Published online Nov 9, 2022. doi: 10.5409/wjcp.v11.i6.455
Peer-review started: July 31, 2022
First decision: September 5, 2022
Revised: September 19, 2022
Accepted: October 27, 2022
Article in press: October 27, 2022
Published online: November 9, 2022
Processing time: 99 Days and 6.6 Hours
Childhood obesity represents a complex disease with a well-known cardio
Core Tip: In addition to the well-known cardiometabolic consequences of obesity (including fatty liver, type 2 diabetes, metabolic syndrome, and cardiovascular disease), early kidney damage has been also demonstrated in children with obesity. As a consequence of the dysmetabolism, the occurrence of microalbuminuria as an early marker of kidney dysfunction has been widely described in these subjects and closely linked to insulin resistance. Given the lack of extensive pediatric data and the prognostic implications of this intriguing association, a better knowledge in this field is needed to counteract the intrinsic increased cardiometabolic risk of children with obesity.
- Citation: Colasante AM, Bartiromo M, Nardolillo M, Guarino S, Marzuillo P, Mangoni di S Stefano GSRC, Miraglia del Giudice E, Di Sessa A. Tangled relationship between insulin resistance and microalbuminuria in children with obesity. World J Clin Pediatr 2022; 11(6): 455-462
- URL: https://www.wjgnet.com/2219-2808/full/v11/i6/455.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v11.i6.455
Microalbuminuria (MA) has been largely recognized as an independent predictive and prognostic marker not only of renal dysfunction but also of cardiovascular morbidity and mortality[1-3] not only in adults with diabetes or obesity but also in healthy subjects[1]. MA is diagnosed when urinary albumin excretion (UAE) is 30-300 mg/24 h in a 24-h urine collection or 20-200 μg/min in a night time collection and ratio of urinary albumin to creatinine concentrations (UACR) is 30-300 mg/g or 3-30 mg/mmol in a first-morning urine sample random urine[2]. Estimates from cross-sectional studies found a prevalence of MA in healthy adult subjects of approximately 4%[3,4] (3.7% in males and 4.6% in females, respectively[4]) with a significant increase up to 6.2% in patients with obesity[4]. In particular, visceral obesity has been linked to MA, since the negative role of visceral adipose tissue on cardiovascular outcomes[5,6]. In this context, MA and obesity had a similar additive effect on the risk of death, independently of other common risk factors (e.g., diabetes, smoking, hypertension)[7].
Owing to its clinical relevance as an early marker of glomerular damage preceding the onset of overt diabetic nephropathy by 10-14 years[8], MA prevalence has been studied also in adults with diabetes, ranging from 7.4% to 11.4%[3,4]. Given the beneficial influence of an optimal glycemic control on the entire spectrum of renal impairments diabetes-related (MA, nephropathy, micro- and macrovascular consequences), MA screening has been recommended in these subjects[9,10].
In contrast to robust evidence supporting the clinical significance of MA in morbid adults, pediatric data in this field are still very limited[1].
The evaluation of MA in healthy children and adolescents represents a pitfall since its potential relationship with strenuous exercise or febrile illness[11].
Based on cross-sectional data from NHANES III[2], MA prevalence in the first years of life has been found to be approximately 5.7%-7.3% in boys and 12.7%-15.1% in girls[1]. The higher percentage of MA in girls than boys could be attributed to the lower muscle mass and urinary creatinine excretion resulting in higher UACR values.
Remarkably, some studies reported an increased prevalence of MA in normal weight adolescents than in those with obesity[12-14]. Higher albumin excretion rate (ACR) levels have been significantly associated with a lower body mass index standard deviation score[15] and a higher height z-score[16]. In fact, healthy adolescents with MA are commonly thin and tall[16] and in these subjects MA might be directly related to the increased physical activity of thin children.
More, an increase of MA has been found in small for gestational-age children[1] and in school-age children[13]. In particular, the microalbumin/creatinine ratio in spot urine of healthy children decreased with increasing age[16]. A positive correlation between ACR and pubertal development stage has been also reported[17].
Of note, glucose metabolism impairments [e.g., insulin resistance (IR)], pro-atherosclerotic pathways (e.g., obesity), and haemodynamic load (e.g., hypertension) have been largely accepted as the main cardiometabolic risk factors for the onset of MA in childhood[1,13,18].
Certain pathophysiological mechanisms such as low-grade inflammation, diffuse vascular damage with endothelial dysfunction[19], and increased permeability of the glomerular basement membrane[20] have been implied in urinary albumin loss.
Although there is a direct association between MA and metabolic syndrome in adults[21,22], no similar consensus has been currently reached in childhood[13,23,24] . As a consequence of pediatric obesity epidemic[21], MA has been described in these children but its relationship with metabolic milieu is still debating[25,26](Table 1).
| Ref. | Study design | Population | Main findings |
| Savino et al[44] | Case Control | One hundred seven OB Caucasian prepubertal and pubertal children and adolescents of both sexes (M 52, F 55). Fifty normal weight Caucasian children as control group (M 26, F 24) | A modest significant difference was seen in AER values, which were higher in the OB group, even if mostly within normal range. AER showed a positive correlation with central adiposity, insulin resistance indexes and hypertension |
| Sanad and Gharib[43] | Cross – Sectional | One hundred fifty prepubertal obese children. Exclusion criteria: fever, infections, renal diseases, LES, endocrine disorders, albuminuria associated with urinary tract infections | There were significant positive correlations between MA and BMI, WC, systolic and diastolic BP, TG and LDL-c levels, insulin resistance and fasting glucose level. In contrast, there was a negative correlation between MA and HDL-c levels (P < 0.01). No significant correlations of MA with age and sex were found (P > 0.05) |
| Csernus et al[45] | Case-Control | Eighty-six obese children. Seventy-nine normal weight children as a control group. children with secondary obesity were excluded | OB children with obesity had a significantly higher U-ACR and U-BMCR as compared to the normal weight children. OB children with no more than one of cardiovascular risk factors (e.g., hyperinsulinemia, fasting or post-prandial glucose, dyslipidemia and hypertension) had a significantly lower U-ACR than those with two or more features. U-ACR was positively correlated with body weight and with the fasting plasma glucose concentrations measured during the OGTT. U-ACR was increased in OB children with hypercholesterolemia. No association of U-ACR with TG and HDL-c levels was found |
| Goknar et al[30] | Case-Control | Eighty-four OB individuals aged 4-16 yr as study (case) group. Sixty-four normotensive healthy children as control group | No statistically significant differences were found in urine microalbumin/creatinine (P = 0.740) |
| Hirschler et al[12] | Retrospective Study | One thousand five hundred sixty-four children aged 5-14 yr, 220/1564 OB (14.1%), 300/1564 OW (19.2%), 1044/1564 (66.7%) normal weight, 318/1564 (20.3%) central OB | U-ACR decreased with increasing z-BMI for boys and girls. Median ACR and urinary albumin levels were significantly higher in normal weight children than in OW/OB children. Median ACR and urinary albumin levels was higher in OB girls than in OB boys |
| Radhakishun et al[28] | Retrospective | Four hundred eight OB children aged 3-19 yr, 50 % males | A low prevalence of MA (2.7%) was found. All subjects with MA were obese |
| Oz-Sig et al[33] | Retrospective | One hundred and five obese children (M 39) aged 4-18 yr. The cohort was divided into three groups as solely obese, with metabolic syndrome and with type 2 diabetes. MA was tested in 24 h collected urine (MA: 30-300 mg) | MA was significantly higher in type 2 diabetic group; statistical significance was reached in the group with metabolic syndrome and type 2 diabetic group. MA was not detected in the solely obese group |
| Lurbe et al[32] | Retrospective | One hundred and thirty-four OB children aged 9-18 yr. Obesity: z score > 2, Moderate obesity: z score 2-2.5. Severe obesity: z score > 2.5. UAE was measured in the first voiding urine of the morning | No differences between different groups of obesity degree were found. Increased UAE was linked to fasting Insulin HOMA Index, higher WC, and TG levels |
| Cho et al[15] | Retrospective | One thousand four hundred and fifty-nine adolescents aged 12-18 yr | MA was detected in 3.6% of subjects (53/1459). The Height z score of the MA group was greater than that of the NA group. The Weight z score of the MA group did not differ from that of NA group. The MA group had a lower BMI z score. MA group had higher HDL-c and lower TG levels. No significant differences in BP, fasting glucose, total cholesterol, and LDL levels were reported. UACR was associated with younger age, lower weight z score, lower BMI z score, lower W/Hr, but not with the height z score. UACR was associated with higher HDL level and lower TG values |
| Burgert et al[34] | Cohort Study | Two hundred seventy-seven children and adolescents | MA was found in 10.1 % of subjects (28/277). No significant differences between the two groups (MA e NA) in term of the anthropometrical and common cardiovascular risk factors were reported. Subjects with MA had higher plasma glucose and insulin levels during OGTT |
| Nguyen et al[29] | Cross Sectional | Two thousand five hundred fifteen adolescents aged 12-19 yr. 310/2515 children with BMI > 95 pc. | MA was detected in 8.9% of the study population. UACR girls was significantly higher in girls than in boys. MA was prevalent among NON-OW adolescents. Similarly, MA was prevalent among adolescents without abdominal obesity, and without insulin resistance |
| Martin-Del-Campo et al[38] | Cross Sectional | One hundred seventy-two children and adolescents aged 6-16 yr, 46/172 (27%) normal weight, 55/172 (32%) overweight, 71/172 (41%) obesity | MA was observed in children with OW (3.6%) and with OB (9.9%) more than in normal weight children |
In cross-sectional studies, MA prevalence in children and adolescents with obesity has been found to be approximately 6.4%[27]. Several clinical and cardiometabolic risk factors linked to MA in children and adolescents with obesity have been studied including age, sex, body mass index, waist circumference, triglycerides, high-density lipoprotein cholesterol, hypertension, and glycated hemoglobin (HbA1c)[28-30].
A large pediatric Korean study[23] demonstrated a significant association of MA with hyperglycemia [odds ratio (OR) 2.62, 95% confidence interval (CI): 1.09-6.30, P < 0.001] in normal weight children, while hypertension (OR 14.10, 95%CI: 1.12-177.98, P < 0.001) was found to be correlated to MA in children with obesity. Interestingly, HbA1c was associated with MA in both groups (OR 3.34, 95%CI: 1.09-10.17, P < 0.001, and OR 6.68, 95%CI: 1.87-23.95, P < 0.001, respectively)[21].
Nguyen et al[29] showed that overweight adolescents with impaired glucose tolerance, IR, and hypertension had MA, as previously demonstrated in adults[22,23]. From a pathogenic point of view, the increased intraglomerular capillary pressure consequent to the excess weight status might result in glomerular hyperfiltration. Consequently, it may potentially lead to MA through endothelial dysfunction triggered by specific “hits” as hypertension, impaired fasting glucose, diabetes mellitus, or smoking[31].
Lurbe et al[32] found that elevated urinary albumin excretion was correlated with higher waist circumference and insulin levels, by emphasizing the role of metabolic derangements in this relationship.
Cho and Kim[24] in a study on 1976 children and adolescents without diabetes mellitus, found a prevalence of MA in subjects with obesity of 3%. More, authors reported an association of HbA1c with MA regardless of weight status. In particular, a significant association of MA with hypertension was demonstrated in these patients, supporting the usefulness of MA as a marker of cardiovascular risk.
Another study[33] examined 105 pediatric patients with obesity divided into three groups as subjects with obesity only, subjects with obesity and metabolic syndrome, and subjects with obesity and type 2 diabetes. MA and increased levels of serum cystatin-C were found in patients with type 2 diabetes. In addition to glomerular damage IR-related, the tubule-interstitial injury might be further aggravated by glucose homeostasis dysregulation in patients with type 2 diabetes.
Burgert et al[34] reported a significant correlation of MA with post challenge alterations in glucose metabolism and insulin sensitivity loss in pediatric patients with obesity and normal glucose tolerance. In line with previous evidence[36], no association between metabolic syndrome and MA was reported, but results are affected by the lack of a control group.
Similarly, Bartz et al[35], in a study on 58 adolescents described an intriguing link between obesity-related IR (calculated through the euglycemic hyperinsulinemic clamp), and early MA onset. Indeed, insulin enhanced the effects of angiotensin II, contributing to hypertension, raised intraglomerular pressure, exacerbation of proteinuria, production of inflammatory cytokines, and apoptosis[36].
In contrast to the low prevalence of MA in the context of pediatric obesity reported in these studies, data on kidney function from adolescents with severe obesity reported a higher MA prevalence (17.3%)[37], suggesting a greater risk of chronic kidney disease for these patients.
In a study by Martin-Del-Campo et al[38] subjects with kidney alterations had higher body fat markers (including body mass index, waist circumference, fat percentage, subscapular skinfold, etc.) values and lower high-density lipoprotein cholesterol levels. Commonly, all these factors have been strongly associated with the development of kidney disease in adults[39-41]. In fact, fat deposition in the glomerulus may alter renal production of vasoactive and inflammatory mediators related to glomerular damages[42].
Also, in the study of Sanad and Gharib[43], MA was proposed as a marker of the endothelial dysfunction related to obesity and its metabolic consequences. Indeed, a positive correlation between MA and a worse cardiometabolic profile (including abdominal obesity, dyslipidemia, hypertension, IR, and impaired glucose tolerance) was demonstrated in children with obesity.
Similar evidence was provided by Savino et al[44]. Although no clinical evidence of kidney dysfunction was found in youths with obesity compared to healthy controls, MA was confirmed to be associated with hypertension, adiposity, and IR.
Csernus et al[45], found that children with obesity and glucose homeostasis abnormalities (including hyperinsulinemia and impaired glucose tolerance) and had a higher urinary albumin/creatinine ratio than normal weight children, supporting the central role of these factors in the development of kidney damage.
On the other hand, there is some contrasting evidence[29] reporting no association between MA and cardiometabolic risk factors in childhood obesity. To explain this, it could be supposed that a longer dysmetabolism (including duration of obesity and IR status) is required for renal dysfunction development.
An Italian study[46] involved 901 children and adolescents subdivided according to estimated glomerular filtration (eGFR). Children with mild-low eGFR (< 20th percentile) and high eGFR (> 80th percentile) had an increased presence of cardio-metabolic risk factor. Between these, children with reduced eGFR levels had a worse cardio-metabolic profile. Considering this, authors suggested eGFR as a useful tool to identify children at greater cardiometabolic risk.
Similar findings were reported in another cross-sectional study examining 360 children with obesity[28]. Subjects with eGFR > 1 SD had higher systolic blood pressure, glucose, and insulin levels in response to oral glucose tolerance test. No significant association was demonstrated between MA (reported in 6.4% of subjects) and other cardiometabolic markers in children with obesity, although a lower insulinogenic index in subjects with MA was reported.
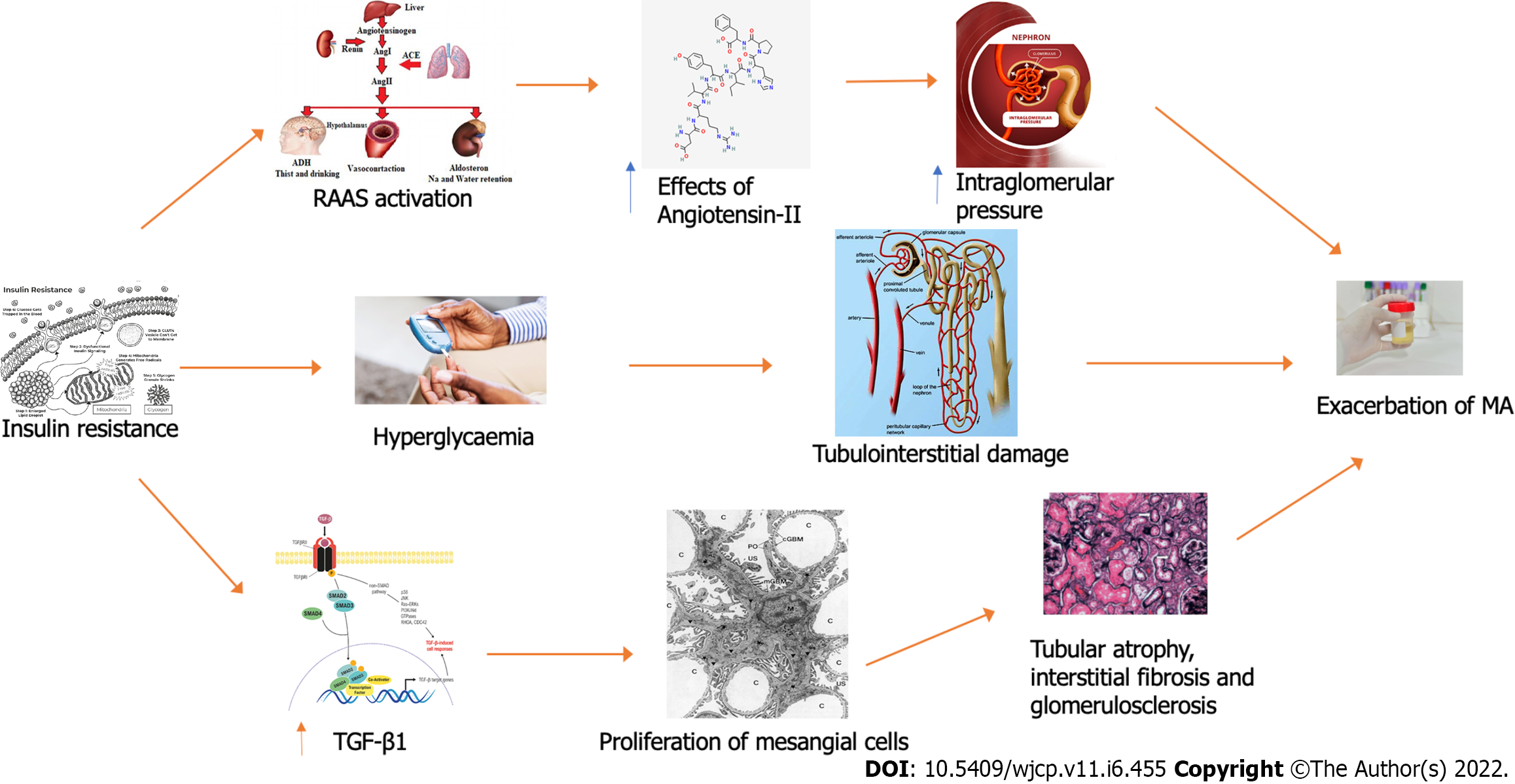
As observed adults[7,39], early renal damage (expressed as MA) has been demonstrated as a consequence of obesity in childhood[1,12,26]. Robust evidence has shown that MA represents a close reflection of the systemic vascular endothelial damage status[26,34,35]. In this tangled framework, a pivotal pathogenic role in the development of the underlying renal hemodynamic abnormalities has been widely recognized for IR[26,32,35] (Figure 1). As a consequence of a reduced insulin sensitivity, hyperinsulinemia- through the well-documented conflicting effects of insulin (both antinatriuretic and at tubular and glomerular level, respectively), acts as a key player in the tangled dysregulation of renal hemodynamics (including glomerular hyperperfusion, hyperfiltration, etc.) occurring in children with obesity[47,48]. To complicate matter, IR seems to mediate the intertwined relationship of MA with obesity and early renal impairment[35]. Classically, several different signaling pathways are involved in IR development and have been found to act also in the early stages of renal injury[26,34,35]. Therefore, MA represents an early predictive cardiometabolic risk marker as its close relationship with endothelial dysfunction reflecting both renal and systemic endovascular damage[26,34,35]. Although the current paucity of data examining the association of IR with MA in children with obesity, there is some pediatric evidence linking this latter to cardiometabolic risk factors[23,25,26,43]. IR represents a central player in Metabolic syndrome, in turn closely related to obesity, realizing a dangerous vicious circle[26,43,46]. In particular, the adiposity-related IR might lead to endothelial dysfunction with subsequent increased permeability responsible for the loss of albumin and other molecules involved in lipid accumulation and inflammation in the wall of vessels[26,43]. Taken together, these derangements might represent a potential pathophysiological explanation of kidney damage observed in children with obesity[26,43,47].
As the relevant prognostic implications of the relationship between MA and IR on the cardio
Within the spectrum of the pediatric obesity-related consequences, recent data have focused on the risk of early kidney damage in these children. Although still contrasting, a large body of evidence supported a complex relationship of MA with IR. Noteworthy, this latter represents an intriguing shared risk factor between obesity and early renal impairment.
Taking into account the adverse prognostic implications of the association of MA with IR not only on renal function but also on the general health status of children with obesity, we believe that MA evaluation should be included in the overall assessment of these patients as subjects with an intrinsic higher cardiometabolic risk.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Geng TY, China; Liao Z, Singapore S-Editor: Gao CC L-Editor: A P-Editor: Zhang XD
| 1. | Tsioufis C, Mazaraki A, Dimitriadis K, Stefanidis CJ, Stefanadis C. Microalbuminuria in the paediatric age: current knowledge and emerging questions. Acta Paediatr. 2011;100:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Jones CA, Francis ME, Eberhardt MS, Chavers B, Coresh J, Engelgau M, Kusek JW, Byrd-Holt D, Narayan KM, Herman WH, Jones CP, Salive M, Agodoa LY. Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39:445-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 315] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Tanaka S, Takase H, Dohi Y, Kimura G. The prevalence and characteristics of microalbuminuria in the general population: a cross-sectional study. BMC Res Notes. 2013;6:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Yan L, Ma J, Guo X, Tang J, Zhang J, Lu Z, Wang H, Cai X, Wang L. Urinary albumin excretion and prevalence of microalbuminuria in a general Chinese population: a cross-sectional study. BMC Nephrol. 2014;15:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Grundy SM, Hansen B, Smith SC Jr, Cleeman JI, Kahn RA; American Heart Association; National Heart, Lung, and Blood Institute; American Diabetes Association. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004;109:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 564] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 6. | Kim H, Kim HJ, Shin N, Han M, Park H, Kim M, Kwon H, Choi SY, Heo NJ. Visceral obesity is associated with microalbuminuria in nondiabetic Asians. Hypertens Res. 2014;37:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Klausen KP, Parving HH, Scharling H, Jensen JS. Microalbuminuria and obesity: impact on cardiovascular disease and mortality. Clin Endocrinol (Oxf). 2009;71:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Correa-Rotter R, Naicker S, Katz IJ, Agarwal SK, Herrera Valdes R, Kaseje D, Rodriguez-Iturbe B, Shaheen F, Sitthi-Amorn C; ISN-COMGAN Bellagio Study Group 2004. Demographic and epidemiologic transition in the developing world: role of albuminuria in the early diagnosis and prevention of renal and cardiovascular disease. Kidney Int Suppl. 2004;S32-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S135-S151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 275] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 10. | Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark Insights. 2016;11:95-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 792] [Cited by in RCA: 636] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 11. | Sanchez-Bayle M, Rodriguez-Cimadevilla C, Asensio C, Ruiz-Jarabo C, Baena J, Arnaiz P, Villa S, Cocho P. Urinary albumin excretion in Spanish children. Niño Jesus Group. Pediatr Nephrol. 1995;9:428-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Hirschler V, Molinari C, Maccallini G, Aranda C. Is albuminuria associated with obesity in school children? Pediatr Diabetes. 2010;11:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Mazaraki A, Tsioufis C, Dimitriadis K, Tsiachris D, Stefanadi E, Zampelas A, Richter D, Mariolis A, Panagiotakos D, Tousoulis D, Stefanadis C. Adherence to the Mediterranean diet and albuminuria levels in Greek adolescents: data from the Leontio Lyceum ALbuminuria (3L study). Eur J Clin Nutr. 2011;65:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Tsioufis C, Tsiachris D, Dimitriadis K, Thomopoulos C, Syrseloudis D, Andrikou E, Chatzis D, Taxiarchou E, Selima M, Mazaraki A, Chararis G, Tolis P, Gennadi A, Andrikou I, Stefanadi E, Fragoulis V, Tzamou V, Panagiotakos D, Tousoulis D, Stefanadis C. Leontio Lyceum ALbuminuria (3L Study) epidemiological study: aims, design and preliminary findings. Hellenic J Cardiol. 2009;50:476-483. [PubMed] |
| 15. | Cho MH, Kim KS, Chung S. Microalbuminuria Is Associated with Lower Weight and Taller Height in Adolescence. Tohoku J Exp Med. 2017;243:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Kwak BO, Lee ST, Chung S, Kim KS. Microalbuminuria in normal Korean children. Yonsei Med J. 2011;52:476-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Bangstad HJ, Dahl-Jørgensen K, Kjaersgaard P, Mevold K, Hanssen KF. Urinary albumin excretion rate and puberty in non-diabetic children and adolescents. Acta Paediatr. 1993;82:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Okpere AN, Anochie IC, Eke FU. Prevalence of microalbuminuria among secondary school children. Afr Health Sci. 2012;12:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Tsioufis C, Dimitriadis K, Chatzis D, Vasiliadou C, Tousoulis D, Papademetriou V, Toutouzas P, Stefanadis C, Kallikazaros I. Relation of microalbuminuria to adiponectin and augmented C-reactive protein levels in men with essential hypertension. Am J Cardiol. 2005;96:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Kwak BO, Chung S, Kim KS. Microalbuminuria in children with urinary tract infection. Korean J Pediatr. 2010;53:840-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Sheng CS, Hu BC, Fan WX, Zou J, Li Y, Wang JG. Microalbuminuria in relation to the metabolic syndrome and its components in a Chinese population. Diabetol Metab Syndr. 2011;3:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Lee HO, Bak HJ, Shin JY, Song YM. Association between Metabolic Syndrome and Microalbuminuria in Korean Adults. Korean J Fam Med. 2015;36:60-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Cho H, Kim JH. Prevalence of microalbuminuria and its associated cardiometabolic risk factors in Korean youth: Data from the Korea National Health and Nutrition Examination Survey. PLoS One. 2017;12:e0178716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Lambers Heerspink HJ, Brantsma AH, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort RT; PREVEND Study Group. Albuminuria assessed from first-morning-void urine samples vs 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol. 2008;168:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 25. | Folić N, Folić M, Marković S, Andjelković M, Janković S. Risk factors for the development of metabolic syndrome in obese children and adolescents. Srp Arh Celok Lek. 2015;143:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Savino A, Pelliccia P, Chiarelli F, Mohn A. Obesity-related renal injury in childhood. Horm Res Paediatr. 2010;73:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Ricotti R, Genoni G, Giglione E, Monzani A, Nugnes M, Zanetta S, Castagno M, Marolda A, Bellomo G, Bona G, Bellone S, Prodam F. High-normal estimated glomerular filtration rate and hyperuricemia positively correlate with metabolic impairment in pediatric obese patients. PLoS One. 2018;13:e0193755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Radhakishun NN, van Vliet M, von Rosenstiel IA, Beijnen JH, Diamant M. Limited value of routine microalbuminuria assessment in multi-ethnic obese children. Pediatr Nephrol. 2013;28:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Nguyen S, McCulloch C, Brakeman P, Portale A, Hsu CY. Being overweight modifies the association between cardiovascular risk factors and microalbuminuria in adolescents. Pediatrics. 2008;121:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Goknar N, Oktem F, Ozgen IT, Torun E, Kuçukkoc M, Demir AD, Cesur Y. Determination of early urinary renal injury markers in obese children. Pediatr Nephrol. 2015;30:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Nenov VD, Taal MW, Sakharova OV, Brenner BM. Multi-hit nature of chronic renal disease. Curr Opin Nephrol Hypertens. 2000;9:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Lurbe E, Torro MI, Alvarez J, Aguilar F, Fernandez-Formoso JA, Redon J. Prevalence and factors related to urinary albumin excretion in obese youths. J Hypertens. 2013;31:2230-6; discussion 2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Oz-Sig O, Kara O, Erdogan H. Microalbuminuria and Serum Cystatin C in Prediction of Early-Renal Insufficiency in Children with Obesity. Indian J Pediatr. 2020;87:1009-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Burgert TS, Dziura J, Yeckel C, Taksali SE, Weiss R, Tamborlane W, Caprio S. Microalbuminuria in pediatric obesity: prevalence and relation to other cardiovascular risk factors. Int J Obes (Lond). 2006;30:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Bartz SK, Caldas MC, Tomsa A, Krishnamurthy R, Bacha F. Urine Albumin-to-Creatinine Ratio: A Marker of Early Endothelial Dysfunction in Youth. J Clin Endocrinol Metab. 2015;100:3393-3399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Rüster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006;17:2985-2991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 37. | Xiao N, Jenkins TM, Nehus E, Inge TH, Michalsky MP, Harmon CM, Helmrath MA, Brandt ML, Courcoulas A, Moxey-Mims M, Mitsnefes MM; Teen-LABS Consortium. Kidney function in severely obese adolescents undergoing bariatric surgery. Obesity (Silver Spring). 2014;22:2319-2325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Martin-Del-Campo F, Batis-Ruvalcaba C, Ordaz-Medina SM, Martínez-Ramírez HR, Vizmanos-Lamotte B, Romero-Velarde E, Cortes-Sanabria L, Cueto-Manzano AM. Frequency and Risk Factors of Kidney Alterations in Children and Adolescents who Are Overweight and Obese in a Primary Health-care Setting. J Ren Nutr. 2019;29:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 453] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 40. | Hayashi K, Takayama M, Abe T, Kanda T, Hirose H, Shimizu-Hirota R, Shiomi E, Iwao Y, Itoh H. Investigation of Metabolic Factors Associated with eGFR Decline Over 1 Year in a Japanese Population without CKD. J Atheroscler Thromb. 2017;24:863-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Ribstein J, du Cailar G, Mimran A. Combined renal effects of overweight and hypertension. Hypertension. 1995;26:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 214] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 42. | Kasiske BL, O'Donnell MP, Cowardin W, Keane WF. Lipids and the kidney. Hypertension. 1990;15:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Sanad M, Gharib A. Evaluation of microalbuminuria in obese children and its relation to metabolic syndrome. Pediatr Nephrol. 2011;26:2193-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Savino A, Pelliccia P, Giannini C, de Giorgis T, Cataldo I, Chiarelli F, Mohn A. Implications for kidney disease in obese children and adolescents. Pediatr Nephrol. 2011;26:749-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Csernus K, Lanyi E, Erhardt E, Molnar D. Effect of childhood obesity and obesity-related cardiovascular risk factors on glomerular and tubular protein excretion. Eur J Pediatr. 2005;164:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Di Bonito P, Sanguigno E, Forziato C, Di Fraia T, Moio N, Cavuto L, Sibilio G, Iardino MR, Di Carluccio C, Capaldo B. Glomerular filtration rate and cardiometabolic risk in an outpatient pediatric population with high prevalence of obesity. Obesity (Silver Spring). 2014;22:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol. 2000;278:F817-F822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 416] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 48. | DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975;55:845-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 699] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 49. | Di Bonito P, Valerio G, Licenziati MR, Campana G, Del Giudice EM, Di Sessa A, Morandi A, Maffeis C, Chiesa C, Pacifico L, Baroni MG, Manco M. Uric acid, impaired fasting glucose and impaired glucose tolerance in youth with overweight and obesity. Nutr Metab Cardiovasc Dis. 2021;31:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |