such as TGF-β1, and this allows exaggerated effects of the VEGF and subsequently the development of fibrous component, providing a scaffold for the high levels of VEGF to act and cause a contracture. This is why crunch phenomenon is often seen in babies with higher PMA[15].
The mean PMA at the time of injection in our cases was 35.1 week which explains the resolution of FVP in our series. In most of the reported cases of crunch phenomenon, the IVA has been given between 37-42 wk[6,27,28]. Thus, we can assume when the anti- VEGF injection given prior 37 week PMA, the baby is believed to be in phase 2 where the vascular component predominates and no contracture of FVP occurs. On the contrary, if anti-VEGF injection is given near or at term, then the disease is already in Phase 3 and anti-VEGF would hasten the already set-in fibrosis leading to contracture with progression of disease to stages 4b and 5. Although determining an exact cut off requires studies with larger sample size, it may still lack direct evidence to establish such a cut off, due to prior knowledge of crunch phenomenon in older babies and ethical considerations. However, it must be kept in mind that the timing of IVA is key and it is unlikely to cause a contracture of pre-existing FVP prior to 37 week PMA, as per indirect evidence.
The effect of systemic absorption of IVA and effect on the other eye has been noted by the development of crunch effect in the fellow eye[28]. Theoretically a shorter acting IVA is preferred due to lesser suppression of systemic VEGF and we feel it could be preferred in cases with significant FVP as well, as this would limit the development of contracture or atleast shorten the critical time when contracture is likely to occur. In our cohort however, the choice of IVA was as per the patient’s preference which in turn was mostly influenced by the cost of the injection. The drawback of this study includes comparison across different anti VEGF that are known to have different half-life in the vitreous cavity. Also, we included babies with significant FVP (> 4 clock hours) treated with IVA injections, without performing ultrasound B scan to rule out anteroposterior traction.
The subset of severe posterior ROP with FVP pose therapeutic dilemma. This study shows favorable outcomes when treated with IVA injections, in babies with PMA < 37 weeks. The present study also shows the importance of extended period of follow up post IVA injections, as we found a high percentage (55.5%) of babies having incomplete vascular maturity at 1 year follow up. However, none of the babies developed anteroposterior traction or tractional retinal detachment or required rescue vitrectomy at any time until the last follow up 5 years post IVA. But we advise caution, especially in a setting without vitreoretinal surgery back up, and recommend a multi-center prospective study with a larger sample size.
There is always a dilemma whether or not intravitreal anti-vascular endothelial growth factor (IVA) injections can be given in severe retinopathy of prematurity (ROP) with fibro vascular proliferation (FVP).
The outcome of laser alone in treating severe posterior ROP with FVP is poor. Hence we wanted to test if IVA injections could be a better option for these subset of cases.
To test if early IVA injections in severe posterior ROP with FVP would cause worsening of the disease.
It is a retrospective study which included 36 eyes of 18 babies, which were given IVA injections for severe posterior ROP with FVP and followed up till 5 years of age.
There was no contraction of the FVP in any of the eyes after the IVA injections. All eyes showed very good regression. The mean post menstrual age of injection was 35.5 wk. Five eyes showed disease reactivation on follow up which was treated by laser in 3 eyes and repeat IVA injections in 2 eyes.
IVA injections can be tried as an initial treatment in severe posterior ROP with FVP, if the post menstrual age at the time if injection is < 37 wk.
IVA injections could be used as a first line treatment in the management of severe posterior ROP with FVP.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen X, United States; Wongwai P, Thailand S-Editor: Liu JH L-Editor: A P-Editor: Zhang XD
| 1. | Terry TL. Fibroblastic Overgrowth of Persistent Tunica Vasculosa Lentis in Infants Born Prematurely: II. Report of Cases-Clinical Aspects. Trans Am Ophthalmol Soc. 1942;40:262-284. [PubMed] |
| 2. | Hansen ED, Hartnett ME. A review of treatment for retinopathy of prematurity. Expert Rev Ophthalmol. 2019;14:73-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Mintz-Hittner HA, Kennedy KA, Chuang AZ; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1132] [Cited by in RCA: 1022] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 4. | Stahl A, Lepore D, Fielder A, Fleck B, Reynolds JD, Chiang MF, Li J, Liew M, Maier R, Zhu Q, Marlow N. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet. 2019;394:1551-1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 340] [Cited by in RCA: 263] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 5. | Wu AL, Wu WC. Anti-VEGF for ROP and Pediatric Retinal Diseases. Asia Pac J Ophthalmol (Phila). 2018;7:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Honda S, Hirabayashi H, Tsukahara Y, Negi A. Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2008;246:1061-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Yonekawa Y, Wu WC, Nitulescu CE, Chan RVP, Thanos A, Thomas BJ, Todorich B, Drenser KA, Trese MT, Capone A Jr. Progressive retinal detachment in infants with retinopathy of prematurity treated with intravitreal bevacizumab or ranibizumab. Retina. 2018;38:1079-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 1343] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 9. | Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, Binenbaum G, Blair M, Peter Campbell J, Capone A Jr, Chen Y, Dai S, Ells A, Fleck BW, Good WV, Elizabeth Hartnett M, Holmstrom G, Kusaka S, Kychenthal A, Lepore D, Lorenz B, Martinez-Castellanos MA, Özdek Ş, Ademola-Popoola D, Reynolds JD, Shah PK, Shapiro M, Stahl A, Toth C, Vinekar A, Visser L, Wallace DK, Wu WC, Zhao P, Zin A. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology. 2021;128:e51-e68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 392] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 10. | Chen TA, Shields RA, Bodnar ZH, Callaway NF, Schachar IH, Moshfeghi DM. A Spectrum of Regression Following Intravitreal Bevacizumab in Retinopathy of Prematurity. Am J Ophthalmol. 2019;198:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Micelli Ferrari T, Furino C, Lorusso VV, Dammacco R, Sborgia G, Sborgia L, Besozzi G. Three-port lens-sparing vitrectomy for aggressive posterior retinopathy of prematurity: early surgery before tractional retinal detachment appearance. Eur J Ophthalmol. 2007;17:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012;130:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 13. | Snyder LL, Garcia-Gonzalez JM, Shapiro MJ, Blair MP. Very Late Reactivation of Retinopathy of Prematurity After Monotherapy With Intravitreal Bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2016;47:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Arevalo JF, Maia M, Flynn HW Jr, Saravia M, Avery RL, Wu L, Eid Farah M, Pieramici DJ, Berrocal MH, Sanchez JG. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 289] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 15. | Park DW III, Capone A Jr. In: AAO Interviews [Internet]. San Francisco: 2019. Available from: https://www.aao.org/education/interview/crunch-detachment-after-anti-vegf-therapy-rop. |
| 16. | Kychenthal A, Dorta P. Vitrectomy after intravitreal bevacizumab (Avastin) for retinal detachment in retinopathy of prematurity. Retina. 2010;30:S32-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Wu WC, Yeh PT, Chen SN, Yang CM, Lai CC, Kuo HK. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: a multicenter study in taiwan. Ophthalmology. 2011;118:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Sun HJ, Choi KS, Lee SJ. Adjunctive effect of intravitreal bevacizumab prior to lens-sparing vitrectomy in aggressive posterior retinopathy of prematurity: a case report. Jpn J Ophthalmol. 2012;56:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Xu Y, Zhang Q, Kang X, Zhu Y, Li J, Chen Y, Zhao P. Early vitreoretinal surgery on vascularly active stage 4 retinopathy of prematurity through the preoperative intravitreal bevacizumab injection. Acta Ophthalmol. 2013;91:e304-e310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Connor TB Jr, Roberts AB, Sporn MB, Danielpour D, Dart LL, Michels RG, de Bustros S, Enger C, Kato H, Lansing M. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest. 1989;83:1661-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 331] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Carroll L, Owen LA. Current evidence and outcomes for retinopathy of prematurity prevention: insight into novel maternal and placental contributions. Explor Med. 2020;1:4-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Smith LE. IGF-1 and retinopathy of prematurity in the preterm infant. Biol Neonate. 2005;88:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL, Albertsson-Wikland K, Carlsson B, Niklasson A, Sjodell L, LeRoith D, Senger DR, Smith LE. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci U S A. 2001;98:5804-5808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 386] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 24. | Lofqvist C, Chen J, Connor KM, Smith AC, Aderman CM, Liu N, Pintar JE, Ludwig T, Hellstrom A, Smith LE. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci U S A. 2007;104:10589-10594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015;122:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 260] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 26. | Capone A Jr, Trese MT. Evolution of stage 4 retinopathy of prematurity. In: Hartnett ME, Trese MT, Capone A Jr., Keats BJB, Caputo G, eds. Pediatric Retina. 2nd ed. Philadelphia: Wolters Kluwer, 2014: 568-572. |
| 27. | Zepeda-Romero LC, Liera-Garcia JA, Gutiérrez-Padilla JA, Valtierra-Santiago CI, Avila-Gómez CD. Paradoxical vascular-fibrotic reaction after intravitreal bevacizumab for retinopathy of prematurity. Eye (Lond). 2010;24:931-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Wood EH, Rao P, Moysidis SN, Dedania VS, Elman MJ, Drenser KA, Capone A Jr, Trese MT. Fellow Eye Anti-VEGF 'Crunch' Effect in Retinopathy of Prematurity. Ophthalmic Surg Lasers Imaging Retina. 2018;49:e102-e104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |