Published online Sep 9, 2022. doi: 10.5409/wjcp.v11.i5.429
Peer-review started: April 22, 2022
First decision: May 31, 2022
Revised: June 10, 2022
Accepted: August 22, 2022
Article in press: August 22, 2022
Published online: September 9, 2022
Processing time: 138 Days and 6.6 Hours
A leukocyte adhesion defect (LAD) is a rare primary immunodeficiency disorder. LAD type 1 (LAD-1) is the most common, which is caused by ITGB2 mutation resulting in dysfunction of β2 integrin, which impairs leukocyte adherence to the endothelium.
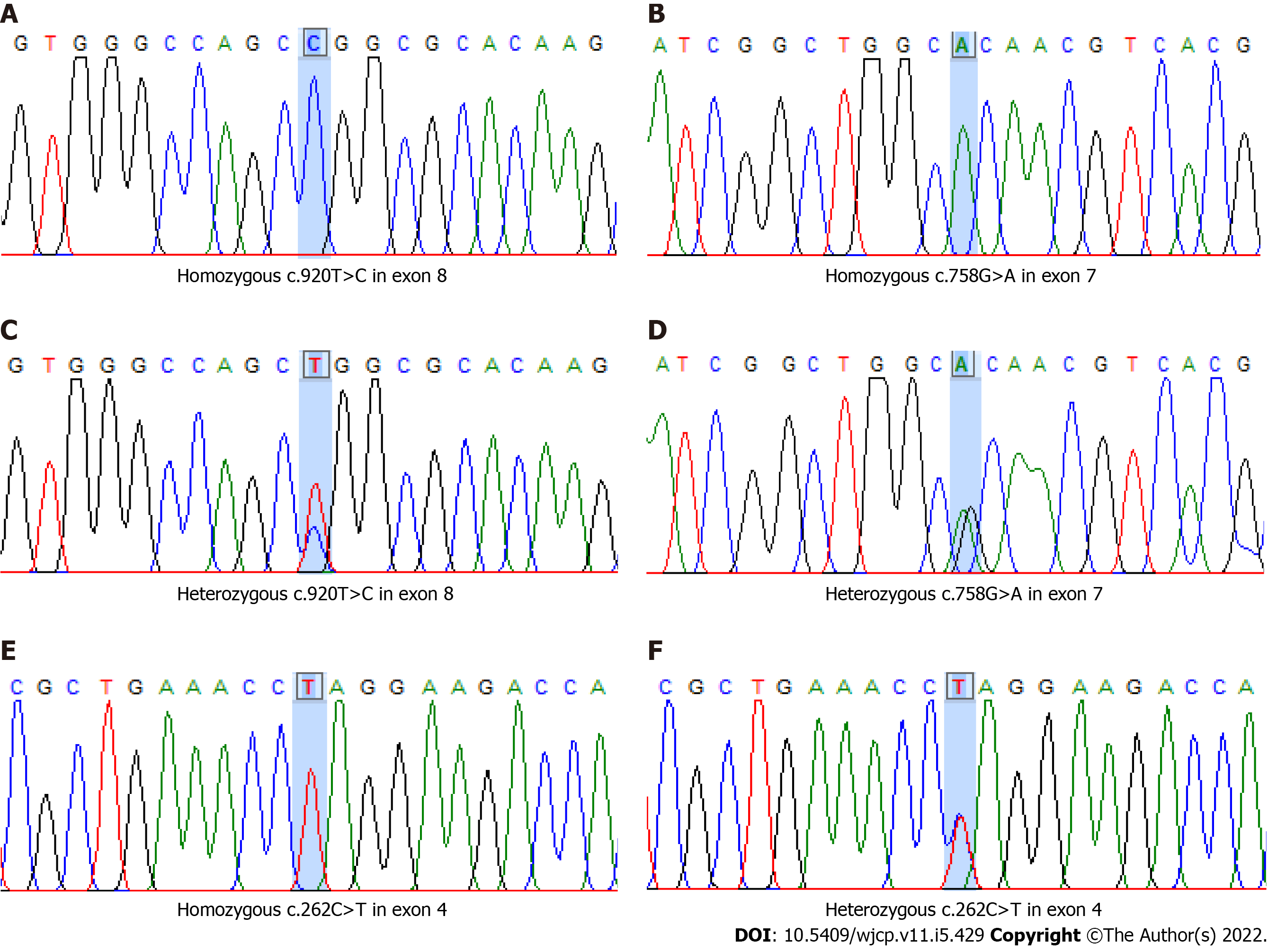
The first two cases of LAD-1 in Thailand presented with recurrent omphalitis, soft tissue infection, marked leukocytosis, and neutrophilia. One patient experienced delayed umbilical cord separation. Mutation analysis was performed by direct DNA sequencing of the ITGB2 gene. The results revealed two novel homozygous missense mutations, c.920C>T (p.Leu307Pro) in exon 8 and c.758G>A (p.Arg
Molecular analysis is essential for definitive diagnosis, early treatment implementation, and prevention of LAD-1 in future pregnancy.
Core Tip: Leukocyte adhesion defect (LAD) is a rare autosomal recessive primary immunodeficiency disorder characterized by defects in the leukocyte recruitment cascade. LAD type 1, caused by a mutation in ITGB2, is the most common form. Here, we report the first two cases of LAD type 1 with a molecularly confirmed ITGB2 mutation in Thailand. At the time of initial presentation, both patients had recurrent omphalitis, bacterial soft tissue infection, and marked leukocytosis. Molecular analysis revealed two missense variants and one nonsense mutation. Early identification of these patients by molecular analysis was proven essential for definitive diagnosis, proper antibiotic prophylaxis, and initiation of matched donor hematopoietic stem cell transplantation.
- Citation: Suksawat Y, Pacharn P, Siripipattanamongkol N, Boonyawat B. Three novel homozygous ITGB2 mutations among two patients with leukocyte adhesion defect type-1: Two case reports. World J Clin Pediatr 2022; 11(5): 429-436
- URL: https://www.wjgnet.com/2219-2808/full/v11/i5/429.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v11.i5.429
Leukocyte adhesion defect (LAD) is a rare autosomal recessive primary immunodeficiency disorder affecting one in every 100000 individuals. A deficiency in the leukocyte adhesion cascade to the blood vessel wall is the pathogenesis of LAD and is classified into three types. LAD type 1 (LAD-1) (OMIM#116920), which is caused by a mutation in the ITGB2 gene, is the most common form. The ITGB2 gene (OMIM*600065) is located on 21q22.3 and encoded the common β subunit of the β2 integrin protein (CD18). Dysfunctional β2 integrin is the main defect in LAD-1 and is attributable to impaired neutrophil firm adhesion[1]. The hallmark characteristics are recurrent bacterial skin and soft tissue infections, omphalitis, and delayed umbilical cord separation. In addition, the absence of pus formation is a distinctive feature[2]. LAD-1 diagnosis can be confirmed by flow cytometric expression of CD18 and CD11 on leukocytes or ITGB2 mutation analysis[2,3]. To date, more than 110 mutations have been identified[3]. Here, we report the first two cases of LAD-1 with molecularly confirmed ITGB2 mutation in Thailand. The study protocol was approved by the Institutional Review Board of the Royal Thai Army.
Case 1: A 21-mo-old Burmese boy presented with prolonged fever and multiple whitish ulcers in the oropharynx (Patient #1, P1).
Case 2: A 9-d-old Thai girl presented with redness around the umbilical stump (Patient #2, P2).
Case 1: The patient had fever and dyspnea for 3 wk. Additionally, he had been previously treated in Myanmar and Laos; however, his clinical condition had deteriorated, and he was referred from a rural hospital near the Thai border. He was on the verge of respiratory failure due to acute upper airway obstruction. Emergency tracheostomy was performed, and pus with debris extending to the oropharynx, larynx, and epiglottis was discovered intraoperatively. Streptococcus viridans and Staphylococcus epidermidis were identified in the pus cultures. Although intravenous antibiotics were administered, healing of the wound was difficult. He was re-admitted several times with chronic wound infections around the tracheostomy site, Pseudomonas aeruginosa pneumonia, oral candidiasis, and cellulitis. Several organisms, including Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Salmonella gr.B, and carbapenem-resistant Enterobacteriaceae Klebsiella pneumoniae were identified in pus cultures.
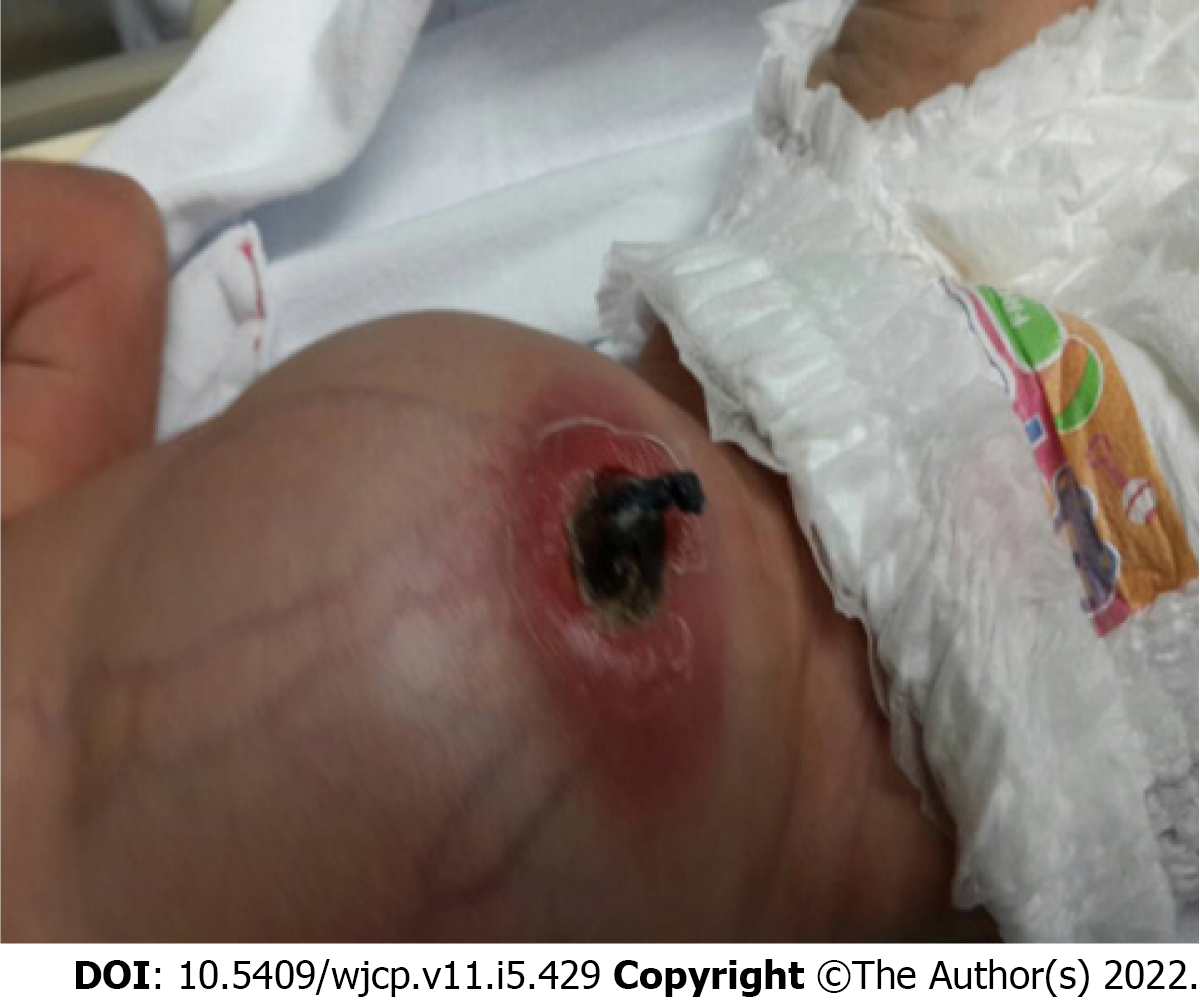
Case 2: At 9 d of age, she presented with fever and abdominal distension. Physical examination revealed minimal pus with redness around the umbilical stump (Figure 1). The patient was then diagnosed with omphalitis. Intravenous cloxacillin and metronidazole were administered; however, her clinical condition worsened. Omphalitis persisted until 4 wk of age. Pus culture revealed Staphylococcus epidermidis, Escherichia coli, and extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. The intravenous antibiotics were switched to carbapenems, with some clinical improvement. She was re-admitted because of recurrent omphalitis at 6 wk of age. After administration of intravenous antibiotics for 3 d, her symptoms subsided. At 2 mo of age, she was referred to our university hospital for assessment of recurrent omphalitis.
Case 1: The patient had a delayed umbilical cord detachment 30 d after birth and delayed wound healing, resulting in difficult-to-treat omphalitis.
Case 2: The patient’s umbilical cord was separated 20 d after birth.
Case 1: The patient was born at term with an uneventful pregnancy and delivery history. The patient completed an extended immunization program based on his age. His sister had no health issues despite being the second child of consanguineous parents.
Case 2: The patient was born at term with an uneventful pregnancy and perinatal history. Her birth weight was 2650 g. BCG and HBV vaccines were administered after birth. She was the first child of a couple who had denied consanguinity.
Case 1: Physical examination of P1 revealed a weight of 9.5 kg (3rd percentile) and a height of 82 cm (25th–50th percentile). Multiple whitish ulcers were present on both lips and the oral cavity, and a BCG scar was observed on the left shoulder. Mild hepatosplenomegaly was observed.
Case 2: Physical examination of P2 revealed normal growth parameters. Periumbilical redness with a lack of pus formation was observed, and a BCG scar was observed on the left shoulder.
Case 1: Complete blood count revealed leukocytosis (WBC 94600/mm3) and neutrophil predominance (67166/mm3), while anti-HIV was negative. Bone marrow aspiration revealed no evidence of hematological malignancy. His parents were asked for permission to conduct immunologic tests, but they denied due to financial concerns.
Case 2: Complete blood count showed significant leukocytosis (65300/mm3) and neutrophilia (46800/mm3). The lymphocyte populations determined using flow cytometry, serum immunoglobulin levels, and dihydrorhodamine 123 assays were normal.
Case 1: Chest radiography showed patchy infiltration in both lower lungs. Computed tomography of the neck showed obstruction of the upper airway due to an infection involving the mucosa of the oropharynx, hypopharynx, glottic, and subglottic levels.
Case 2: Anatomical abnormalities of the umbilicus were excluded by abdominal ultrasound, which revealed neither a patent urachus nor an omphalomesenteric cyst.
The diagnosis of LAD-1 was suspected in both patients.
After informed consent was obtained from the patients and their parents, genomic DNA was extracted from peripheral blood lymphocytes using commercially available kits, according to the manufacturer’s protocol. Sixteen coding exons and exon-intron boundaries of the ITGB2 gene were amplified by PCR using primers as described previously[4,5]. Each 50 μL PCR mixture contained 1.5 mmol/L MgCl2, 200 μmol/L of each dNTP, 0.5 μmol/L of each primer, 100 to 200 ng of genomic DNA and 1.25 units of Taq DNA polymerase. The PCR conditions were as follows: Initial denaturation at 95°C for 5 min; 30 cycles of 95°C for 30 s, 62 °C to 64°C for 30 s, and 72°C for 45 s; and a final extension at 72°C for 5 min. All PCR products were purified and directly sequenced in both the forward and reverse directions. The reference sequences were NM_000211.5 and NP_000202.3 for ITGB2 cDNA and β2 integrin amino acid positions, respectively.
Homozygous of two novel “likely pathogenic” missense variants, c.920T>C (p.Leu307Pro) in exon 8 and c.758G>A (p.Arg
| Information/computation (in silico) predictive programs | c.920T>C (p.Leu307Pro) in exon 8 | c.758G>A (p.Arg253His) in exon 7 | c.262C>T (p.Gln88Ter) in exon 4 |
| Human gene mutation database (HGMD) | Not identified | Not identified | Not identified |
| National center for biotechnology information (NCBI): dbSNP and ClinVar | Not identified | Uncertain significance rs200423927 | Not identified |
| Exome aggregation consortium (ExAC) and 1000 genomes project | Not identified | ExAC 0.0002817 heterozygous (only) | Not identified |
| Mutation taster (http://www.mutationtaster.org/) | Disease causing | Disease causing | Disease causing |
| PolyPhen (http://genetics.bwh.harvard.edu/pph2/) | Probably damaging | Benign | - |
| SIFT (http://sift.jcvi.org/) | Damaging | Damaging | - |
| ACMG classification (2015) | Likely pathogenic (PM2, PM3, PP2, PP3) | Likely pathogenic (PM2, PM3, PP2, PP3) | Pathogenic (PVS1, PM2, PM3, PP3) |
Clinical and laboratory information, including ITGB2 mutation analysis results for both patients, are summarized in Table 2.
| Clinical information | Patient 1 | Patient 2 |
| Age of onset | 1 mo | 9 d |
| Clinical characteristics | Omphalitis | Omphalitis |
| Soft tissue infection | Gastroenteritis (occasional) | |
| Delayed wound healing | ||
| Pneumonia | ||
| Delay separation of the umbilical cord | Yes | No |
| Family history of consanguineous marriage | Yes | No |
| Organisms | Streptococcus viridans | Staphylococcus epidemidis |
| Staphylococcus epidermidis | Escherichia coli | |
| Staphylococcus aureus | Klebsiella pneumonia, ESBL | |
| Pseudomonas aeruginosa and others | ||
| White blood cells (WBC) | 94600/mm3 | 65300/mm3 |
| Absolute neutrophil counts (ANC) | 67166/mm3 | 46800/mm3 |
| ITGB2 mutation | c.920T>C (p.Leu307Pro) in exon 8 and c.758G>A (p.Arg253His) in exon 7 | c.262C>T (p.Gln88Ter) in exon 4 |
| Outcome (at present) | Alive | Alive |
The final diagnosis for both patients was LAD-1 with the carrier status in the parents.
Intravenous cloxacillin, ceftazidime, and amikacin were administered for chronic wound and soft tissue infections, resulting in good clinical responses.
The use of intravenous gentamycin and ciprofloxacin for recurrent omphalitis improved her clinical outcome. She has not presented with omphalitis since that time.
Currently, hematopoietic stem cell transplantation cannot be performed because of the unavailability of matched donors. Sulfamethoxazole-trimethoprim with itraconazole prophylaxis was initiated and a significant decline in infection was noted. P1 developed a soft tissue infection around the tracheostomy, and P2 had occasional gastroenteritis and chronic otitis media in response to antibiotics. Several follow-up visits were conducted for both patients. They are currently six years old.
LAD-1 was first identified in 1979[6]. To date, more than 400 LAD-1 cases have been reported with the highest prevalence in Iran, the United States, and India in over 100 publications[7,8]. Our report is the first case series of LAD-1 diagnosed in Thailand. Patients with LAD-1 usually present in infancy with delayed umbilical cord separation, omphalitis, and skin infection in both mucosal and subcutaneous tissues. LAD-1 is classified as mild (LAD-1+), moderate (LAD-1-), or severe (LAD-10) depending on CD18 expression in neutrophils. Although the CD18 expression study remains unavailable at our institution, our patients were suspected of having LAD-10 due to the early age of onset[3,7-9]. Both patients experienced omphalitis at the first presentation, which was also the most common initial manifestation among patients with LAD-10 in related studies[8,10,11]. However, delayed cord separation after three weeks was only observed in P1, suggesting that this clinical feature may not be an essential characteristic of LAD-10[8-10,12].
Other common infections that have been widely reported in related studies, including respiratory tract infection and sepsis[5-8,10], were not identified in P2, which could possibly be explained by early diagnosis and antibiotic prophylaxis in this patient. The spectra of infectious organisms in our patients were similar to those of other reported cohorts in which bacterial infections, including gram-positive cocci (Staphylococci or Streptococci) and gram-negative bacteria (Pseudomonas aeruginosa or Klebsiella pneumoniae) were predominantly identified[8,9,11]. Marked neutrophilic leukocytosis, which is the hallmark of LAD-1, was found in both patients.
The BCG vaccine is routinely prescribed for newborns as part of the national immunization program in Thailand. Vaccine-associated serious BCG infection has been reported among people with immunodeficiency, particularly severe combined immunodeficiency and chronic granulomatous disease (CGD)[13]. Furthermore, we previously described a case of X-linked CGD with disseminated BCG infection[14]. According to the recent vaccination recommendations for primary immunodeficiency disease, live bacterial vaccines, such as BCG vaccine, are not recommended for patients with LAD[15]. Nevertheless, no BCG vaccine-related complications were reported among patients with LAD in the most recent systematic review[13], except for one reported Japanese girl with LAD-1 who had necrotizing ulcers after BCG vaccination[16]. This phenomenon is explained by a study in mice with abnormal integrins, CD11a and CD18, which were susceptible to Mycobacterium tuberculosis infection, indicating that adhesion molecules are essential for mycobacterial immunity[17]. Because BCG vaccination was administered to both patients at birth, BCG-related complications were monitored. To date, BCG-related complications have not been observed.
The diagnosis of LAD-1 is based on either the flow cytometric expression study of CD18 on leukocytes or molecular confirmation. Due to the unavailability of the CD18 expression study at our institution, molecular analysis by direct DNA sequencing of ITGB2 was performed. Mutations in the ITGB2 gene are heterogeneous, and missense mutations are the main cause of LAD-1 deficiency. Most mutations are located in the highly conserved domain, Von Willebrand Factor type A (VWFA), which consists of 240 amino acids encoded by exons 5 to 9 of the ITGB2 gene. This domain is required for the enzymatic activity of the β2 integrin (CD18). Other mutations are scattered throughout the gene[3,4,7,8,10].
This study identified three novel mutations: two likely deleterious missense mutations and one deleterious nonsense mutation. The two missense variants, c.920C>T (p.Leu307Pro) in exon 8 and c.758G>A (p.Arg253His) in exon 7, which were identified in P1, are located in the VWFA domain, which is highly conserved in the β2 integrin protein. The c.920C>T variant has never been identified in either the Exome Aggregation Consortium (ExAC) or the 1000 Genomes Project population databases. c.758G>A is a rare variant identified in approximately 4 of 8632 individuals of East Asian ancestry according to the ExAC database and was identified only in the heterozygous state. Additionally, these two homozygous missense mutations were not identified in the Thai Reference Exome variant database (1092 unrelated Thai individuals; T-Rex, https://trex.nbt.or.th/).
Most of the in silico analysis tools consistently predicted these two variants that may damage protein function (Table 1). Many missense pathogenic variants in nearby residues in the ITGB2 gene have been reported to be associated with LAD-1[3]. These reasons support the possibility of “likely pathogenic” for both missense variants in Myanmar patient with LAD-1. Further in vitro studies may address the possible impact of amino acid substitutions on the function of β2 integrin. Unfortunately, functional studies cannot be performed at our institution. Two mutations were detected simultaneously in some of the previously reported patients[4]. One nonsense mutation, c.262C>T (p.Gln88Ter) in exon 4, which was identified in P2, leads to a premature stop at codon 88 which normally encodes for glutamine and results in loss of both active VWFA and cysteine rich domains of β2 integrin protein. This nonsense mutation has never been reported in the ExAC or 1000 Genomes Project databases. The VWFA domain is crucial for the structural association of a β-integrin subunits for heterodimer formation on the cell surface and functional activity. Any significant alterations in this region will definitely have a deleterious effect on the expression and function of β2 integrin and result in the LAD-10 phenotype among most patients with LAD-1[8,10]. Even though consanguineous marriage was denied by the parents of P2, consanguinity could not be excluded based on the identification of a novel nonsense mutation in the homozygous state in the patient and the heterozygous state in both parents.
LAD-10 has an extremely poor prognosis, with the majority of patients dying within two years of life[7,12]. Early hematopoietic stem cell transplantation remains the treatment of choice, but this is unavailable for the majority of affected children in developing countries, including our patients. Thus, antibiotics used for prophylaxis and treatment of infections are the mainstay of treatment, while waiting for matched donors.
Herein, we report two classic cases of severe LAD-1. Early onset and recurrent omphalitis were common pathognomonic signs in our patients. A significant increase in white blood cell counts combined with neutrophilia should increase the awareness of LAD-1. Mutation analysis of the ITGB2 gene remains the gold standard for the diagnosis of LAD-1. Three novel homozygous ITGB2 mutations were identified in these patients. Molecular investigation is essential for definitive diagnosis, early treatment implementation, and prenatal diagnosis in future pregnancies.
The authors express their gratitude to the patients and their families who participated in this study. This study was supported by Phramongkutklao College of Medicine.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Genetics and heredity
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shuang W, China; Zhao GH, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Hanna S, Etzioni A. Leukocyte adhesion deficiencies. Ann N Y Acad Sci. 2012;1250:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 2. | van de Vijver E, van den Berg TK, Kuijpers TW. Leukocyte adhesion deficiencies. Hematol Oncol Clin North Am. 2013;27:101-116, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | van de Vijver E, Maddalena A, Sanal Ö, Holland SM, Uzel G, Madkaikar M, de Boer M, van Leeuwen K, Köker MY, Parvaneh N, Fischer A, Law SK, Klein N, Tezcan FI, Unal E, Patiroglu T, Belohradsky BH, Schwartz K, Somech R, Kuijpers TW, Roos D. Hematologically important mutations: leukocyte adhesion deficiency (first update). Blood Cells Mol Dis. 2012;48:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 4. | Yassaee VR, Hashemi-Gorji F, Boosaliki S, Parvaneh N. Mutation spectra of the ITGB2 gene in Iranian families with leukocyte adhesion deficiency type 1. Hum Immunol. 2016;77:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Taghizade Mortezaee F, Esmaeli B, Badalzadeh M, Ghadami M, Fazlollahi MR, Alizade Z, Hamidieh AA, Chavoshzadeh Z, Movahedi M, Heydarzadeh M, Sadeghi Shabestari M, Tavassoli M, Nabavi M, Nasiri Kalmarzi R, Pourpak Z. Investigation of ITGB2 gene in 12 new cases of leukocyte adhesion deficiency-type I revealed four novel mutations from Iran. Arch Iran Med. 2015;18:760-764. [PubMed] |
| 6. | Hayward AR, Harvey BA, Leonard J, Greenwood MC, Wood CB, Soothill JF. Delayed separation of the umbilical cord, widespread infections, and defective neutrophil mobility. Lancet. 1979;1:1099-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 82] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Almarza Novoa E, Kasbekar S, Thrasher AJ, Kohn DB, Sevilla J, Nguyen T, Schwartz JD, Bueren JA. Leukocyte adhesion deficiency-I: A comprehensive review of all published cases. J Allergy Clin Immunol Pract. 2018;6:1418-1420.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Kambli PM, Bargir UA, Yadav RM, Gupta MR, Dalvi AD, Hule G, Kelkar M, Sawant-Desai S, Setia P, Jodhawat N, Nambiar N, Dhawale A, Gaikwad P, Shinde S, Taur P, Gowri V, Pandrowala A, Gupta A, Joshi V, Sharma M, Arora K, Pilania RK, Chaudhary H, Agarwal A, Katiyar S, Bhattad S, Ramprakash S, Cp R, Jayaram A, Gornale V, Raj R, Uppuluri R, Sivasankaran M, Munirathnam D, Lashkari HP, Kalra M, Sachdeva A, Sharma A, Balaji S, Govindraj GM, Karande S, Nanavati R, Manglani M, Subramanyam G, Sampagar A, Ck I, Gutha P, Kanakia S, Mundada SP, Krishna V, Nampoothiri S, Nemani S, Rawat A, Desai M, Madkaikar M. Clinical and Genetic Spectrum of a Large Cohort of Patients With Leukocyte Adhesion Deficiency Type 1 and 3: A Multicentric Study From India. Front Immunol. 2020;11:612703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Shaw JM, Al-Shamkhani A, Boxer LA, Buckley CD, Dodds AW, Klein N, Nolan SM, Roberts I, Roos D, Scarth SL, Simmons DL, Tan SM, Law SK. Characterization of four CD18 mutants in leucocyte adhesion deficient (LAD) patients with differential capacities to support expression and function of the CD11/CD18 integrins LFA-1, Mac-1 and p150,95. Clin Exp Immunol. 2001;126:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Madkaikar M, Italia K, Gupta M, Chavan S, Mishra A, Rao M, Mhatre S, Desai M, Manglani M, Singh S, Suri D, Agrawal A, Ghosh K. Molecular characterization of leukocyte adhesion deficiency-I in Indian patients: identification of 9 novel mutations. Blood Cells Mol Dis. 2015;54:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Parvaneh N, Mamishi S, Rezaei A, Rezaei N, Tamizifar B, Parvaneh L, Sherkat R, Ghalehbaghi B, Kashef S, Chavoshzadeh Z, Isaeian A, Ashrafi F, Aghamohammadi A. Characterization of 11 new cases of leukocyte adhesion deficiency type 1 with seven novel mutations in the ITGB2 gene. J Clin Immunol. 2010;30:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Wolach B, Gavrieli R, Wolach O, Stauber T, Abuzaitoun O, Kuperman A, Amir Y, Stepensky P, Somech R, Etzioni A. Leucocyte adhesion deficiency-A multicentre national experience. Eur J Clin Invest. 2019;49:e13047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Fekrvand S, Yazdani R, Olbrich P, Gennery A, Rosenzweig SD, Condino-Neto A, Azizi G, Rafiemanesh H, Hassanpour G, Rezaei N, Abolhassani H, Aghamohammadi A. Primary Immunodeficiency Diseases and Bacillus Calmette-Guérin (BCG)-Vaccine-Derived Complications: A Systematic Review. J Allergy Clin Immunol Pract. 2020;8:1371-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Boonyawat B, Suksawat Y, Pacharn P, Suwanpakdee P, Traivaree C. X-Linked Chronic Granulomatous Disease: Initial Presentation with Intracranial Hemorrhage from Vitamin K Deficiency in Infant. Case Rep Pediatr. 2018;2018:7041204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 15. | Sobh A, Bonilla FA. Vaccination in Primary Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4:1066-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Kurosawa H, Mizukami T, Nunoi H, Kato M, Sato Y, Okuya M, Fukushima K, Katsuyama Y, Arisaka O. Necrotizing Ulcer After BCG Vaccination in a Girl With Leukocyte-adhesion Deficiency Type 1. J Pediatr Hematol Oncol. 2018;40:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Ghosh S, Chackerian AA, Parker CM, Ballantyne CM, Behar SM. The LFA-1 adhesion molecule is required for protective immunity during pulmonary Mycobacterium tuberculosis infection. J Immunol. 2006;176:4914-4922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |