Published online May 9, 2022. doi: 10.5409/wjcp.v11.i3.295
Peer-review started: October 11, 2021
First decision: November 7, 2021
Revised: November 25, 2021
Accepted: April 2, 2022
Article in press: April 2, 2022
Published online: May 9, 2022
Processing time: 208 Days and 6.4 Hours
Studies in Africa, Asia, and Latin America are needed to provide a comprehensive picture of the global incidence of celiac disease (CD).
To describe the serology, endoscopic and histological findings in typical and atypical presentations of pediatric CD at a tertiary referral hospital in an African low/middle income country (LMIC).
This observational study was conducted on 199 patients with CD from 2010 to 2019. The patients were divided into typical and atypical groups according to the presenting symptoms including 120 and 79 patients respectively. Serology, upper gastrointestinal endoscopy with duodenal biopsy were performed for patients who had symptoms suggestive of CD. The severity of the intestinal damage was graded according to the histo-pathologic Marsh-Oberhuber classification.
Chronic diarrhea was the main intestinal presentation in the typical group. Anemia was the most common extraintestinal symptom in both the typical and atypical group. Marsh-Oberhuber type 3b and 3c was significantly higher in the seropositive patients with a P value of 0.007. A significant correlation was observed between the histological grade of the biopsied duodenal mucosa and the clinical presentation (P < 0.001). Age was significantly higher in the atypical group (P value < 0.001).
Although typical CD was observed in 120 patients in this study, the clinical variability of the condition was frequently observed. Age only was a significant predictor for the appearance of atypical CD. Therefore, CD presentations in LMIC are not different from industrialized countries.
Core Tip: This study included 199 patients diagnosed with celiac disease (CD) over a 10-year period from 2010 to 2019 and was conducted at our tertiary hospital. Age, sex, clinical presentation, serological tests, and endoscopic findings were evaluated. We used the Marsh-Oberhuber classification to define the histopathological findings of the duodenal biopsies. The histopathological evaluation of intestinal biopsies revealed a statistically significant correlation between the histological grade of biopsied duodenal mucosa and the clinical presentation (P < 0.001). Those typical and atypical CD are not different from industrialized countries regarding age, clinical presentations, serology and pathology.
- Citation: Mansour HH, Mohsen NA, El-Shabrawi MH, Awad SM, Abd El-Kareem D. Serologic, endoscopic and pathologic findings in pediatric celiac disease: A single center experience in a low/middle income country. World J Clin Pediatr 2022; 11(3): 295-306
- URL: https://www.wjgnet.com/2219-2808/full/v11/i3/295.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v11.i3.295
Celiac disease (CD) is an immune-mediated enteropathy caused by permanent sensitivity to gluten in genetically susceptible individuals. CD patients sustain an autoimmune reaction to the gliadin protein fraction of gluten[1]. The incidence of CD has been rising significantly in the second half of the 20th century and into the 21st century throughout the Western industrialized world. Population-based studies in Africa, Asia, and Latin America are needed to provide a comprehensive picture of the global incidence of CD[2].
CD is one of the most common causes of chronic malabsorption. This results from the injury to the small intestine with loss of absorptive surface area, reduction of digestive enzymes, and consequential impaired absorption of micronutrients, such as fat-soluble vitamins, iron, and potentially B12 and folic acid. Moreover, the inflammation exacerbates the symptoms of malabsorption by causing a net secretion of fluid that can result in diarrhea[3]. The clinical features of CD vary considerably. Typical or classic CD patients have predominant gastrointestinal manifestations, such as chronic diarrhea, abdominal distension, and failure to thrive. Typical CD is common in children diagnosed within the first 2 years of life. Many cases of CD present with predominant non-GI signs and symptoms, such as anemia, short stature, aphthous stomatitis, recurrent abdominal pain, pica, delayed puberty, osteopenia, and dental enamel hypoplasia and are called atypical or non-classic CD. The most common atypical presentations of CD are iron deficiency anemia unresponsive to iron therapy and short stature[4].
CD should be considered in the diagnosis of patients with an appropriate clinical history and patients from high-risk populations. Serological tests are used as initial tests for CD, and duodenal biopsies obtained during esophago-gastroduo-denoscopy (EGD) are considered the standard for diagnosis[5]. Endoscopy reveals grossly visible abnormalities in the proximal portion of the small intestine (scalloping of duodenal folds, mosaic mucosal pattern, and mucosal atrophy). The diagnosis is confirmed via histologic evaluation as per the Marsh-Oberhuber classification[6]. However, many recent studies have shown that the ingestion of uncontaminated oats is not only safe but can also improve the quality of the diet in most patients with CD or dermatitis herpetiformis. Other natural foods, such as vegetables, salads, pulses, fruits, nuts, meat, fish, poultry, cheese, eggs, and milk, can be consumed in a gluten free diet (GFD) without limitations[7].
This observational study aims to compare the serological, gastrointestinal endoscopic, and histopathologic findings in typical and atypical presentations of pediatric CD at a tertiary referral hospital in the capital city of Egypt; an African low/middle income country (LMIC). We also aimed to find whether these findings are different from presentations in industrialized countries.
This hospital-based, cross-sectional observational study was conducted at Cairo University Children Hospital which is the largest pediatric tertiary hospital in the capital city of Egypt and one of the largest in the Middle East and North Africa (MENA) region. Data of the patients diagnosed with CD was collected in the period from January 2010 to December 2019. The study included 199 patients diagnosed with celiac disease; they were divided into two groups based upon the presenting symptoms: typical or classic CD (Group A) and atypical or non-classic CD patients (Group B). Patients with predominant GI features, such as chronic diarrhea, abdominal distension, and failure to thrive, were included in the typical CD group, whereas the patients with atypical intestinal or extraintestinal symptoms were included in the atypical CD group. The age of patients ranged from less than 1 year up to 18 years. Patients that were within the age group but on gluten-free diet, those within the age group without a history of gluten introduction before 6 mo, and those with other GIT pathology, such as inflammatory bowel disease (IBD) (e.g., Crohn’s disease) were excluded from the study. Detailed history was taken from each patient and/or care-takers with a special focus on the age of disease onset, history of diarrhea or constipation, abdominal distension, weight loss, anemia, bone pain, and neurological symptoms. Anthropometric measurements (height, weight and head circumference), and full systemic examination were performed.
Investigations of all patients included complete blood cell count, measurement and serum calcium and celiac serology (total immunoglobulin A, anti-tissue transglutaminase (anti-tTG) antibody IgA). EGD and duodenal biopsy were also performed. For the endoscopy to be accurate, the patients should have been on a gluten-containing diet. The patients were asked to fast (no food or drink) for 6-8 h before endoscopy. The amount and type of sedation are dependent on the patient’s age, weight, and coexisting medical conditions. In the GI endoscopy laboratory of our tertiary hospital, UGI endoscopy was performed using pediatric-size flexible gastro-duodenoscopes with compatible biopsy forceps (standard gastroscope manufactured by KARL STORZ group (Tuttlingen, Germany). Four biopsies were obtained from the second and third part of the duodenum and one from the duodenal bulb. The endoscopic duodenal specimens were processed as formalin-fixed specimens embedded in paraffin blocks. The sections were cut with a thickness of 5 microns and stained with Hematoxylin and Eosin. The severity of the intestinal damage was graded by the pathologist as per the Marsh-Oberhuber classification[8]. After endoscopy was performed, the patient was transferred to a recovery room until any medication or sedation wears off. Most of the children were able to resume eating food within a few hours, after they fully recovered.
Diagnostic criteria for celiac disease patients in this study include (at least 4 of 5 or 3 of 4 if the HLA genotype is not performed) [9]
Typical symptoms of celiac disease: Chronic diarrhea, growth faltering and iron deficiency anemia.
Positivity of serum celiac disease IgA class autoantibodies at high titer: Both IgA class anti-tTG and endomyseal antibody (EMA) in IgA-sufficient subjects or IgG class anti-tTG and EMA in IgA deficient subjects. The finding of IgG class anti-deamidated gliadin peptide adds evidence to the diagnosis.
HLA-DQ2 or DQ8 genotypes: HLA-DQ2 positivity includes subjects with only half the heterodimer (HLA-DQB1*02 positive).
Celiac enteropathy at the small intestinal biopsy: Including Marsh-Oberhuber 3 Lesions, Marsh-Oberhuber 1-2 Lesions associated with positive celiac antibodies positive at low/high titer, or Marsh-Oberhuber 1-3 Lesions associated with IgA subepithelial deposits.
Response to the GFD: Data was statistically described in terms of mean ± SD, or frequencies (number of cases) and percentages when appropriate. Comparison of age between the study groups was done using Student t test for independent samples. For comparing categorical data, Chi-square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5. Two-sided P values less than 0.05 was considered statistically significant. All statistical calculations were done using computer program IBM SPSS (Statistical Package for the Social Science; IBM Corp, Armonk, NY, United States) release 22 for Microsoft Windows.
This observational study enrolled 199 patients diagnosed as CD according to clinical presentations, serology and histopathology at our tertiary referral hospital. Data of the patients diagnosed with CD was collected in the period from January 2010 to December 2019. They were further divided into two groups: typical CD and atypical CD groups. The typical group (Group A) included 120 cases of CD patients who had typical intestinal symptoms, whereas the atypical group (Group B) included 79 cases of celiac disease patients who had atypical intestinal or extraintestinal symptoms. The mean age was 3.74 ± 2.93 years in Group A (typical) and 5.74 ± 4.0 years in Group B (atypical), being significantly higher in the atypical group (P value < 0.001).
Overall, 51.7% (n = 62) males and 48.3% (n = 58) females presented with typical symptoms (Group A), whereas 51.9% (n = 41) males and 48.1% (n = 38) females presented with atypical symptoms (Group B) without statistically significant difference (P value was 0.9).
Chronic diarrhea (increased frequency and/or fluidity of the stool for more than 4 wk) was the main intestinal presentation in the typical group (99.2%) (n = 119) than in the atypical group (1.3%) (n = 1), whereas constipation (infrequent passage of stool or passage of hard stool) was more common in the atypical group (16.5%) (n = 13) than in the typical group (5.0%) (n = 6). Overall, 69.2% (n = 83) of the patients in the typical group and 41.8% (n = 33) in the atypical group presented with abdominal distention. Such symptoms were statistically significant as shown in Table 1.
| Clinical presentations | Typical, n = 120 | Atypical, n = 79 | P value | ||
| n | % | n | % | ||
| Male | 62 | 51.7 | 41 | 51.9 | 0.974 |
| Female | 58 | 48.3 | 38 | 48.1 | |
| Chronic diarrhea | 119 | 99.2 | 1 | 1.3 | < 0.001 |
| Abdominal distention | 83 | 69.2 | 33 | 41.8 | < 0.001 |
| Constipation | 6 | 5 | 13 | 16.5 | 0.007 |
| Weight loss | 81 | 67.5 | 49 | 62 | 0.427 |
| Anemia | 119 | 99.2 | 79 | 100 | 0.931 |
| Short stature | 74 | 61.7 | 52 | 65.8 | 0.552 |
| Hypocalcemia | 85 | 70.8 | 52 | 65.8 | 0.455 |
| Depression | 0 | 0 | 2 | 2.5 | 0.08 |
| Skin lesion | 20 | 16.7 | 11 | 13.9 | 0.764 |
Anemia (low hemoglobin level for age and sex) was the most common extraintestinal symptom in both the typical and atypical group (99.5%) (n = 198). There were 99.2% (n = 119) of the typical group cases discovered during patient follow-up and 100% (n = 79) of the atypical group cases presented with anemia. The type of anemia was determined by mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH). The most common type of anemia was microcytic hypochromic anemia, detected in 93.3% (n = 111/119) of the patients in the typical group and 93.7% (n = 74/79) in the atypical group. Normocytic normochromic anemia was detected in 5.9% (n = 7) of the patients in the typical group and 5.1% (n = 4) in the atypical group. Four cases of normocytic normochromic anemia were diagnosed as glucose-6-phpsphate dehydrogenase (G6PD) deficiency, two of them in the typical group and two in the atypical group. Macrocytic anemia was detected in two patients, one was typical and the other was atypical (Table 2).
| Microcytic hypochromic | Normocytic normochromic | Macrocytic hypochromic | P value | ||||
| n | % | n | % | n | % | 0.931 | |
| Typical | 111 | 93.3 | 7 | 5.9 | 1 | 0.8 | |
| Atypical | 74 | 93.7 | 4 | 5.1 | 1 | 1.3 | |
Hypocalcemia was reported in 70.8% (n = 85) of the typical group cases and 65.8% (n = 52) of the atypical group cases. Delayed puberty was detected in 75% (n = 9/12) of the typical group cases and detected in 73.1% (n = 19/26) of the atypical group cases. Dermatitis was detected in 12.5% (n = 15) of the typical group cases and 7.6% (n = 6) of the atypical group cases.
Failure to thrive was common in the typical group, whereas short stature was common in the atypical group. Failure to thrive (weight < 5 percentile) was found in 67.5% (n = 81) of the typical group cases and 62% (n = 49) of the atypical group cases. Short stature (height < 5 percentile) was found in 61.7% (n = 74) of the typical group cases and 65.8% (n = 52) of the atypical group cases as shown in Table 1.
The majority of our patients had normal to high total IgA level (84.9%) (n = 169/199): 81.7% (n = 98) of the typical group and 89.9% (n = 71) of the atypical group. Low level of total IgA was observed in 18.3% (n = 22) of the typical group cases and 10.1% (n = 8) of the atypical group cases. Anti-tTG IgA was positive in 48.3% (n = 58) of the typical group cases and 60.8% (n = 48) of the atypical group cases (Table 3).
| Typical, n = 120 | Atypical, n = 79 | P value | |||
| n | % | n | % | ||
| Total IgA deficient | 22 | 18.3 | 8 | 10.1 | 0.074 |
| Anti-tTG IgA-positive | 58 | 48.3 | 48 | 60.8 | 0.086 |
| Anti-tTG IgA-negative | 40 | 33.3 | 23 | 29.1 | 0.315 |
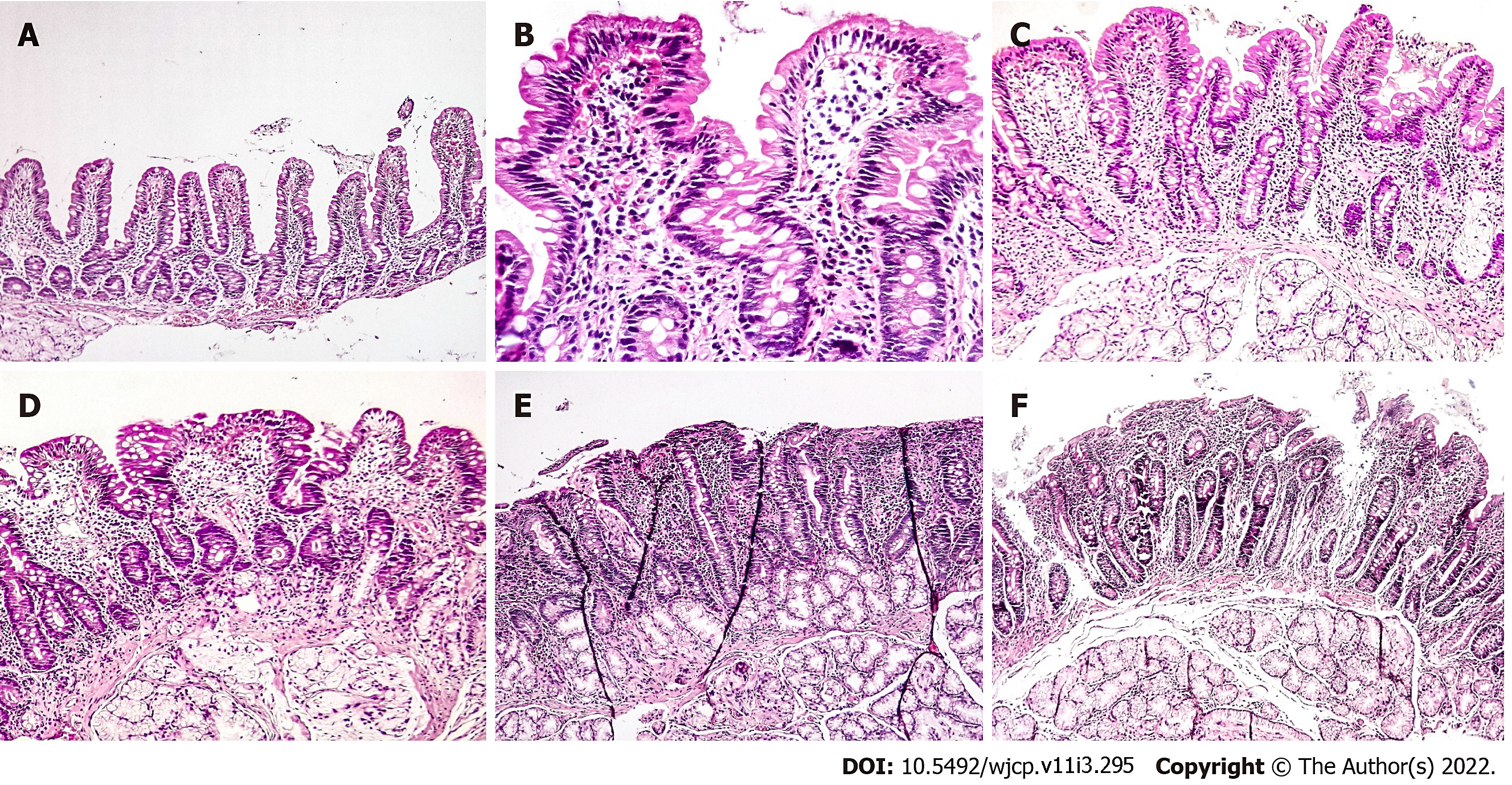
Histopathological evaluation of the intestinal biopsies (Figure 1) revealed Marsh-Oberhuber type 0 in 8 patients (4.0%), Marsh-Oberhuber type 1 in 18 (9.0%), Marsh-Oberhuber type 3a in 53 (26.6%), Marsh-Oberhuber type 3b in 56 (28.1%), Marsh-Oberhuber type 3c in 57 (28.6%), and Marsh-Oberhuber type 3b-c in 7 (3.5%). Marsh-Oberhuber type 3a was more common in the atypical group, whereas Marsh-Oberhuber type 3c was more common in the typical group. Marsh-Oberhuber type 3a was diagnosed in 24 patients (20.0%) in the typical group and 29 (36.7%) in the atypical group. The intestinal biopsies revealed Marsh-Oberhuber type 3c in 47 patients (39.2%) of the typical group and only 10 (12.7%) of the atypical group. The intestinal biopsies revealed Marsh-Oberhuber type 3b in 31 patients (25.8%) of the typical group and 25 (31.6%) of the atypical group. Marsh-Oberhuber type 3b-c was found in four patients (3.3%) of the typical group and three (3.8%) of the atypical group. No cases were classified as Marsh-Oberhuber type 2. Six patients (5%) of the typical group and two (2.5%) of the atypical group were classified as Marsh-Oberhuber type 0, whereas eight patients (6.7%) of the typical group and ten (12.7%) of the atypical group were classified as Marsh-Oberhuber type 1 (Table 4).
| Marsh-Oberhuber classification | Typical, n = 120 | Atypical, n = 79 | P value | ||
| n | % | n | % | ||
| 0 | 6 | 5 | 2 | 2.5 | 0.386 |
| 1 | 8 | 6.7 | 10 | 12.7 | 0.149 |
| 2 | 0 | 0 | 0 | 0 | |
| 3a | 24 | 20 | 29 | 36.7 | 0.009 |
| 3b | 31 | 25.8 | 25 | 31.6 | 0.372 |
| 3c | 47 | 39.2 | 10 | 12.7 | < 0.001 |
| 3b-c | 4 | 3.3 | 3 | 3.8 | 0.862 |
Marsh-Oberhuber type 0 was found only in seropositive patients. Total and subtotal villous atrophy (VA) (Marsh-Oberhuber type 3b and 3c) were more common in seropositive patients. Overall, 54% of the seronegative patients (n = 34) had Marsh-Oberhuber type 3b and 3c, whereas 67% of the seropositive patients (n = 71) had Marsh-Oberhuber type 3b-c, being significantly higher in the seropositive patients (P value = 0.007) (Table 5).
| Marsh-Oberhuber grade | CD seronegative, n = 63 | CD seropositive, n = 106 | P value | ||
| n | % | n | % | 0.007 | |
| 0 | 0 | 0 | 7 | 6.6 | |
| 1 | 8 | 12.7 | 8 | 7.5 | |
| 2 | 0 | 0 | 0 | 0 | |
| 3a | 21 | 33.3 | 20 | 18.9 | |
| 3b | 23 | 36.5 | 29 | 27.4 | |
| 3c | 10 | 15.9 | 36 | 34 | |
| 3b-c | 1 | 1.6 | 6 | 5.7 | |
Among the seronegative patients, no cases were classified as Marsh-Oberhuber type 0 or type 2 based on their histological findings. In the seronegative patients with typical symptoms, 4 patients (10%) had Marsh-Oberhuber type 1, whereas 13 patients (32.5%), 13 patients (32.5%), 9 patients (22.5%), and 1 patient (2.5%) had Marsh-Oberhuber type 3a, 3b, 3c, and 3b-c, respectively. In the seronegative patients with atypical symptoms, four patients (17.4%) had Marsh-Oberhuber type 1, whereas eight patients (34.8%), ten patients (43.5%), and one patient (4.3%) had Marsh-Oberhuber type 3a, 3b, and 3c, respectively (Table 6).
| Marsh-Oberhuber classification | Typical, n = 40 | Atypical, n = 23 | P value | ||
| n | % | N | % | 0.315 | |
| 0 | 0 | 0 | 0 | 0 | |
| 1 | 4 | 10 | 4 | 17.4 | |
| 2 | 0 | 0 | 0 | 0 | |
| 3a | 13 | 32.5 | 8 | 34.8 | |
| 3b | 13 | 32.5 | 10 | 43.5 | |
| 3c | 9 | 22.5 | 1 | 4.3 | |
| 3b-c | 1 | 2.5 | 0 | 0 | |
In seropositive patients with typical symptoms 27 patients (46.6%) had Marsh-Oberhuber type 3c, in seropositive patients with atypical symptoms 15 patients (31.3%) had Marsh-Oberhuber type 3b with P value = 0.022 (Table 7).
| Marsh-Oberhuber classification | Typical, n = 58 | Atypical, n = 48 | P value | ||
| n | % | n | % | 0.022 | |
| 0 | 5 | 8.6 | 2 | 4.2 | |
| 1 | 3 | 5.2 | 5 | 10.4 | |
| 2 | 0 | 0 | 0 | 0 | |
| 3a | 6 | 10.3 | 14 | 29.2 | |
| 3b | 14 | 24.1 | 15 | 31.3 | |
| 3c | 27 | 46.6 | 9 | 18.8 | |
| 3b-c | 3 | 5.2 | 3 | 6.3 | |
Logistic regression analysis for the predictors of atypical CD, including all statistically significant items including age, diarrhea, constipation, abdominal distention, Marsh-Oberhuber type 3a, and Marsh-Oberhuber type 3c) was conducted. Age only was significant with a P value = 0.013 (as age progresses, the predictors of atypical CD will increase).
This study included 199 patients diagnosed with CD over a 10-year period from January 2010 to December 2019 and was conducted at our tertiary hospital. Age, sex, clinical presentation, serological tests, and endoscopic findings were evaluated. We used the Marsh-Oberhuber classification to define the histopathological findings of the duodenal biopsies.
The mean age was 3.74 ± 2.93 years in Group A (typical) and 5.74 ± 4.0 years in Group B (atypical), being significantly higher in the atypical CD group than in the typical CD group (P < 0.001). The study conducted by Semwal et al[4] reported a mean age of 4.83 ± 3.05 years in the typical group and 7.71 ± 3.46 years in the atypical group, being significantly higher in the atypical group (P < 0.001). Dinler et al[10] reported a mean age of 6.2 ± 4.4 years in the typical group and 11.5 ± 3.4 years in the atypical group, being significantly higher in the atypical group (P < 0.001). Moreover, Kuloğlu et al[11]. reported that the age of children with typical type (7.5 ± 4.3 years) was significantly lower than that of those with atypical type (10.8 ± 4.3 years) (P = 0.001).
This difference is apparently due to the late diagnosis of the atypical cases because the typical presentations (diarrhea, abdominal distension) are usually noticed by the caretaker easily and therefore CD is diagnosed at an early age. Atypical presentations are diagnosed at a later age, due to the less awareness of the varied non-GI presentations of CD[4].
Of the studied cases, 51.7% (n = 103/199) were males and 48.2% (n = 96/199) were females, with a male/female ratio of 1.1:1. In the study conducted by Semwal et al[4], 48 males and 53 females had a male/female ratio of 1:1.1, whereas in the study of Dinler et al[10], 33 males and 54 females had a male/female ratio of 1:1.6. Kuloğlu et al[11] evaluated the features of 109 children with CD, in which the disease is known to be more frequent among females, with a male/female ratio of 1:1.5.
Anemia was the most common symptom (99.5%) (n = 198/199), followed by failure to thrive (65.3%) (n = 130/199), short stature (63.3%) (n = 126/199), chronic diarrhea (60.3%) (n = 120/199), and abdominal distention (58.3%) (n = 116/199).
In the study of Semwal et al[4], the most common symptom was anemia (54.5%) (n = 55/101) followed by chronic diarrhea (51.5%) (52/101) and short stature (50.5%) (51/101); in that of Dinler et al[10], chronic diarrhea (60.9%) (n = 53/87) followed by failure to thrive (49.4%) (n = 43/87), abdominal distention (41.3%) (n = 36/87), and short stature (33.3%) (n = 29/87); and in that of Kuloğlu et al[11], diarrhea (53.2%) (n = 58/109) followed by failure to thrive (45.9%) (n = 50/109), short stature (42.2%) (n = 46/109), and abdominal distention (26.6%) (n = 44/109).
Chronic diarrhea (n = 119/120) and abdominal distension (n = 83/120) were the most common presentations in the typical CD cases, whereas anemia (n = 79/79) and short stature (n = 52/79) were the most common presentations in the atypical CD cases. In the study of Semwal et al[4], chronic diarrhea (n = 52/55) and abdominal distention (n = 32/55) were the common presentations in the typical CD cases, whereas anemia (n = 36/46) and short stature (n = 29/46) were the most common presentations in the atypical CD cases. In the study of Dinler et al[10], chronic diarrhea (n = 53/55) and abdominal distention (n = 36/55) were the common presentations in the typical CD cases, whereas anemia (n = 10/32) and short stature (n = 20/32) were the most common presentations in the atypical CD cases. Moreover, in the study of Kuloğlu et al[11], chronic diarrhea (n = 58/66) and abdominal distention (n = 29/66) were the common presentations in the typical CD cases, whereas anemia (n = 23/41) and short stature (n = 22/41) were the most common presentations in the atypical CD cases.
In this study, the other common atypical presentations were constipation (9.5%) (n = 19/199), recurrent aphthous ulcers (5%) (n = 10/199), and bone changes in the form of rickets (10%) (n = 20/199). In the study conducted by Semwal et al[4], the most common atypical presentations were constipation (6.9%) (n = 7/101), recurrent aphthous ulcers (3.9%) (n = 4/101), and bone changes in the form of rickets (3.9%) (n = 4/101). Moreover, in the study conducted by Dinler et al[10], the most common atypical presentations were constipation (3.4%) (n = 3/87) and recurrent aphthous ulcers (2.2%) (n = 2/87), and in the study conducted by Kuloğlu et al[11], constipation (2.7%) (n = 3/109) and recurrent aphthous ulcers (1.8%) (n = 2/109).
Delayed puberty (14%) (n = 28/199), dermatitis (10.6%) (n = 21/199), and depression (1%) (n = 2/199). In the study of Semwal et al[4], delayed puberty (2.0%) (n = 2/101), chronic urticaria (1.0%) (n = 1/101), and depression (1.0%) (n = 1/101) were observed; in that of Dinler et al[10], chronic urticaria (1.1%) (n = 1/87) and delayed puberty (2.2%) (n = 2/87); and in that of Kuloğlu et al[11], delayed puberty (5.5%) (n = 6/109).
Anemia in CD is primarily caused by iron deficiency but also by the lack of other nutritional factors necessary for normal erythropoiesis, such as folic acid, vitamin B12, proteins, and copper[11]. Anemia was the most common presenting feature in the present study and was multifactorial. Based on the laboratory evaluation, anemia was prevalent in 198/199 patients (99.5%), with the microcytic hypochromic type being the most common in 185/198 patients (93.4%). In the study conducted by Berry et al[12], anemia was the most common presenting feature in their study and was multifactorial. Based on the laboratory evaluation, anemia was prevalent in 96/103 patients (93.2%), with iron deficiency anemia (IDA) being the most common in 84/103 patients (81.5%). In the study conducted by Dinler et al[10], iron deficiency with low iron stores was the most common presentation of anemia in both groups; IDA was found in 48/85 patients (56.5%). In the study conducted by Kuloğlu et al[11], iron deficiency anemia was a frequent finding in CD patients. It is seen in the majority of patients with one or more other findings and can also be the single finding of the disease. IDA was found in 80/98 patients (81.6%). Normocytic normochromic anemia was found in 5.6% of the patients (11 patients), one of them diagnosed with autoimmune hemolytic anemia and 4 cases (2%) diagnosed with G6PD deficiency. In the study conducted by Hosnut et al[13], the association between G6PD deficiency and CD was coincidental. The gene frequency of enzyme deficiency was 0.70 in the Mediterranean region. The prevalence of G6PD deficiency was high, reaching up to 10% in some ethnic populations in Turkey. Since G6PD deficiency is common in their country, the presence of CD and G6PD deficiency in their patients would be expected[13].
The prevalence of vitamin B12 deficiency is variable in different studies and ranges from 8 to 41% of the patients[14,15]. In this study, macrocytic hypochromic anemia was found in 2/199 patients (1%). One of them was diagnosed with megaloblastic anemia (vitamin B12 deficiency) by bone marrow biopsy (BMB). In the study conducted by Dinler et al[10] and Kuloğlu et al[11], vitamin B12 deficiency was observed in 3.4% (3/87) and 5.5% (6/109) of the child patients with CD, respectively. In the study conducted by Berry et al[12], Wierdsma et al[16], and McGowan et al[17], vitamin B12 deficiency was observed in 2.9% (3/103), 11% (5/50), and 19% (15/80) of the adult patients with CD, respectively. The mechanism of vitamin B12 deficiency in CD remains unclear. Various postulated mechanisms include ileal VA, pancreatic insufficiency in CD, autoimmune gastritis, small intestinal bacterial overgrowth, and decreased efficiency of mixing with transfer factors in the small intestine[12].
In this study, IgA deficiency was observed in 15% (n = 30/199) of the cases. In the study conducted by Kuloğlu et al[11], IgA deficiency was detected in 9.1% (n = 10/109) of the cases. The prevalence of CD in patients with selective IgA deficiency ranges from 10% to 30%[18]. Moreover, 53.3% (n = 106/199) of the cases had positive anti-tTG IgA. In the study conducted by Wolf et al[19], 76.4% (n = 404/529) of the patients had positive anti-tTG IgA.
In this study, we found a higher but non-significant proportion of patients with typical CD symptoms among the IgA anti-tTG-seronegative patients (63.5%) (n = 40/63) compared with the atypical CD seronegative cases (36.5%) (n = 23/63). In the study conducted on adults by Sugai et al[20], the proportion of patients with typical CD symptoms among the IgA anti-tTG-seronegative patients was 26.3% (n = 5/19) compared with the atypical CD seronegative cases (68.4%) (n = 13/19).
Furthermore, the histopathological evaluation of intestinal biopsies revealed total or subtotal VA (Marsh-Oberhuber type 3b and 3c) in 82/120 patients (68.3%) in the typical group and 38/79 (48%) in the atypical group. Partial VA (Marsh-Oberhuber type 3a) was observed in 24/120 patients (20%) in the typical group and 29/79 (36.7%) in the atypical group. The presence of total VA in the intestinal biopsies was significantly higher in the typical group than that in the atypical group (P < 0.001). In the study conducted by Dinler et al[10], the histopathological evaluation of intestinal biopsies revealed total or subtotal VA in 40/55 patients (72.7%) in the typical group and 12/32 (37.5%) in the atypical group. Partial VA was observed in 15/55 patients (27.3%) in the typical group and 20/32 (62.5%) in the atypical group. The presence of total or subtotal VA in the intestinal biopsies was significantly higher in the typical group than that in the atypical group (P < 0.02). Moreover, Boskovica et al[21] reported that the histopathological evaluation of intestinal biopsies revealed total or subtotal VA in 44/66 patients (66.6%) in the typical group and 24/37 (64.8%) in the atypical group. Partial VA was observed in 5/66 patients (7.5%) in the typical group and 1/37 (2.7%) in the atypical group.
Total and subtotal VA (Marsh-Oberhuber type 3b and 3c) were more common in the seropositive patients. Overall, 34/63 patients (54%) had Marsh-Oberhuber type 3b and 3c in the seronegative patients, whereas 71/106 patients (67%) had Marsh-Oberhuber type 3b and 3c in the seropositive patients, being significantly higher in the seropositive patients with a P value = 0.007.
In the study conducted on children by Hawamdeh et al[22], 40/51 seropositive patients (78.4%) had Marsh-Oberhuber type 3, whereas 9/30 seronegative patients (30%) had Marsh-Oberhuber type 3. A significant association between anti-tTG IgA titer and Marsh-Oberhuber grading was observed (P value 0.000). In the study of Donaldson et al[23], 3/26 seronegative patients (11.5%) had Marsh-Oberhuber type 3b and 3c, whereas 31/56 seropositive patients (55.3%) had Marsh-Oberhuber type 3b and 3c. IgA anti-tTG was significantly correlated with the Marsh-Oberhuber grades (P value 0.001). In the study conducted by Boskovica et al[21], the levels of tTG antibody were correlated significantly with Marsh-Oberhuber types in the entire population (P < 0.0001) and separately for typical (P < 0.001) and atypical (P < 0.0001) groups. In the study conducted on adults by Sugai et al[20], 7/19 seronegative patients (36.8%) had Marsh-Oberhuber type 3b and 3c, whereas 33/45 seropositive patients (73.3%) had Marsh-Oberhuber type 3b and 3c. In the study of Dore et al[24], severe duodenal mucosal damage (Marsh-Oberhuber type 2-3) was observed less frequently in patients with seronegative CD (n = 28/48) than in those with seropositive CD (n = 66/85) (58% vs 78%, P = 0.019).
In this study, 8/199 patients (4%) had Marsh-Oberhuber 0 and positive serology (potential CD): 6/120 patients had typical symptoms (5%) and 2/79 had atypical symptoms (2.5%). In the study conducted by Hawamdeh et al[22], 15/81 patients (18.5%) had Marsh-Oberhuber 0 and positive serology and were considered potential CD patients. In the study conducted by Boskovica et al[21], there were 12/37 patients with positive serology and Marsh-Oberhuber 0 (potential CD) (32.4%) had atypical CD symptoms and 11/66 (16.6%) had typical CD symptoms. In the present study, the histopathological evaluation of intestinal biopsies revealed Marsh-Oberhuber type 1 in 18/199 patients (9%), type 3a in 53/199 (26.6%), type 3b in 56/199 (28.1%), type 3c in 57/199 (28.6%), and type 3b-c in 7/199 (3.5%). Typical CD was more likely to have a significantly higher Marsh-Oberhuber grade based on the histological findings (P < 0.001). In the study conducted by Semwal et al[4], the histopathological evaluation of intestinal biopsies revealed Marsh-Oberhuber type 2 in 2/101 patients (2%), type 3a in 45/101 (44.6%), type 3b in 34/101 (33.7%), and type 3c in 20/101 (19.8%). Typical CD was more likely to have a significantly higher Marsh-Oberhuber grade based on the histological findings (P < 0.001).
In this study, the histopathological evaluation of intestinal biopsies revealed a statistically significant correlation between the histological grade of biopsied duodenal mucosa and the clinical presentation (P < 0.001). Dinler et al[10] and Semwal et al[4] also found that in children, total/subtotal VA was significantly higher in the typical group than in the atypical group. On the other hand, Brar et al[25] found that the degree of VA in the duodenal biopsies did not correlate with the mode of presentation, which might be due to the differences in the age of the study population since we studied children, while Brar et al[25] studied adult CD patients. Those typical and atypical CD in LMIC are not different from industrialized countries regarding age, clinical presentations, serology and pathology.
Limitations of our study: We didn’t collect data about family history (as we focus on histopathology and clinical finding) and vomiting (as vomiting was not a common presentation in our patients and this was reported in Abu-Zekry[26]. We didn’t collect data for laboratory test as anemia (hemoglobin, MCV, MCH) were taken through interpretation of result according to age and sex.
In conclusion, CD presentations in LMIC are not different from industrialized countries and late diagnosis is more common in atypical cases.
Celiac disease (CD) is defined as an immune mediated systemic disorder elicited by gluten and related prolamines in genetically susceptible individuals. CD is one of the most common causes of chronic malabsorption. CD results from injury to the small intestine with loss of absorptive surface area, reduction of digestive enzymes, and consequential impaired absorption of micronutrients such as fat-soluble vitamins, iron, and potentially B12 and folic acid. Celiac disease presented with chronic diarrhea, failure to thrive and abdominal distention usually observed within the first 1-2 years of life. At the older age, atypical features such as anemia, short stature, bone disease and liver failure may occur. It should be considered in patients with an appropriate clinical history as well as in patients from high-risk populations. Serological tests are used as initial tests for CD and duodenal biopsies obtained during esophagogastroduodenoscopy (EGD) are considered the standard for diagnosis. The diagnosis of CD is based on the identification of histological lesions accompanied by clinical and serological consistent data. On the basis of the presence of one or more of these elementary lesions the histopathology of CD is subdivided into different diagnostic categories according to Marsh classification.
Many cases of Celiac disease in our country with different clinical presentations motivate us to search for different histopathological examination in the disease sub-types.
To compare the serological, gastrointestinal endoscopic, and histopathologic findings in typical and atypical presentations of pediatric CD at a tertiary referral hospital in the capital city of Egypt; an African low/middle income country. We also aimed to find whether these findings are different from presentations industrialized countries.
A hospital-based, cross-sectional observational study was conducted at Cairo University Children Hospital which is the largest pediatric tertiary hospital in the capital city of Egypt and one of the largest in the Middle East and North Africa (MENA) region. Data of the patients diagnosed with CD was collected in the period from 2010 to 2019. The study included 199 patients diagnosed with celiac disease; they were divided into two groups based upon the presenting symptoms: typical or classic CD (Group A) and atypical or non-classic CD patients (Group B).
Typical CD is more common than atypical, chronic diarrhea was common in typical group with P value < 0.001. sero-positive cases were 106 (typical 58, atypical 48cases). The most common histological finding in typical seropositive cases were March types 3c (27/58, 46.6%), The most common histological finding in atypical seropositive cases were March types 3b (15/48, 31.3%) (P value 0.022).
CD clinical presentations in low/middle income country are not different from industrialized countries and late diagnosis is more common in atypical cases.
Follow up of the CD cases and their prognosis, if there is changes in histological picture in future.
We would like to thank the parents and children for their cooperation in this study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Han JM, China; Ma L, China S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Ammoury RF, Croffie JM. Malabsorptive disorders of childhood. Pediatr Rev. 2010;31:407-15; quiz 415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | King JA, Jeong J, Underwood FE, Quan J, Panaccione N, Windsor JW, Coward S, deBruyn J, Ronksley PE, Shaheen AA, Quan H, Godley J, Veldhuyzen van Zanten S, Lebwohl B, Ng SC, Ludvigsson JF, Kaplan GG. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2020;115:507-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 3. | Garg K, Gupta RK. What a practitioner needs to know about celiac disease? Indian J Pediatr. 2015;82:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Semwal P, Gupta RK, Sharma R, Garg K. Comparison of Endoscopic and Histological Findings between Typical and Atypical Celiac Disease in Children. Pediatr Gastroenterol Hepatol Nutr. 2018;21:86-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Kasirer Y, Turner D, Lerman L, Schechter A, Waxman J, Dayan B, Bergwerk A, Rachman Y, Freier Z, Silbermintz A. Scalloping is a reliable endoscopic marker for celiac disease. Dig Endosc. 2014;26:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Schuppan D, Zimmer KP. The diagnosis and treatment of celiac disease. Dtsch Arztebl Int. 2013;110:835-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Lionetti E, Catassi C. New clues in celiac disease epidemiology, pathogenesis, clinical manifestations, and treatment. Int Rev Immunol. 2011;30:219-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1205] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 9. | Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Dinler G, Atalay E and Kalayci AG. Celiac disease in 87 children with typical and atypical symptoms in Black Sea region of Turkey. World J Pediatr. 2009;5:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Kuloğlu Z, Kirsaçlioğlu CT, Kansu A, Ensari A, Girgin N. Celiac disease: presentation of 109 children. Yonsei Med J. 2009;50:617-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Berry N, Basha J, Varma N, Varma S, Prasad KK, Vaiphei K, Dhaka N, Sinha SK, Kochhar R. Anemia in celiac disease is multifactorial in etiology: A prospective study from India. JGH Open. 2018;2:196-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Hosnut FO, Canan O, Özcay F, Özbek N. Awareness of glucose-6 phosphate-dehydrogenase deficiency in celiac disease. Acta Paediatr. 2010;99:786-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Dahele A, Ghosh S. Vitamin B12 deficiency in untreated celiac disease. Am J Gastroenterol. 2001;96:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Dickey W. Low serum vitamin B12 is common in coeliac disease and is not due to autoimmune gastritis. Eur J Gastroenterol Hepatol. 2002;14:425-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Bodé S, Gudmand-Høyer E. Symptoms and haematologic features in consecutive adult coeliac patients. Scand J Gastroenterol. 1996;31:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Wierdsma NJ, van Bokhorst-de van der Schueren MA, Berkenpas M, Mulder CJ, van Bodegraven AA. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients. 2013;5:3975-3992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 18. | McGowan KE, Lyon ME, Butzner JD. Celiac disease and IgA deficiency: complications of serological testing approaches encountered in the clinic. Clin Chem. 2008;54:1203-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Wolf J, Petroff D, Richter T, Auth MKH, Uhlig HH, Laass MW, Lauenstein P, Krahl A, Händel N, de Laffolie J, Hauer AC, Kehler T, Flemming G, Schmidt F, Rodrigues A, Hasenclever D, Mothes T. Validation of Antibody-Based Strategies for Diagnosis of Pediatric Celiac Disease Without Biopsy. Gastroenterology. 2017;153:410-419.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Sugai E, Hwang HJ, Vázquez H, Smecuol E, Niveloni S, Mazure R, Mauriño E, Aeschlimann P, Binder W, Aeschlimann D, Bai JC. New serology assays can detect gluten sensitivity among enteropathy patients seronegative for anti-tissue transglutaminase. Clin Chem. 2010;56:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Boskovica A, Kitica L, Prokica D and Stankovica I. Pediatric typical vs. atypical celiac disease: Correlation of duodenal histology with tissue transglutaminase levels. International Journal of Clinical Pediatrics. 2012;1:109-114. [DOI] [Full Text] |
| 22. | Hawamdeh H, Al-Zoubi B, Al Sharqi Y, Qasrawi A, Abdelaziz Y and Barbar M. Association of tissue transglutaminase antibody titer with duodenal histological changes in children with Celiac disease. Gastroenterology research and practice. 2016;2016:6718590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Donaldson MR, Firth SD, Wimpee H, Leiferman KM, Zone JJ, Horsley W, O'Gorman MA, Jackson WD, Neuhausen SL, Hull CM, Book LS. Correlation of duodenal histology with tissue transglutaminase and endomysial antibody levels in pediatric celiac disease. Clin Gastroenterol Hepatol. 2007;5:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Dore MP, Pes GM, Dettori I, Villanacci V, Manca A and Realdi G. Clinical and genetic profile of patients with seronegative coeliac disease: The natural history and response to gluten-free diet. BMJ Open Gastro. 2017;4:1-8. [DOI] [Full Text] |
| 25. | Brar P, Kwon GY, Egbuna II, Holleran S, Ramakrishnan R, Bhagat G, Green PH. Lack of correlation of degree of villous atrophy with severity of clinical presentation of coeliac disease. Dig Liver Dis. 2007;39:26-9; discussion 30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Abu-Zekry M, Kryszak D, Diab M, Catassi C, Fasano A. Prevalence of Celiac Disease in Egyptian Children Disputes the East-West Agriculture-dependent Spread of the Disease. J Pediatr Gastroenterol Nutr. 2008;47:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |