Published online May 9, 2022. doi: 10.5409/wjcp.v11.i3.289
Peer-review started: June 21, 2021
First decision: July 30, 2021
Revised: August 1, 2021
Accepted: March 26, 2022
Article in press: March 26, 2022
Published online: May 9, 2022
Processing time: 319 Days and 14.3 Hours
Rapid molecular testing has revolutionized the management of suspected viral meningitis and encephalitis by providing an etiological diagnosis in < 90 min with potential to improve outcomes and shorten inpatient stays. However, use of molecular assays can vary widely.
To evaluate current practice for molecular testing of pediatric cerebrospinal fluid (CSF) samples across the United Kingdom using a structured questionnaire.
A structured telephone questionnaire survey was conducted between July and August 2020. Data was collected on the availability of viral CSF nucleic acid amplification testing (NAAT), criteria used for testing and turnaround times including the impact of the coronavirus disease 2019 pandemic.
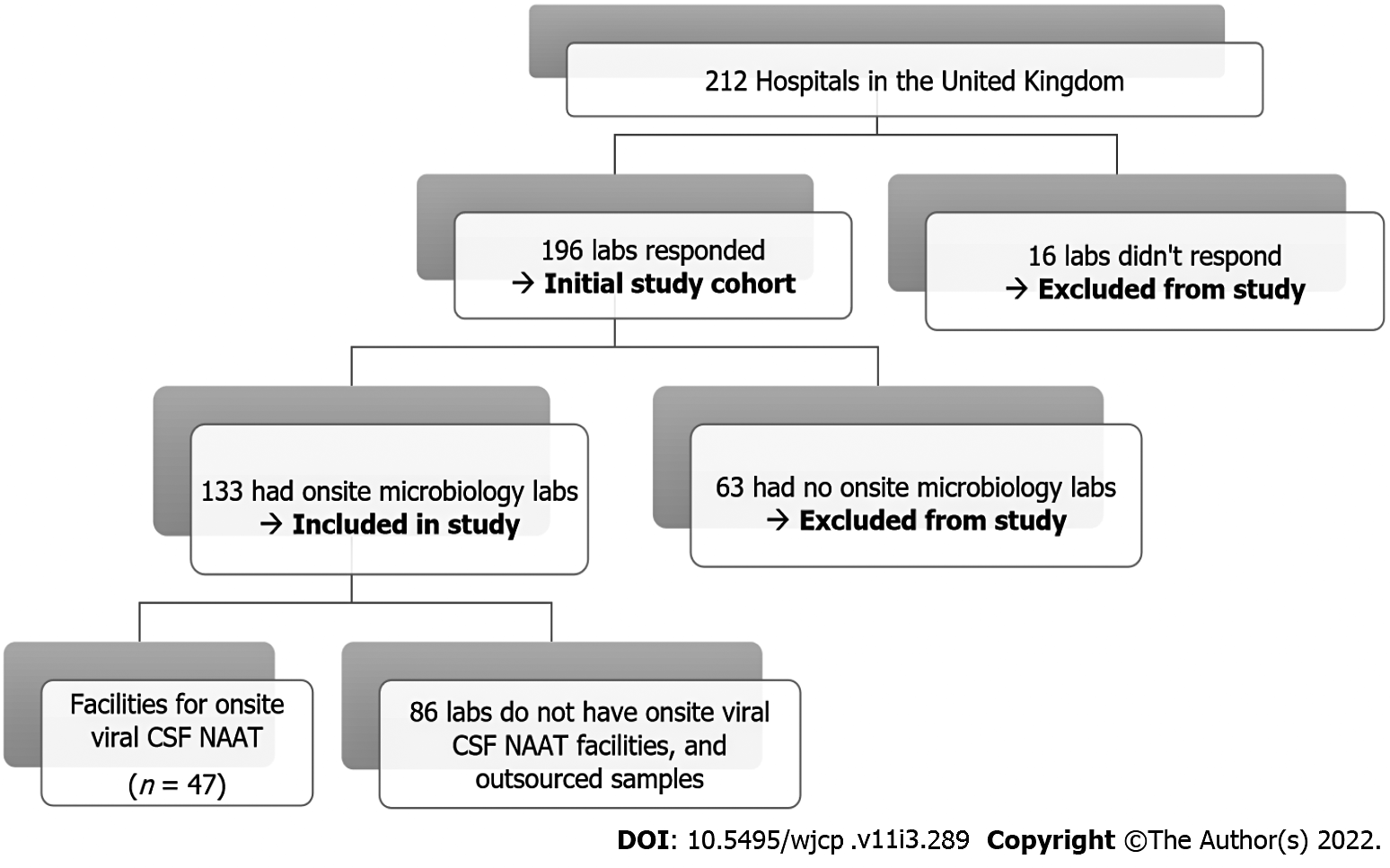
Of 196/212 (92%) microbiology laboratories responded; 63/196 (32%) were excluded from final analysis as they had no on-site microbiology laboratory and outsourced their samples. Of 133 Laboratories included in the study, 47/133 (35%) had onsite facilities for viral CSF NAAT. Hospitals currently undertaking onsite NAAT (n = 47) had much faster turnaround times with 39 centers (83%) providing results in ≤ 24 h as compared to those referring samples to neighboring laboratories (5/86; 6%).
Onsite/near-patient rapid NAAT (including polymerase chain reaction) is recommended wherever possible to optimize patient management in the acute setting.
Core Tip: Rapid diagnosis of viral meningitis in children through nucleic acid amplification testing (NAAT) of cerebrospinal fluid (CSF) can help in establishing a firm diagnosis, allowing early discontinuation of antibiotics and ensuring improved antibiotic stewardship. Turnaround times will be improved through availability of onsite NAAT facilities in the hospitals with inpatient pediatric units. All CSF samples in infants, irrespective of their white cell counts (actual/adjusted) should be offered NAAT, as viral meningitis due to enterovirus or human parechovirus can occur without pleocytosis.
- Citation: Paul SP, Balakumar V, Kirubakaran A, Niharika J, Heaton PA, Turner PC. Turnaround times for molecular testing of pediatric viral cerebrospinal fluid samples in United Kingdom laboratories. World J Clin Pediatr 2022; 11(3): 289-294
- URL: https://www.wjgnet.com/2219-2808/full/v11/i3/289.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v11.i3.289
Timely diagnosis of meningitis is crucial to reduce mortality and long-term neurological disability[1,2]. The introduction of public health initiatives and immunization programs over the last 50 years have significantly decreased the incidence of bacterial meningitis in the United Kingdom[3]. In the United Kingdom, viral pathogens are the commonest cause of meningitis in both adult and pediatric populations[2]. The diagnosis of meningitis involves clinical assessment and a variety of laboratory investigations. Distinguishing between viral and bacterial causes can be challenging at initial presentation. For cases of suspected meningitis, in the absence of contraindications including coagulation disorders or raised intracranial pressure, a lumbar puncture should be performed[4,5]. Nucleic acid amplification testing (NAAT), predominantly through polymerase chain reaction (PCR) technology of the cerebrospinal fluid (CSF), is recognised as the gold standard for diagnosis in viral meningitis[6].
The aim of this study was to evaluate the use and availability of viral NAAT testing of CSF in microbiology laboratories across the United Kingdom.
An electronic search of the National Health Service (NHS) database (http://www.nhs.uk/servicedirectories/pages/nhstrustlisting.aspx) was conducted to identify NHS trusts providing pediatric services across the United Kingdom (n = 212): England (n = 172), Scotland (n = 20), Wales (n = 12) and Northern Ireland (n = 8). Structured telephone surveys were conducted with either a Consultant Microbiologist (n = 3) or a Senior Biomedical Scientist (n = 193) in participating hospitals between July and August 2020. Twenty three of the 196 respondents submitted data via email citing data protection policy for their hospital. Sixteen laboratories did not respond, citing work pressures due to the coronavirus disease 2019 (COVID-19) pandemic, and were excluded from this study. The study was conducted and approved as an outcome audit. Ethical approval was considered not necessary as no confidential patient data was collected.
The survey consisted of a standardized questionnaire delivered by a single interviewer and the responses were collated electronically. The questionnaire asked the following details regarding NAAT of Paediatric CSF samples: (1) Whether the laboratory had onsite facilities to perform viral NAAT on CSF samples, type of assay used, criteria required to perform viral NAAT, the availability of point-of-care (POC) testing, and the approximate turnaround time (TAT); (2) If the laboratory did not perform viral NAAT, they were asked where samples were sent, criteria required to perform testing, and the TAT for NAAT results; and (3) All the laboratories performing onsite testing were questioned about the impact of the COVID-19 pandemic on their ability to process CSF NAAT samples.
Statistical analysis was performed using standard Chi-squared analysis and a P value < 0.05 was considered to indicate significance.
In total, 196/212 hospitals (92%) responded to the questionnaire. Of those responding, 133 (68%) had an onsite microbiology laboratory within the same hospital site as the pediatric facility and were included in the study; 63 hospitals (32%) were covered by offsite microbiology services at a different hospital and were excluded from the study (Figure 1). More than one-third of onsite microbiology laboratories in the United Kingdom (n = 47) had facilities to perform viral CSF NAAT as well as cover neighboring onsite laboratories (n = 88) with no NAAT facilities. Other laboratories with no onsite microbiology laboratories (n = 63) outsourced samples elsewhere. The criteria used to perform viral NAAT amongst the 47 onsite laboratories were as follows: (1) Clinician request in 32% (n = 15); (2) Combination of CSF white blood cell count and clinician request in 28% (n = 13); (3) Performed on all samples if requested (referred to as “blanket testing”) in 19% (n = 9); (4) Entirely dependent on CSF pleocytosis in 6% (n = 3); (5) Approval from a microbiologist in 4% (n = 2); and (6) Respondents unaware of the criteria for testing in 11% (n = 5).
The majority of microbiology laboratories (n = 86) that sent samples away did so on clinical request (n = 51; 59%). Other criteria included: CSF white cell counts (WCC) plus clinical request (n = 22; 26%), blanket testing (n = 8; 9%), and not known to respondent (n = 5; 6%). The TAT varied for CSF viral NAAT samples and is summarised in Table 1. The majority of laboratories (46 of 47) with onsite viral CSF NAAT facilities reported a sample processing time of ≤ 48 h, P < 0.00001. Four centers with onsite microbiology laboratories sent CSF samples to neighboring hospitals for more comprehensive NAAT targets as they offered limited facilities for viral PCR testing (only for enterovirus) performed through POC testing.
| TAT (in hours) | Laboratories with onsite viral NAAT facilities (n = 47) | Laboratories without onsite viral NAAT facilities (n = 86) | P value |
| < 12 | 21 | 4 | < 0.00001 |
| 12-24 | 18 | 1 | < 0.00001 |
| 24-48 | 7 | 40 | < 0.00001 |
| 48-72 | 0 | 23 | NC |
| > 72 | 0 | 15 | NC |
| Variable | 1 | 3 | NC |
Onsite laboratories used a variety of assay kits to perform viral NAAT including BioFire® (n = 22), in-house kits (n = 8), various Multiplex PCR kits (n = 6), LightCycler® (n = 1), Altona diagnostics (n = 1), AusDiagnostics® (n = 3), EliTech® (n = 2), M2000 (n = 1) and kits not specified (n = 3). Most of the kits covered 4 common viruses: Enterovirus, Human parechovirus, Herpes simplex virus (HSV) 1 and 2. There were facilities for testing additional viruses such as Varicella zoster virus, Cytomegalovirus, Adenovirus, Human Herpes Virus-6, Epstein-Barr virus, which varied depending on the kit used.
The COVID-19 pandemic had minor effects on the turnaround time for viral CSF NAAT results for laboratories performing onsite tests (n = 4; 9%), primarily due to the sharing of PCR/NAAT machines for COVID-19 (severe acute respiratory syndrome coronavirus 2) analysis, as well as shortages of staff and/or manufacturer delays.
The diagnosis of pediatric meningitis can be fraught with difficulty, especially in neonates and infants. Although there are several suggestive clinical signs, there is no diagnostic isolated single finding or combination of features[7]. Clinical suspicion, with cytological and microscopic analysis of CSF samples are the mainstay of diagnosis; antibiotic treatment is often started empirically while these results are awaited.
Enterovirus and Human Herpes Virus-6 (HHV-6) are the main pathogens causing viral meningitis in older neonates, infants and children. They usually have a favourable outcome, though neurological impairment has been observed, particularly following certain enterovirus strains such as D68 or human parechovirus[2]. HSV 1 and 2 infections typically cause severe encephalitis with serious sequelae if treatment with antivirals is delayed; evidence of these infections should be confirmed by NAAT as soon as possible.
The use of NAAT in the diagnosis of viral meningitis has been demonstrated to result in briefer parenteral antibiotic courses[5,8]. A positive CSF enterovirus result has also been associated with shorter lengths of hospital stay in infants with viral meningitis[5,9,10]. Most experts recommend that CSF PCR results for HHV-6 (due to potential for reactivation) should be interpreted with caution in the absence of readily attributable symptoms. A recent study reported that following detection of HHV-6 in 25 of 1005 children, five were subsequently diagnosed with either HHV-6 meningitis or meningoencephalitis based on HHV-6 detection in CSF, clinical presentation, and radiographic findings. These results led to early discontinuation of empirical acyclovir treatment in 12 children and appropriate initiation of ganciclovir therapy in 4 as a result of faster establishment of microbiological diagnosis[11]. NAAT remains an underutilised investigation: One observational study of 323 patients with a negative CSF gram stain reported that although PCR had the highest diagnostic yield it was only requested for 39.6% of patients[12].
The overwhelming majority of laboratories with onsite NAAT facilities (n = 47) reported a sample TAT of ≤ 24 h in 39/47 (89%), as compared to only 5/86 (6%) for samples sent elsewhere (P < 0.00001). The impact of transit times meant that only 52% (n = 45) of microbiology laboratories without NAAT facilities had a TAT of ≤ 48 h. Hopefully, as technology develops, turnaround times should improve, especially if POC tests become increasingly available. This has already been demonstrated as feasible for viral respiratory swab testing during the COVID-19 pandemic. A Canadian study using a model-based analysis of a retrospective cohort of all hospitalised children admitted with suspected enterovirus meningitis between November 2013 and 2017 demonstrated that same-day TAT of CSF enterovirus PCR was associated with a cost reduction of 342.83 Canadian dollar per patient in comparison to specimens sent to a reference laboratory. Further benefits such as decreased length of stay (LOS) and antibiotic therapy were also noted[13]. A retrospective study from the United States with 363 children who had HSV PCR tested on CSF samples demonstrated that the median duration of acyclovir therapy was significantly reduced in the group following implementation of a direct sample-to-answer assay technique (leading to faster TAT) as compared to laboratory-developed real-time PCR assay used in pre-implementation group [14.3 h vs 29.2 h (P < 0.01)] and marginal reduction in median LOS [4 d vs 5 d (P 0.23)][14].
There was also a wide variation in the acceptance criteria for performing NAAT analysis in the 133 centers with an onsite microbiology laboratory; the most popular approaches being based on clinician request (66/133, 50%) or a combination of CSF WCC with clinician request (35/133, 26%). Two recently published studies from the United Kingdom have suggested performing viral PCR testing of all CSF samples in infants, irrespective of their adjusted CSF WCC, has potential to reduce length of hospital stay and antibiotic usage[5,9].
The COVID-19 pandemic had minimal effect on TAT with delay in sample analysis reported in 6% centers who had onsite testing facilities. Within the context of pediatrics, the cumulative effect of these delays can be lengthier hospital admissions, prolonged courses of parenteral antibiotics and diagnostic uncertainty.
Despite the widely documented benefits of using NAAT technology to aid the diagnosis and management of pediatric meningitis, onsite testing facilities for viral NAAT are limited in the United Kingdom. The lack of available NAAT facilities may have significant implications on patient outcomes, including increased LOS and duration of parenteral antibiotics. Early discontinuation of antibiotics in cases of viral meningitis should lead to improved antibiotic stewardship. Our study underlines the need for a national consensus on the role of PCR testing and emphasises the desirability of onsite PCR testing equipment for microbiology laboratories in the United Kingdom and elsewhere in the world.
Viral pathogens are considered the major cause for meningitis worldwide. The use of nucleic acid amplification testing (NAAT), predominantly through polymerase chain reaction (PCR) in the diagnosis of meningitis has been demonstrated to result in faster turnaround times, shorter length of stay and briefer course of parenteral antibiotics.
NAAT remains an underutilized investigation and it is important to develop a national consensus on the role of PCR testing for diagnosing viral meningitis in children.
The aim of this study was to evaluate the use and availability of viral NAAT testing of cerebrospinal fluid (CSF) in microbiology laboratories across the United Kingdom.
Structured telephone questionnaire survey was conducted to understand the availability of viral CSF NAAT in the United Kingdom with emphasis on the criteria used for testing and turnaround times including the impact of the coronavirus disease 2019 pandemic.
Onsite facilities for viral CSF NAAT was available in 35% centres with much faster turnaround times of ≤ 24 h as compared to those outsourcing to neighboring laboratories.
Onsite/near-patient rapid NAAT [including polymerase chain reaction (PCR)] is recommended wherever possible to optimize patient management in the acute setting.
Our study underlines the need for a national consensus on the role of NAAT and emphasizes the need for on-site PCR testing equipment for microbiology laboratories in the United Kingdom.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gravina AG, Italy; Matowicka-Karna J, Poland S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | GBD 2016 Meningitis Collaborators. Global, regional, and national burden of meningitis, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:1061-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 232] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 2. | Hudson JA, Broad J, Martin NG, Sadarangani M, Galal U, Kelly DF, Pollard AJ, Kadambari S. Outcomes beyond hospital discharge in infants and children with viral meningitis: A systematic review. Rev Med Virol. 2020;30:e2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Martin NG, Sadarangani M, Pollard AJ, Goldacre MJ. Hospital admission rates for meningitis and septicaemia caused by Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae in children in England over five decades: a population-based observational study. Lancet Infect Dis. 2014;14:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | National Institute for Health and Care Excellence (NICE). Meningitis (bacterial) and meningococcal septicaemia in under 16s: recognition, diagnosis and management. [cited 15 May 2021]. Available from: https://www.nice.org.uk/guidance/cg102. |
| 5. | Turner PC, Brayley J, Downing HC, Homfray GJ, Doolan G, Paul SP. Screening for enteroviral meningitis in infants and children-Is it useful in clinical practice? J Med Virol. 2019;91:1882-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Sanaei Dashti A, Alizadeh S, Karimi A, Khalifeh M, Shoja SA. Diagnostic value of lactate, procalcitonin, ferritin, serum-C-reactive protein, and other biomarkers in bacterial and viral meningitis: A cross-sectional study. Medicine (Baltimore). 2017;96:e7637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Curtis S, Stobart K, Vandermeer B, Simel DL, Klassen T. Clinical features suggestive of meningitis in children: a systematic review of prospective data. Pediatrics. 2010;126:952-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Lyons TW, McAdam AJ, Cohn KA, Monuteaux MC, Nigrovic LE. Impact of in-hospital enteroviral polymerase chain reaction testing on the clinical management of children with meningitis. J Hosp Med. 2012;7:517-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Chakrabarti P, Warren C, Vincent L, Kumar Y. Outcome of routine cerebrospinal fluid screening for enterovirus and human parechovirus infection among infants with sepsis-like illness or meningitis in Cornwall, UK. Eur J Pediatr. 2018;177:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Dewan M, Zorc JJ, Hodinka RL, Shah SS. Cerebrospinal fluid enterovirus testing in infants 56 days or younger. Arch Pediatr Adolesc Med. 2010;164:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Pandey U, Greninger AL, Levin GR, Jerome KR, Anand VC, Dien Bard J. Pathogen or Bystander: Clinical Significance of Detecting Human Herpesvirus 6 in Pediatric Cerebrospinal Fluid. J Clin Microbiol. 2020;58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Nesher L, Hadi CM, Salazar L, Wootton SH, Garey KW, Lasco T, Luce AM, Hasbun R. Epidemiology of meningitis with a negative CSF Gram stain: under-utilization of available diagnostic tests. Epidemiol Infect. 2016;144:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Alghounaim M, Caya C, Cho M, Beltempo M, Yansouni CP, Dendukuri N, Papenburg J. Impact of decreasing cerebrospinal fluid enterovirus PCR turnaround time on costs and management of children with suspected enterovirus meningitis. Eur J Clin Microbiol Infect Dis. 2020;39:945-954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Van TT, Mongkolrattanothai K, Arevalo M, Lustestica M, Dien Bard J. Impact of a Rapid Herpes Simplex Virus PCR Assay on Duration of Acyclovir Therapy. J Clin Microbiol. 2017;55:1557-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |