Published online May 9, 2022. doi: 10.5409/wjcp.v11.i3.270
Peer-review started: June 27, 2021
First decision: July 30, 2021
Revised: August 9, 2021
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: May 9, 2022
Processing time: 313 Days and 23.9 Hours
Bleeding per rectum in children can be seen in congenital as well as acquired conditions that may require medical or surgical management. The present review article is aimed to discuss the imaging findings of some common and uncommon causes of bleeding per rectum in children.
Core Tip: Bleeding per rectum in children can be seen in congenital as well as acquired conditions. The referring clinicians as well the radiologists must be aware of the various radiological findings of common and uncommon causes of bleeding per rectum in children discussed in the article.
- Citation: Chandel K, Jain R, Bhatia A, Saxena AK, Sodhi KS. Bleeding per rectum in pediatric population: A pictorial review. World J Clin Pediatr 2022; 11(3): 270-288
- URL: https://www.wjgnet.com/2219-2808/full/v11/i3/270.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v11.i3.270
Bleeding per rectum in children may be in the form of passage of either bright red or dark red blood (hematochezia) or black tarry stools (melena). It may occur due to congenital as well as acquired causes in the pediatric population which may require medical or surgical management.
The causes of bleeding per rectum in children across different age groups are summarized in Table 1. In the present review, we aim to discuss the imaging findings of some common and uncommon causes of bleeding per rectum in children.
| Up to 2 yr of age | 2-5 yr | 6-15 yr |
| Milk allergy | Polyps | Polyps |
| Necrotizing enterocolitis | Anal fissure | Anal fissure |
| Duplication cyst | Intussusception | Infectious enterocolitis |
| Polyps | Meckel diverticulum | Inflammatory bowel disease |
| Anal fissure | Infectious enterocolitis | Henoch-Schönlein purpura |
| Intussusception | Bleeding diathesis | Hemolytic-uremic syndrome |
| Hirschsprung disease related enterocolitis | Henoch-Schönlein purpura | Bleeding diathesis |
| Meckel diverticulum | Hemolytic-uremic syndrome | Angiodysplasia |
| Infectious enterocolitis | Angiodysplasia | Lymphonodular hyperplasia |
| Bleeding diathesis | Lymphonodular hyperplasia | Extrahepatic portal venous obstruction |
| Extrahepatic portal venous obstruction |
Necrotizing enterocolitis (NEC) refers to acute severe inflammation of the bowel in the newborn. Its incidence ranges from 1%-5% in neonatal intensive care units[1,2]. Greater risk of NEC is noted in extreme preterm (less than 28 wk) and extremely low birth weight (birth weight less than 1000 g) babies[3]. Approximately 10% of NEC cases may be seen in term neonates with associated congenital heart diseases being the major risk factor for these patients[1,4-6].
Clinical findings vary with the severity of involvement, with feed intolerance, vomiting, hematochezia, and abdominal tenderness noted in the early stage. In advanced stages, it can lead to peritonitis, sepsis, and eventually shock.
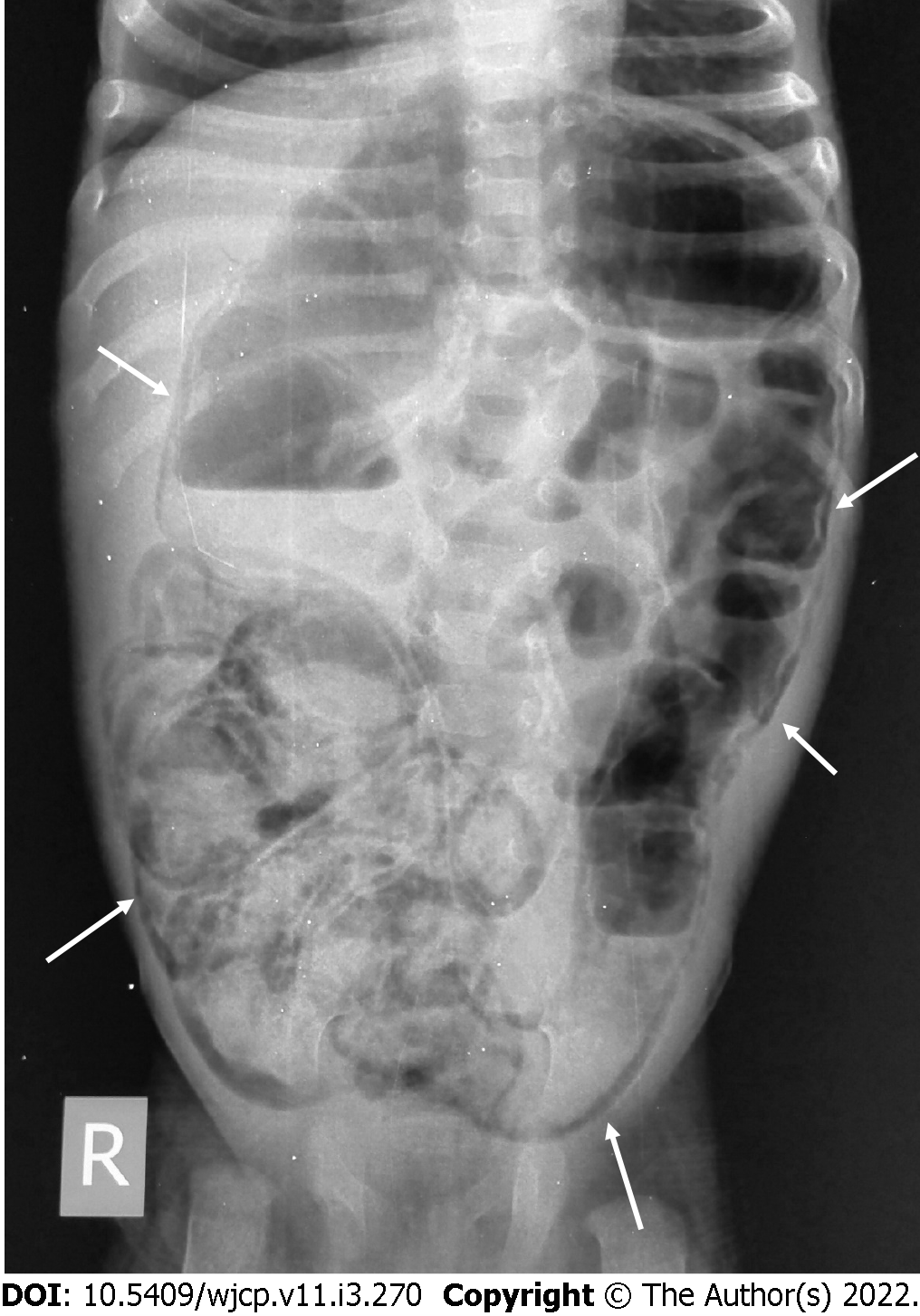
Plain abdominal radiographs are the initial radiological investigation and the mainstay of diagnosis of NEC in an appropriate clinical setting. Both supine views and cross-table lateral views are preferred. The earliest and most common sign of NEC even before clinical findings is the loss of normal “mosaic” pattern of bowel gas in neonates along with tubular or rounded dilatation of the loops. This is seen in 90% of the neonates. The degree of dilatation and clinical severity are correlated[7]. Furthermore, persistent dilated bowel loops are an ominous sign, with the “fixed bowel loop sign” reflecting transmural bowel necrosis and imminent perforation”.
In an appropriate clinical setting, the presence of intramural gas is considered a pathognomonic sign of NEC and is seen in 19%-98% of the cases[8-10] (Figure 1). Two patterns can be seen - a bubbly pattern (representing air in the submucosa) or a linear pattern of intramural gas (suggesting subserosal gas).
Portal venous gas (seen in approximately 30% of cases on X-ray) is seen as linear, branching radiolucent lines radiating from the region of the hilum towards the periphery[11,12]. It must be differentiated from pneumobilia where the gas is seen more centrally as compared to portal venous gas where it extends more peripherally[12].
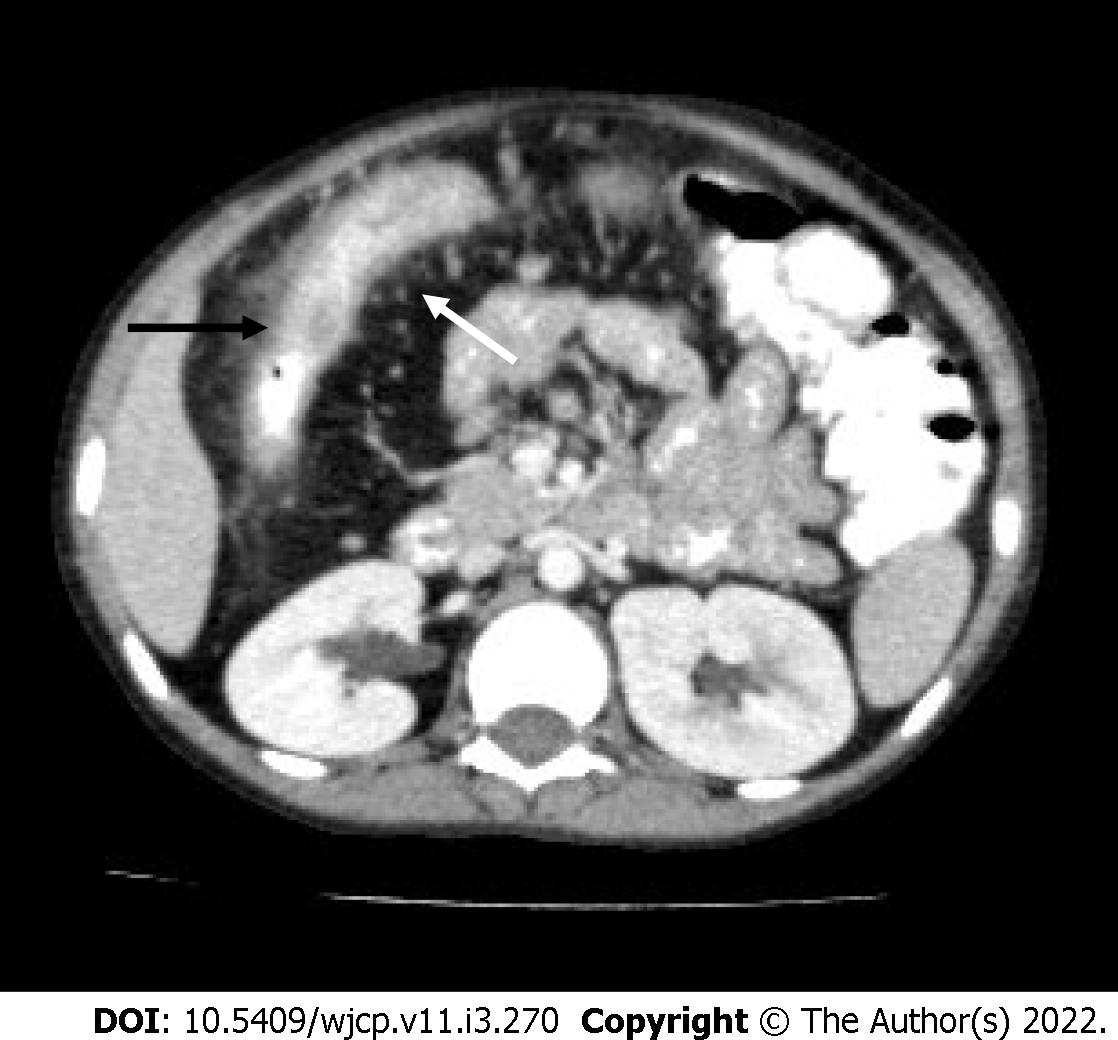
In later stages, bowel perforation may occur and is seen as pneumoperitoneum which becomes an indication for surgical intervention[13] (Figure 2). Various signs have described free intraperitoneal gas in the abdomen, namely, Rigler sign (air lining the bowel wall), football sign, Cupola sign (air under the central diaphragm), inverted V sign (air outlining the lateral umbilical ligaments), etc. Cross table lateral views are especially valuable in detecting small amounts of interbowel gas, which is seen as a triangular lucency between the bowel walls.
Ultrasonography (USG) provides valuable information in patients with NEC in the form of bowel wall thickness and echogenicity, free intraperitoneal fluid and its character, peristalsis, and bowel wall perfusion. Few studies have shown USG to be more sensitive in depicting intramural and portal venous gas[14,15]. However, the major limitation is that it is operator dependent. Early stages of NEC show bowel wall thickening with loss of normal gut signature (the hypoechoic rim of the muscularis propria) with an increase in the wall echogenicity (Figure 3). This is accompanied by an increase in the Doppler color flow in the bowel wall in early stages. Later stages show thinning of the wall with reduced and later absent flow[14]. Free intraperitoneal fluid may be seen. The presence of low-level internal echoes/septations suggests perforation[12,13].
Meckel’s diverticulum is a true diverticulum arising from the terminal ileum and is the result of persistence of the vitello-intestinal duct[16]. The lifetime risks of complications from Meckel’s diverticulum are reported to be 6.4%[17], which include bleeding, diverticulitis, and intussusception. The risk of bleeding is more common in children in than adults[18] and is due to the presence of ectopic gastric mucosa causing ulceration.
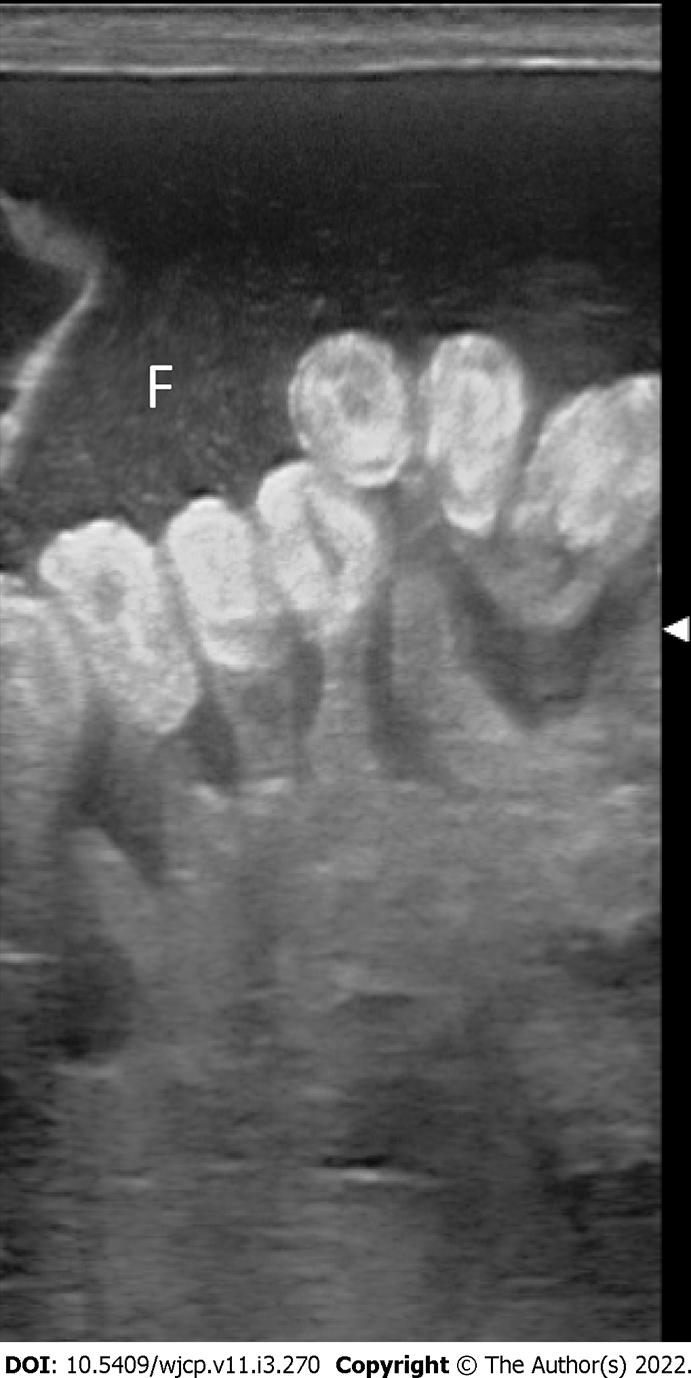
High-resolution USG shows a fluid-filled anechoic blind ending tubular structure in the right lower quadrant with a typical “gut signature”. The other non-blinding end connects to a peristaltic bowel loop.
On Tc99m pertechnate scintigraphy, the ectopic gastric mucosa within the diverticulum appears as a focal area of increased tracer uptake in the right iliac fossa (sensitivity, 85%; specificity, 95%). Other scintigraphy techniques like Tc-99m labeled sulfur colloid scan and RBC scan can also localize the site of LGIB, but neither is specific for Meckel’s diverticulum[19, 20].
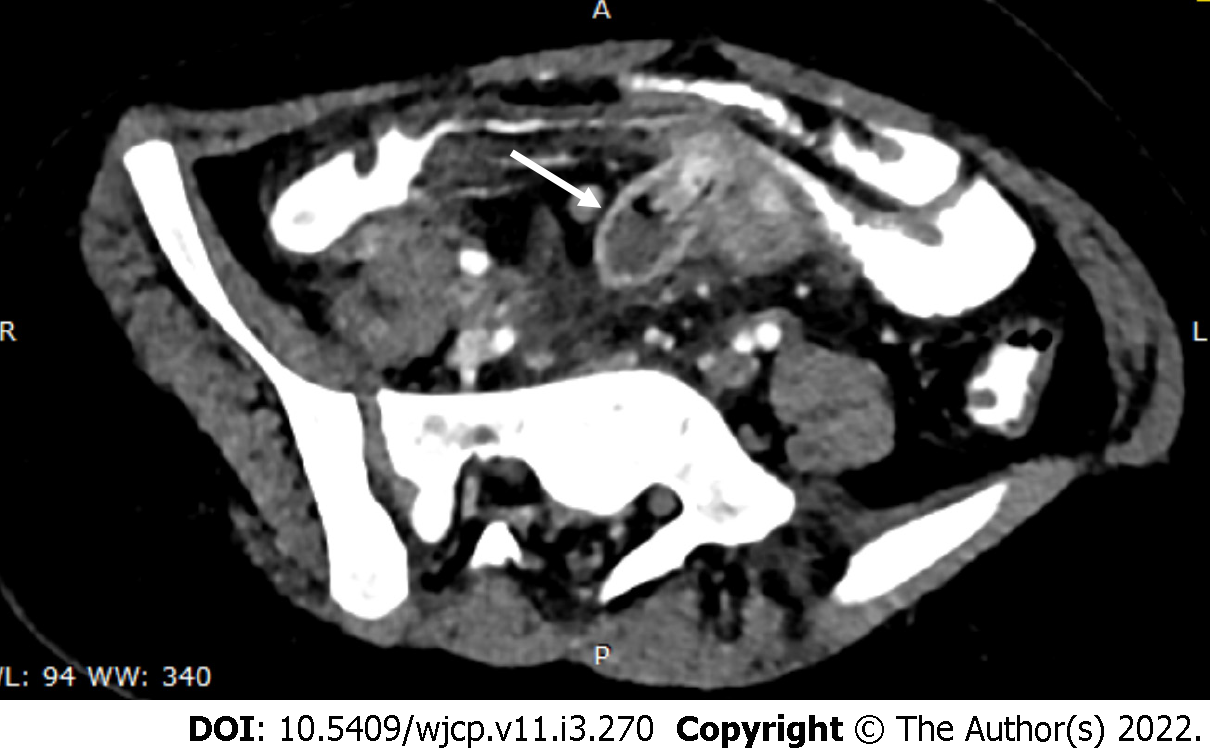
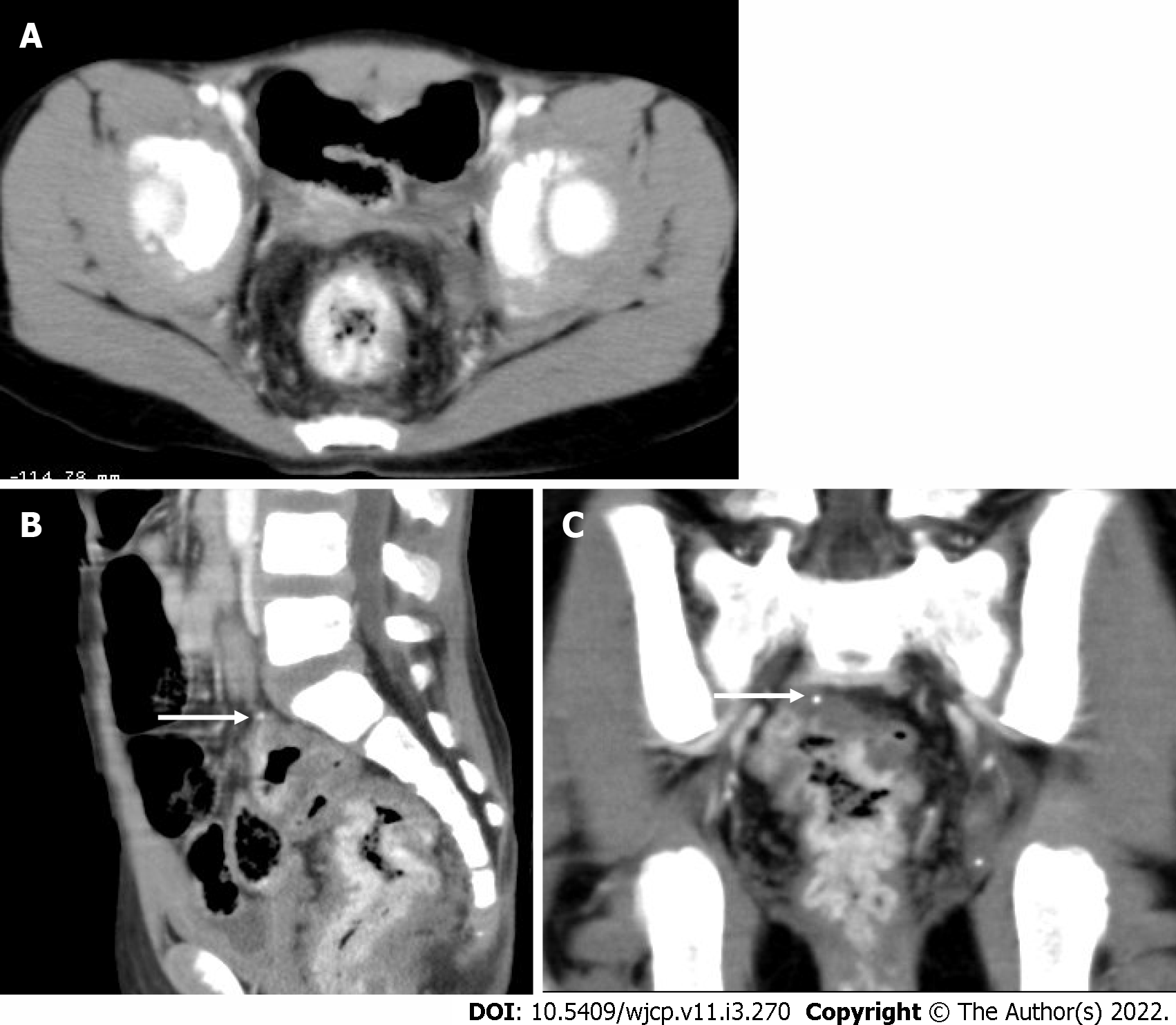
Complications of Meckel’s diverticulum like diverticulitis, bowel obstruction, and in some cases intussusceptions are very well seen on computed tomography (CT)[21]. On CT, it appears as a fluid containing blind-ending pouch with variable mural thickness and adjacent fat stranding (Figure 4). Bowel obstruction can occur in Meckel’s diverticulum secondary to intussusception, volvulus, the inclusion of diverticulum in the hernia, or foreign body impaction. Direct visualization of the diverticulum on computed tomography (CT) is difficult and features are similar to those caused by post-op adhesions, i.e., dilated bowel loops with an abrupt change in caliber with the absence of soft tissue mass at the site of obstruction.
Surgical resection is the treatment of choice for symptomatic Meckel’s diverticulum. However, management in asymptomatic incidentally detected diverticulum is controversial with some authors advocating conservative approaches owing to reduced lifetime risk of complications while others support early prophylactic diverticulectomy[17,18].
Colorectal polyps are an important cause of lower gastrointestinal (GI) bleeding in children and adolescents with an estimated prevalence of 12% during pediatric colonoscopy for lower GI bleeding[22]. The majority of them are juvenile hamartomatous polyps[23] and an overwhelming majority of these are solitary and sporadic not associated with malignancy[24,25]. Most of these present as painless rectal bleeding[26]. Few of them may have lower abdominal pain. Most of these are located in the rectosigmoid and thus present as fresh red blood per rectum[27]. Multiple colonic polyps have been associated with polyposis syndromes.
On abdominal radiographs, they may appear as a rounded soft tissue mass in gas-filled bowel lumen. Barium enema may show polyps as a filling defect on the dependent wall[28]. Double contrast enema (DCE) better outlines the polyps which may be seen as ring shadow with barium coated white rim. “Bowler hat sign” on double contrast air enema refers to the appearance of sessile polyp formed by a ring of barium at the base of the polyp surrounding a domed layer of barium coating the surface of the polyp[29]. “Mexican hat sign” is the analogous appearance of pedunculation on DCE formed by pair of concentric rings with outer and inner rings representing head and stalk of polyp[28].
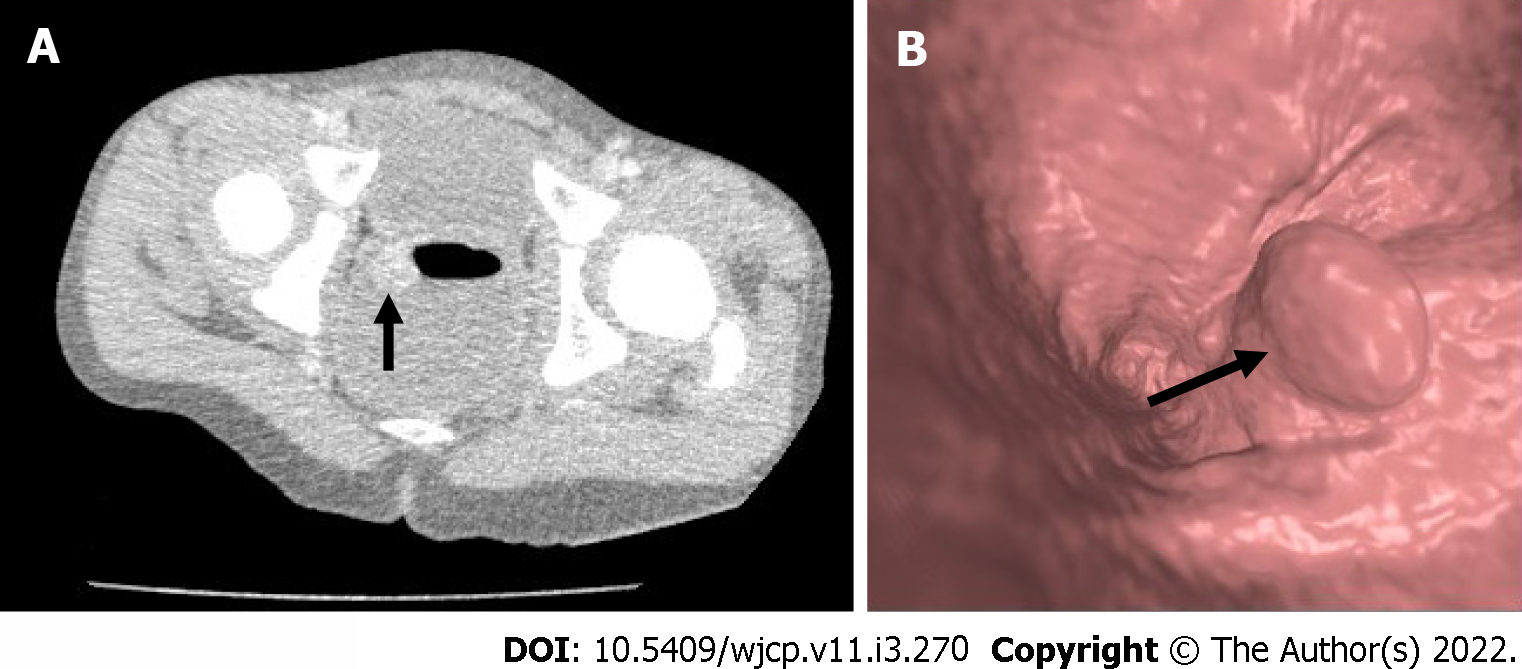
CT colonography with the help of advanced graphic software creates two dimensional and three dimensional images along with volumetric data of the colon[30]. Being non-invasive, it provides a virtual endoluminal image of the polyps which are seen as projections (Figure 5). Bright lumen and dark lumen techniques in magnetic resonance (MR) colonography are used to visualize the colon. In bright lumen technique, polyps are seen as hypointense filling defects in the bright lumen. In dark lumen techniques, polyps appear as enhancing soft- tissue masses against the background of dark intraluminal air/water. Dark lumen techniques have better sensitivity than bright lumen techniques[31,32].
Inflammatory bowel disease is a chronic disease of the gastrointestinal tract consisting of two separate but related entities, namely, ulcerative colitis and Crohn’s disease (CD). The manifestations of CD vary depending upon the extent of the disease with isolated colonic involvement presenting similarly to UC, whereas small bowel CD presents as fever, weight loss, and fatigue more commonly than UC[33]. Symptoms of inflammatory bowel disease wax and wane, resulting in “flares” and “remission”, respectively. Up to 30% of children with CD present with growth failure[34].
Imaging has been used to assess parts of the bowel not accessible by direct endoscopic visualization, namely, the small bowel. Fluoroscopic techniques like small bowel enteroclysis and barium meal follow-through have largely been replaced by non-invasive techniques like CT and MR enterography, which provide both mucosal and extraluminal information as well as extraintestinal manifestations.
Double contrast barium enema allows to obtain greater details of the colonic mucosa and shows irregular thickening and distortion of the valvulae conniventes, widely separated bowel loops due to fibro-fatty mesenteric proliferation and pseudo-sacculations at the ulcer site. Severe cases produce transverse and longitudinal ulcers giving rise to cobblestone appearance. Chronic cases result in multiple strictures, sinus tracts, and fistulae, which can be readily demonstrated on contrast studies.
USG has a limited role and shows predominantly bowel wall thickening with loss of mural stratification, bowel wall hyperemia, reactive mesenteric lymphadenopathy, and ascites[35]. Other complications like abscess formation and bowel obstruction can also be demonstrated on USG.
CT helps in the simultaneous assessment of extraluminal and extraintestinal complications and has emerged as one of the primary imaging modalities. Common signs of active CD include bowel wall thickening (> 3 mm) and mucosal hyperenhancment[36,37]. These are the most common and sensitive findings of CD[38,39]. Increased vascularity in form of “comb sign” (Figure 6) refers to increased vascularity of the distal mesenteric arterial arcades and the vasa recta of the affected ascending colon and small bowel and is a sign of active inflammation[40]. Perienteric fat stranding and engorged vasa recta are the most specific signs of active CD on CT enterography[41]. Chronic CD produces “creeping fat sign” due to fibrofatty proliferation. Complications like strictures, bowel obstruction, fistula formation, and an intra-abdominal abscess can also be readily demonstrated.
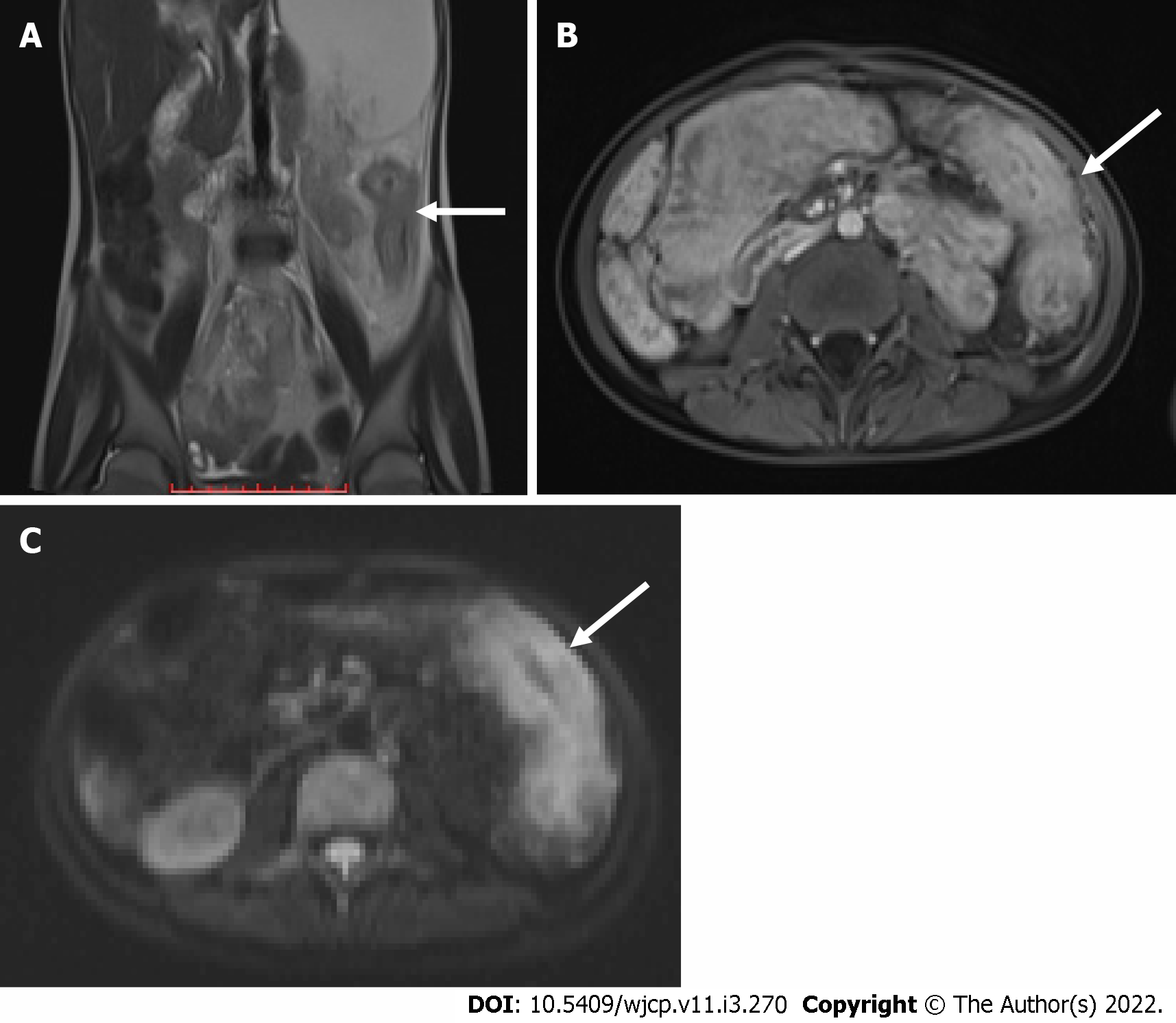
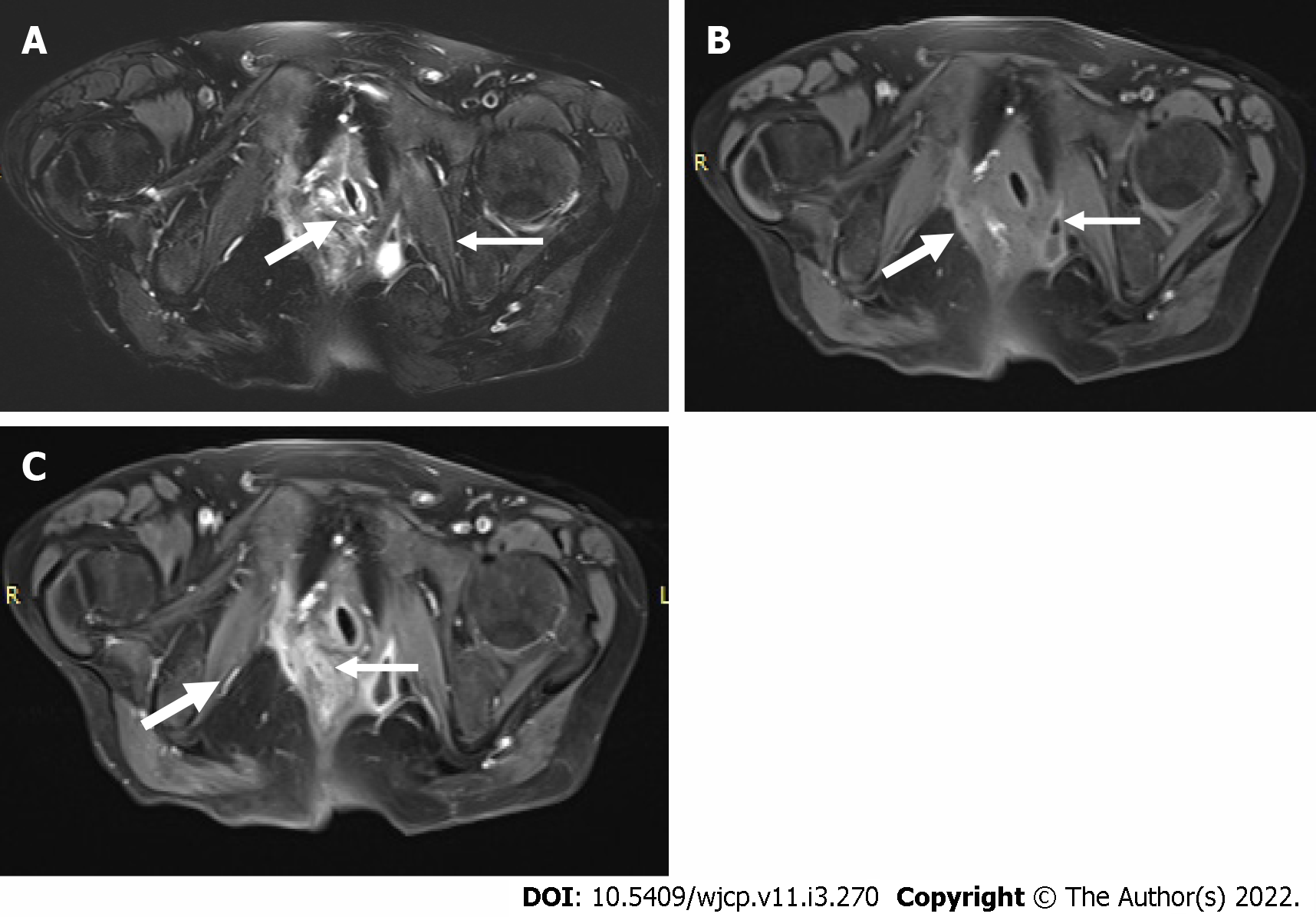
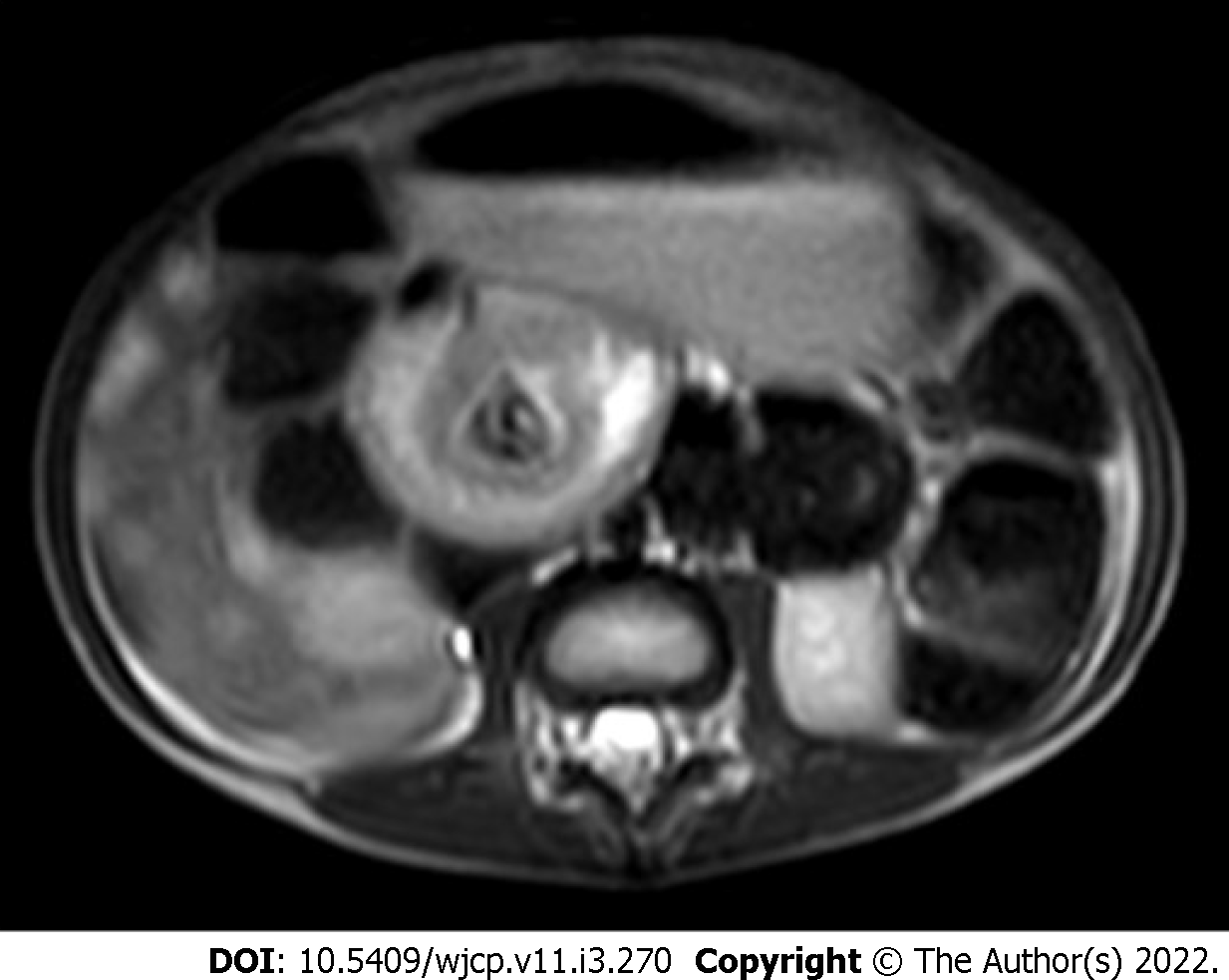
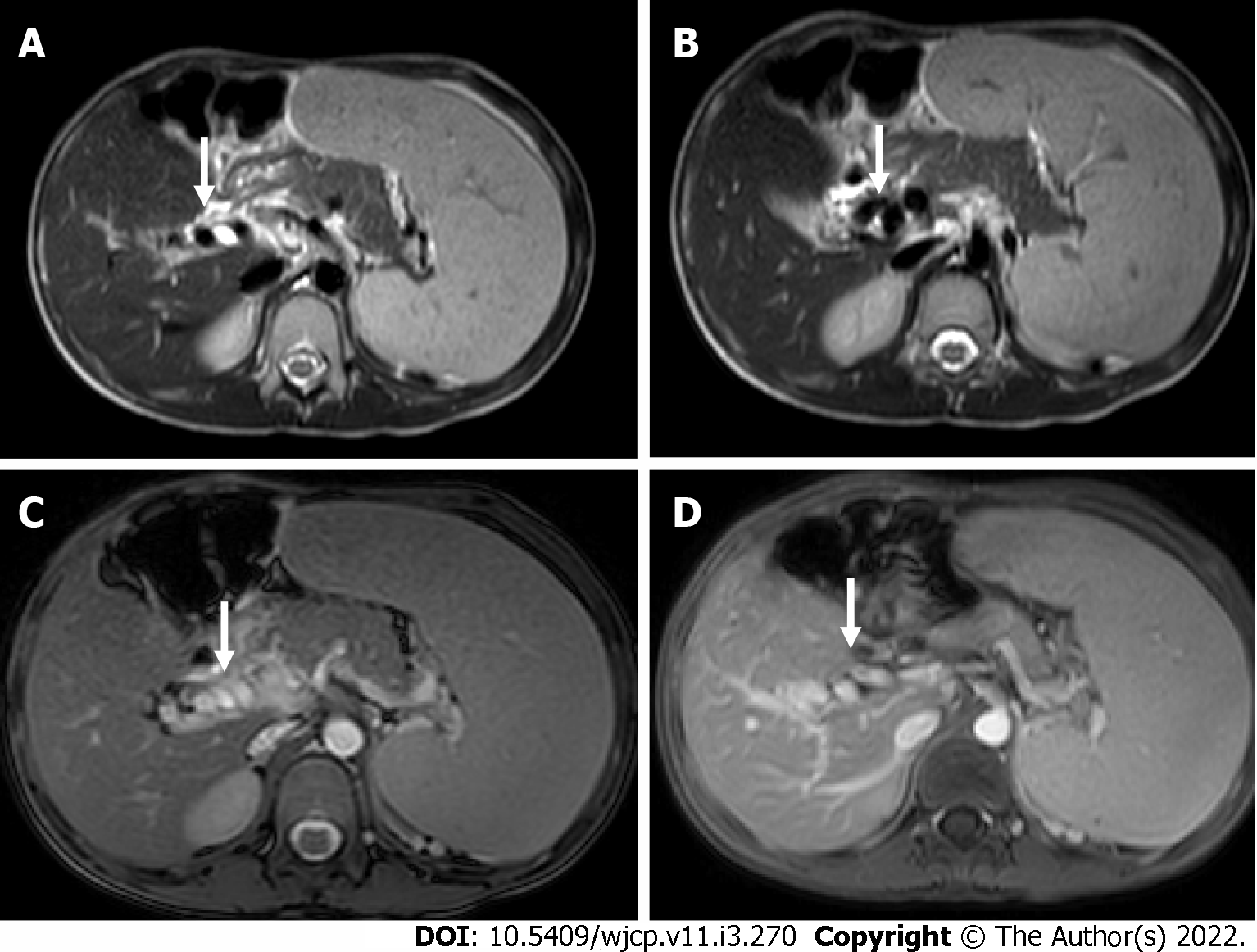
MR enterography offers the advantage of radiation free modality and is of utmost importance in the pediatric population. It is specifically the modality of choice for better evaluation of perianal disease and better distinction of acute disease from chronic disease. Findings on MR enterography such as bowel wall thickening, mucosal hyperenhancement, mural stratification, and perienteric fat stranding denote active disease (Figure 7A and B). Chronic fibrotic CD shows hypointense signal on T2 weighted images. Diffusion weighted imaging is unique and shows restricted diffusion with low apparent diffusion coefficient values in active disease[42](Figure 7C). Strictures are better evaluated on MR enterography due to its dynamic bowel examination in time and CINE imaging[39]. Perianal fistulas and other entero-cutaneous fistulas appear as enhancing tracts best visualized on post- contrast fat-saturated T1 weighted sequences[43,44] (Figure 8).
Midgut volvulus occurs due to intestinal malrotation. In malrotation, due to abnormal 2700 counter-clock rotation of the midgut around the superior mesenteric artery (SMA) axis, an abnormally long, narrow mesenteric pedicle is present from the ligament of Trietz to the ileocaecal valve and is more susceptible for midgut volvulus. It usually presents with bilious vomiting due to proximal small bowel obstruction, but occasionally bloody stools may be seen secondary to intestinal ischemia.
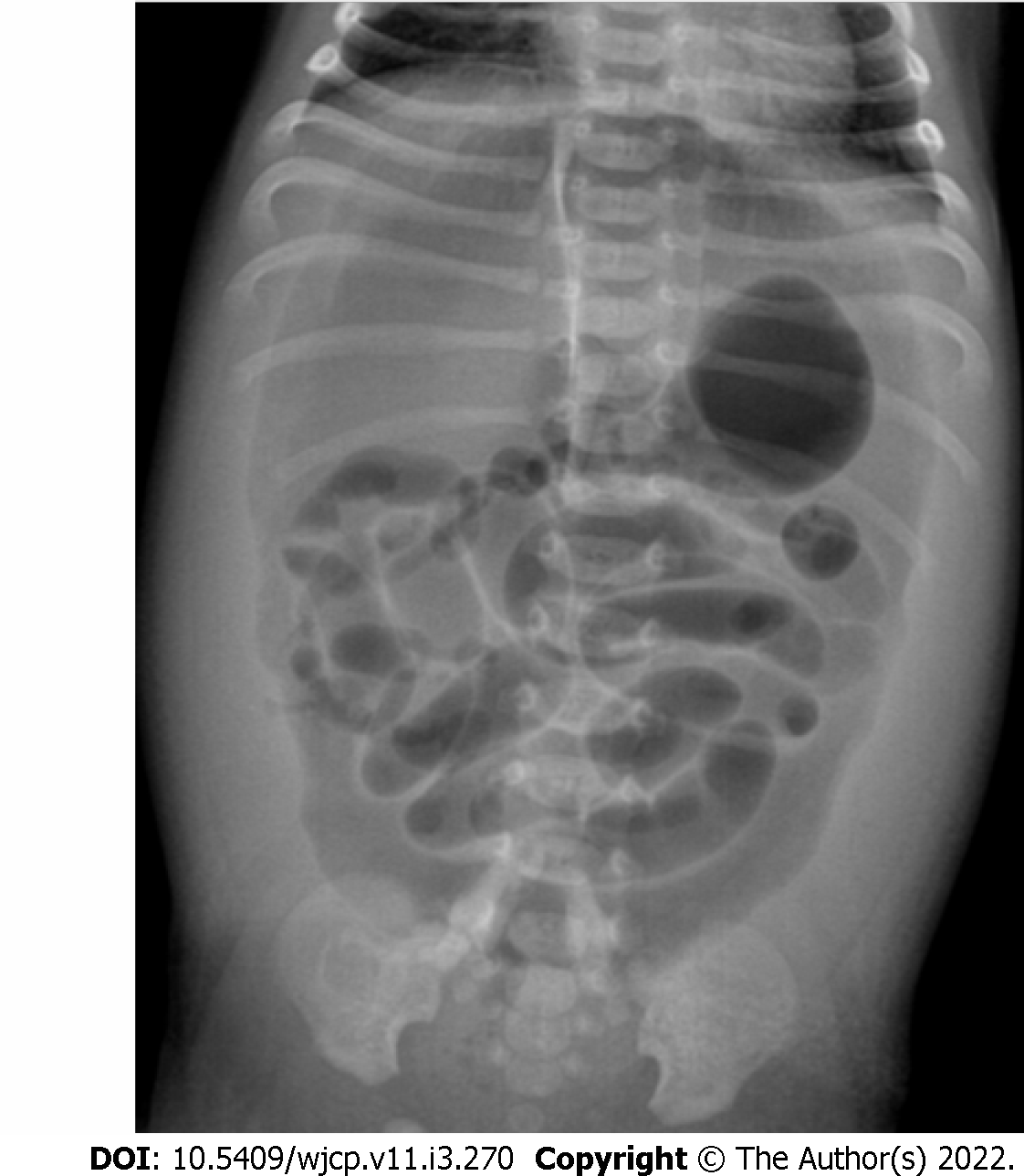
Plain abdominal radiographs show a paucity of bowel gases beyond the stomach and the duodenum and colonic gas if present is seen in the left hemi-abdomen.
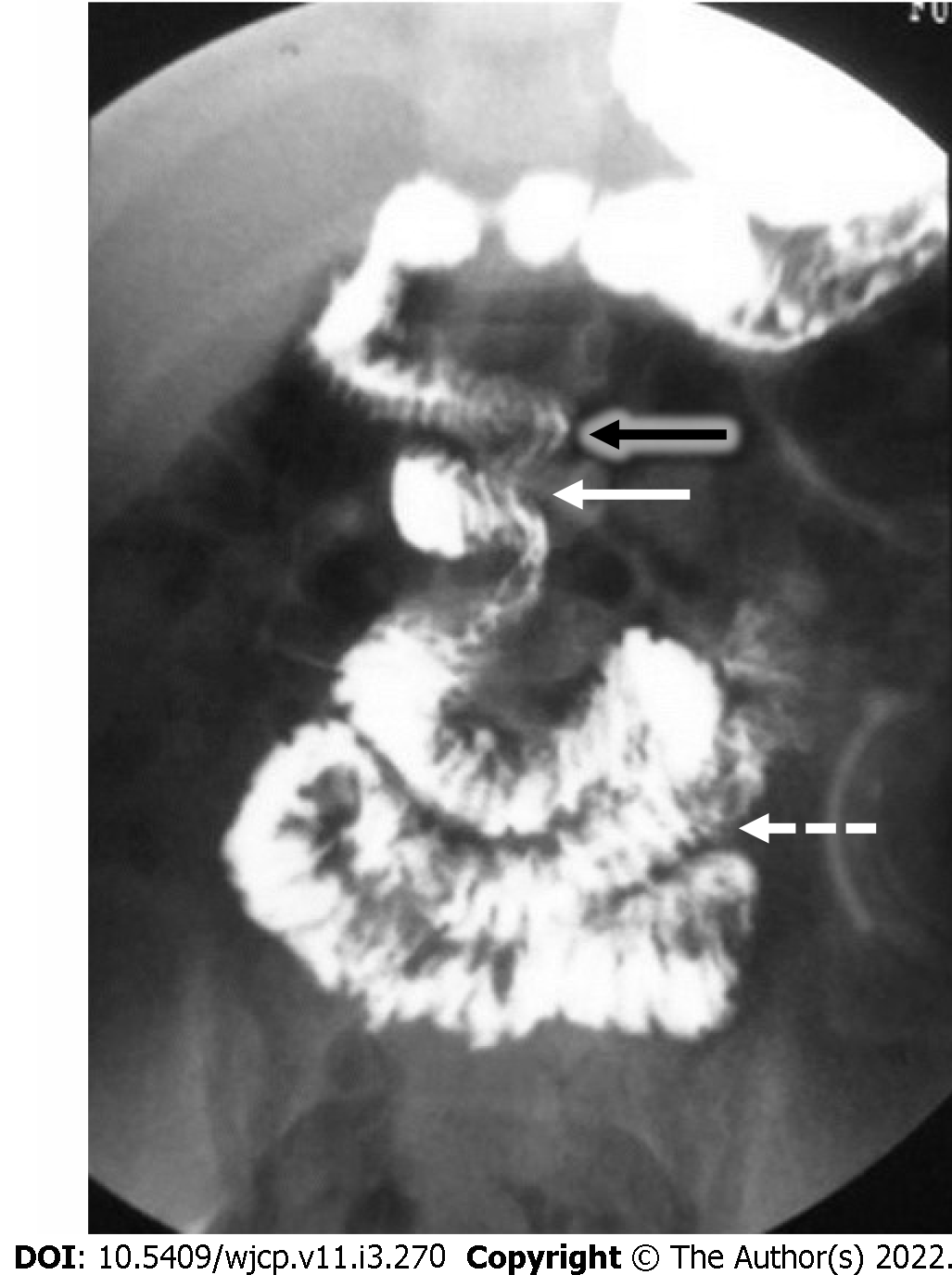
Upper GI contrast studies are the preferred imaging tests for a suspected case. Typical findings on fluoroscopy include a corkscrew appearance of the duodenum with it not crossing the midline and duodeno-jejunal (DJ) flexure present on the right, below the level of the pylorus and to the right of the left pedicle of L1 vertebra[45](Figure 9).
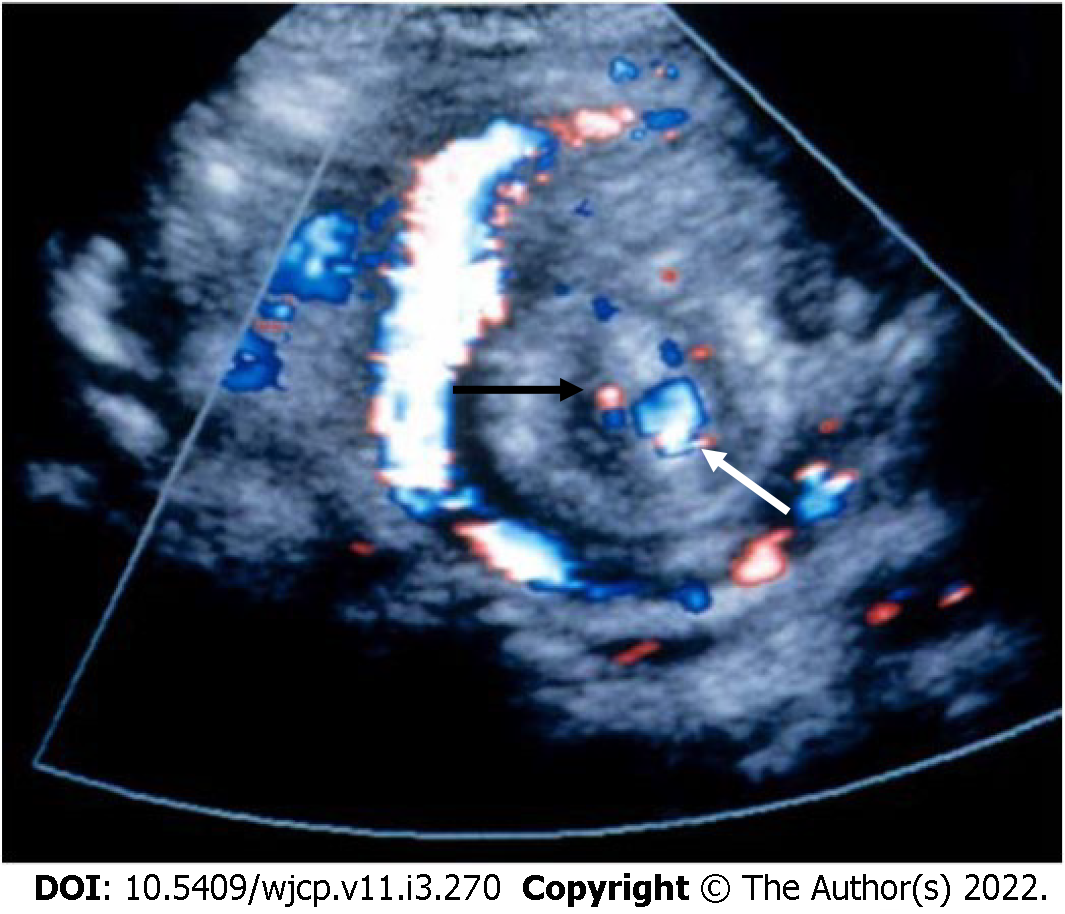
On USG, the superior mesenteric vein (SMV) is present to the left of the SMA[46]; however, the absence of this finding does not rule out malrotation[47,48]. On color Doppler images, twisting or wrapping of the SMV and the mesentery around the SMA in a clockwise direction is suggestive of whirlpool sign (Figure 10). It has a sensitivity, specificity, and positive predictive value of 92%, 100%, and 100%, respectively[46].
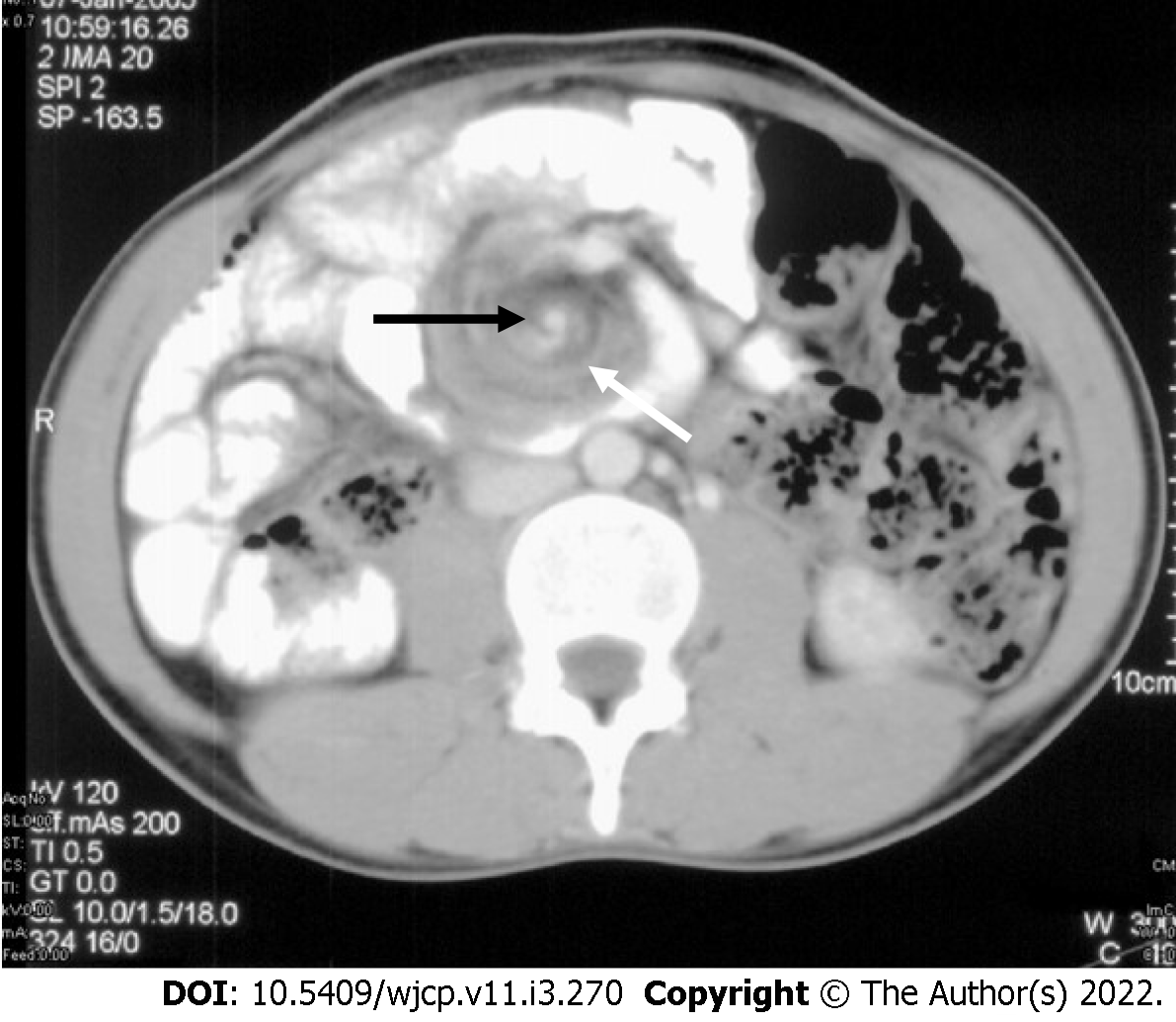
On CT and magnetic resonance imaging (MRI) at the site of volvulus, swirling of mesenteric vessels may be seen[49] (Figures 11 and 12). There is abnormally positioned duodenum, DJ flexure, cecum (in the left upper quadrant), and the large bowel (the majority of colonic loops in the right hemiabdomen) along with distention of the proximal duodenum and the stomach.
Extra-hepatic portal vein obstruction (EHPVO) is an important and common cause of non-cirrhotic portal hypertension (HTN). It is characterized by chronic thrombosis of the main portal vein, with or without intrahepatic portal vein, splenic vein, and SMV involvement with portal vein cavernoma formation. It is a disorder of children and young adults. In developing countries in the Asian region, it is the most common cause of portal HTN and upper GI bleeding in the pediatric population. Hypercoagulable state, infections, inflammation, portal venous anomalies, and perinatal umbilical vein catheterization are the most common etiologies; however, 70% of cases are idiopathic[50-52].
Clinically, these patients present with upper GI variceal bleeding with the associated feature of hypersplenism. Portal cavernous cholangiopathy (PCC) is seen in approximately 70%-100% of cases. Only 5%-28% of these are symptomatic due to biliary obstruction leading to intrahepatic biliary radical dilatation (IHBRD), choledocholithiasis, and hepatolithiasis[50, 52]. Lower GI bleeding is rare with EHPVO (seen in 0.5%-10% of cases), but this is usually torrential and life-threatening. Anorectal varices (63%-95% of cases) and colopathy (approximately 54% of cases), secondary to increased portal venous pressure, are the two main causes for lower GI bleed in these patients[53]. Isolated inferior mesenteric vein portal hypertension secondary to EHPVO has been reported[54].
USG along with Doppler is usually the first investigation in a suspected case of EHPVO. The portal vein is not visualized and is replaced with multiple tortuous vascular channels suggestive of cavernoma formation. Depending on the extent of portal vein involvement, both intra- and extra-hepatic cavernoma formation can be seen. Monophasic hepatopetal flow is noted in the collaterals. Pericholecystic collaterals are seen in approximately 30- 50% of cases. In patients with PCC, IHBRD, hepatolithiasis, and choledocholithiasis may be seen[50-52].
CT demonstrates vascular, biliary, and visceral changes. However, it is not routinely performed in children with EHPVO.
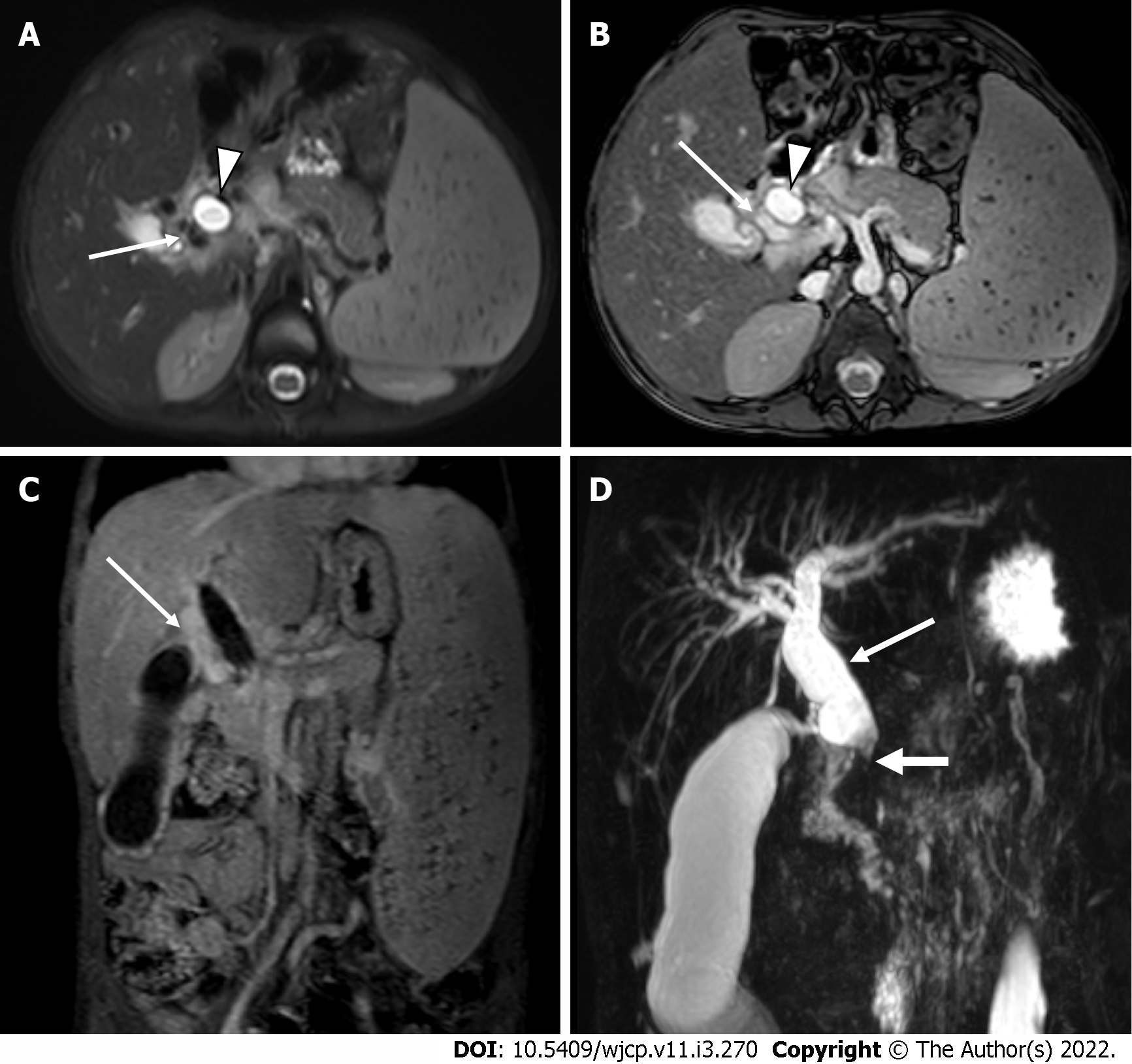
Magnetic resonance cholangiopancreatography (MRCP) and MR portovenography provide valuable information regarding the biliary and splenoportal axis, respectively (Figure 13). MRI features suggestive of PCC include irregular wavy contour of bile ducts, biliary duct narrowing and strictures with or without dilatation, gall bladder and bile duct wall thickening, CBD angulation, hepatolithiasis and cholelithiasis, and choledocholithiasis. Paracholedochal and pericholecystic collaterals (Figure 14) are seen as enhancing tortuous collaterals causing smooth extrinsic impressions on bile duct[50, 52]. MRI also demonstrates the presence of intra-splenic siderotic nodules (Gamna-Gandy bodies), which denotes long standing portal hypertension.
Duplication cysts are a rare gastrointestinal tract developmental anomaly with an incidence of approximately 0.2% of all children and are most commonly seen in infancy. They may be contained within the gastrointestinal tract or lie outside to it and are usually seen on the mesenteric side. They are either cystic (80%) or tubular (20%). The ileum, esophagus, and colon are the common sites. Histologically, they show GI epithelial inner lining and smooth muscle outer layer. Presentation is variable and usually depends on location, size and mass effect, and complications. It may present as vomiting, abdominal distention, bleeding, abdominal mass, and increased urinary frequency and hesitancy. The cysts may be complicated by perforation and can act as a lead point for intussusception, volvulus, and bowel obstruction. GI bleeding occurs primarily because of ulceration of the gastric mucosa, intussusception, or pressure necrosis[55-58].
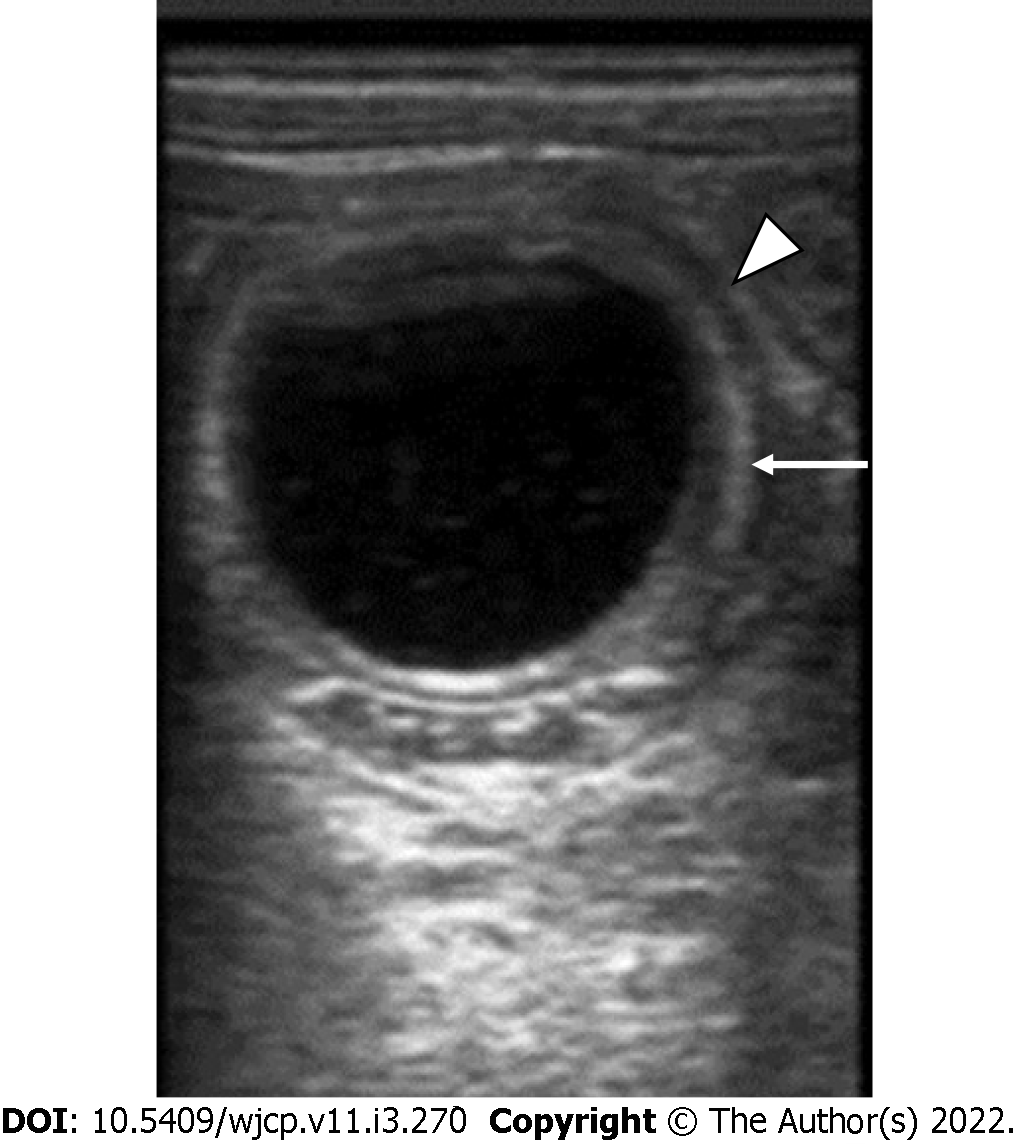
On USG, these are seen as well-defined anechoic lesions that demonstrate a classic gut signature (in about 50% of cases), i.e., mucosal internal echogenic layer and muscular outer hypoechoic layer (Figure 15). This appearance is usually interrupted, due to non-uniform thickness[56]. USG appearance may vary in cases with hemorrhage. Barium contrast studies although not routinely used, may demonstrate a sub-mucosal filling defect with a mass effect on the gastrointestinal tract or rarely communicating with it[56].
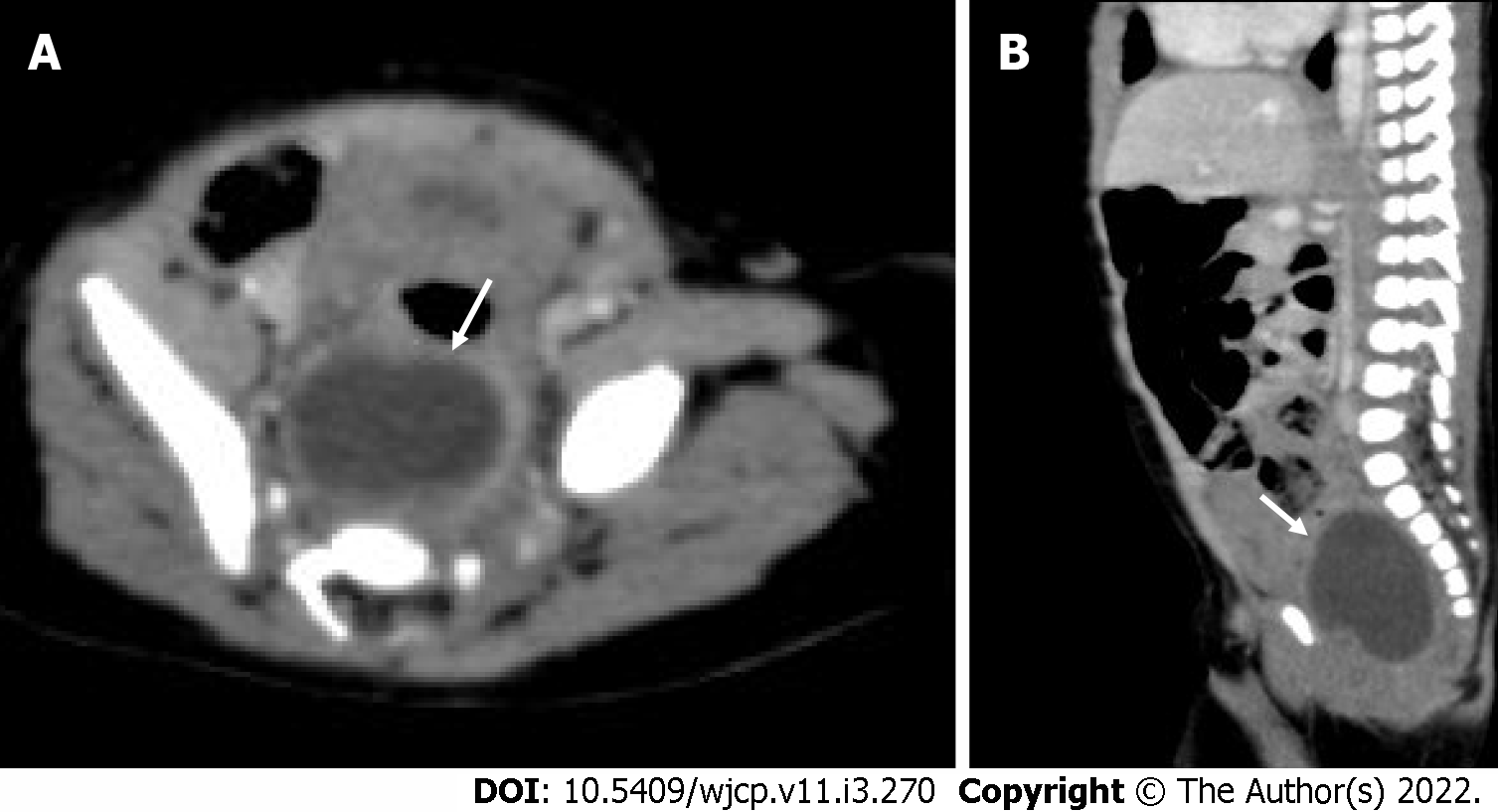
On CT, a duplication cyst is seen as a well-defined non-enhancing mass with cystic attenuation adjacent to the GI tract (Figure 16). However, the central attenuation may vary, depending on hemorrhage or proteinaceous material, which usually show higher central attenuation[56] MRI will show a well-defined cystic lesion with heterogeneous signal density on TI weighted image and T2 homogenous high signal intensity[55].
Gastrointestinal hemangiomas are benign vascular tumors. They are most commonly seen in pediatric and young adults where they present as GI bleeding in about 80% of cases and are a cause of life-threatening anemia. Most commonly are seen in the small bowel. They are also seen in the colon and rectum. They may be seen as part of Klippel-Trénaunay, Maffucci, blue rubber bleb nevus syndrome, and disseminated neonatal hemangiomatosis. Histologically, they can be of cavernous, capillary, and mixed type with the cavernous variety being the most common subtype[59-61].
Plain radiograph being the first routine investigation may show phleboliths (evident in 50% of cases) along the course of the bowel. Extensive phleboliths are rare in young and this gives a clue for further investigations[59, 60].
CT scan provides an intramural and extramural extension of the lesion. On CT, the involved bowel shows asymmetric bowel wall thickening with contrast enhancement. Phleboliths may or may not be seen[60] (Figure 17).
MRI shows rectal wall thickening, increased T2 signal intensity, and prominent perirectal serpiginous vascular channels. The perirectal vascular channels and atypical location help to differentiate rectal hemangiomas from hemorrhoids.
Intussusception refers to telescoping of a bowel segment (intussusceptum) into the distal segment (intussuscipiens). It is among the most common abdominal emergencies in the pediatric age group with most cases (approximately 80%) occurring between 6 mo to 2 years of age[62-64]. It is also one of the most common causes of small bowel obstruction in infants[63]. The clinical triad of acute abdominal pain, palpable abdominal mass, and currant jelly stools/hematochezia is noted only in 50% of patients[62]. Ileo-colic is the most common type, where an ileum segment invaginates across IC junction into the colon for variable length[64].
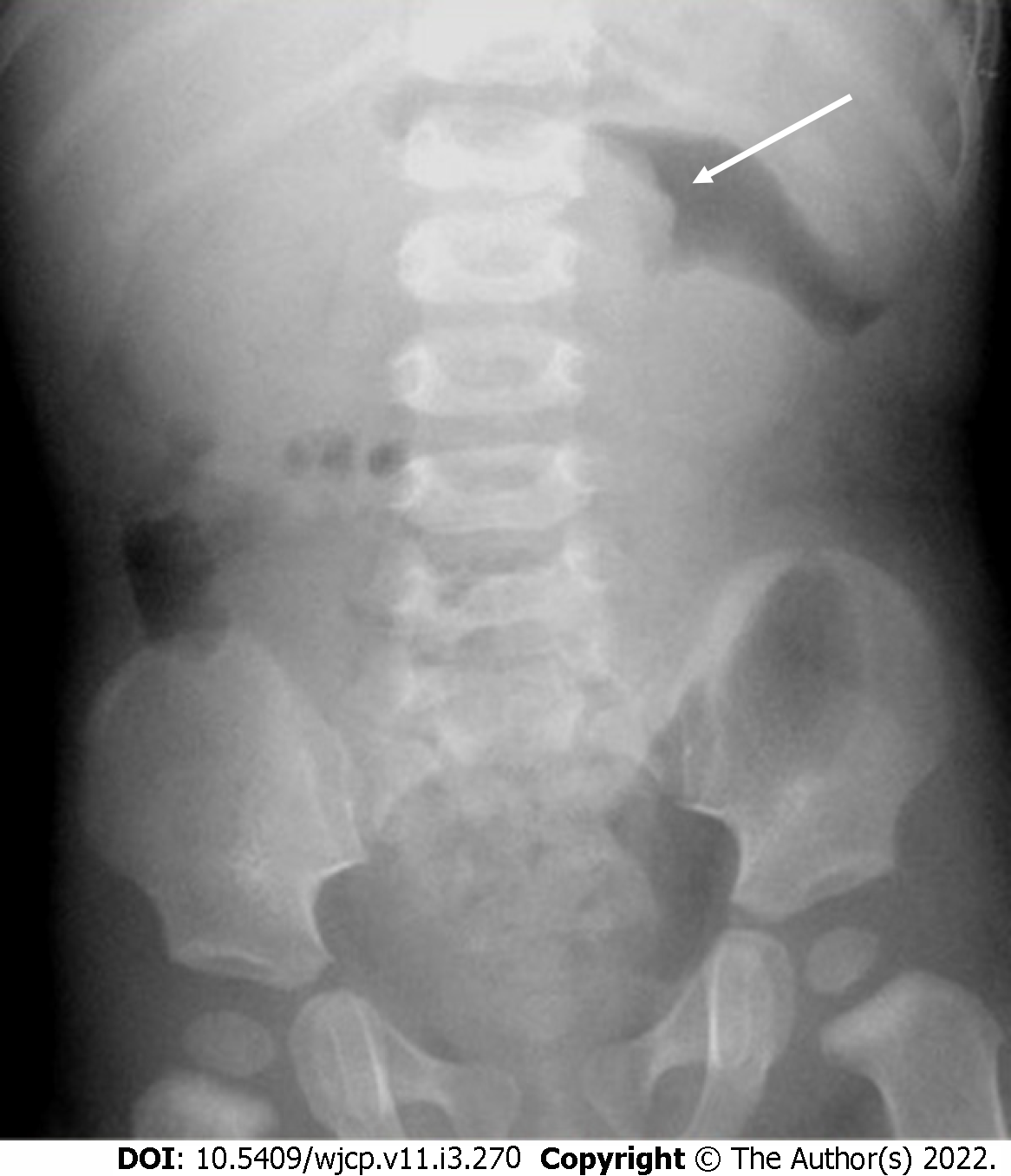
Many radiographic signs of intussusception have been described, which include soft tissue density mass in the right upper quadrant, gasless abdomen, small bowel obstruction, and meniscus sign (Figure 18). X-rays usually are the initial investigation and are primarily used to look for obstruction and perforation and to rule out any other causes of pain abdomen. On barium enema, the meniscus sign and the coiled spring sign are the classic signs explained in intussusception[62].
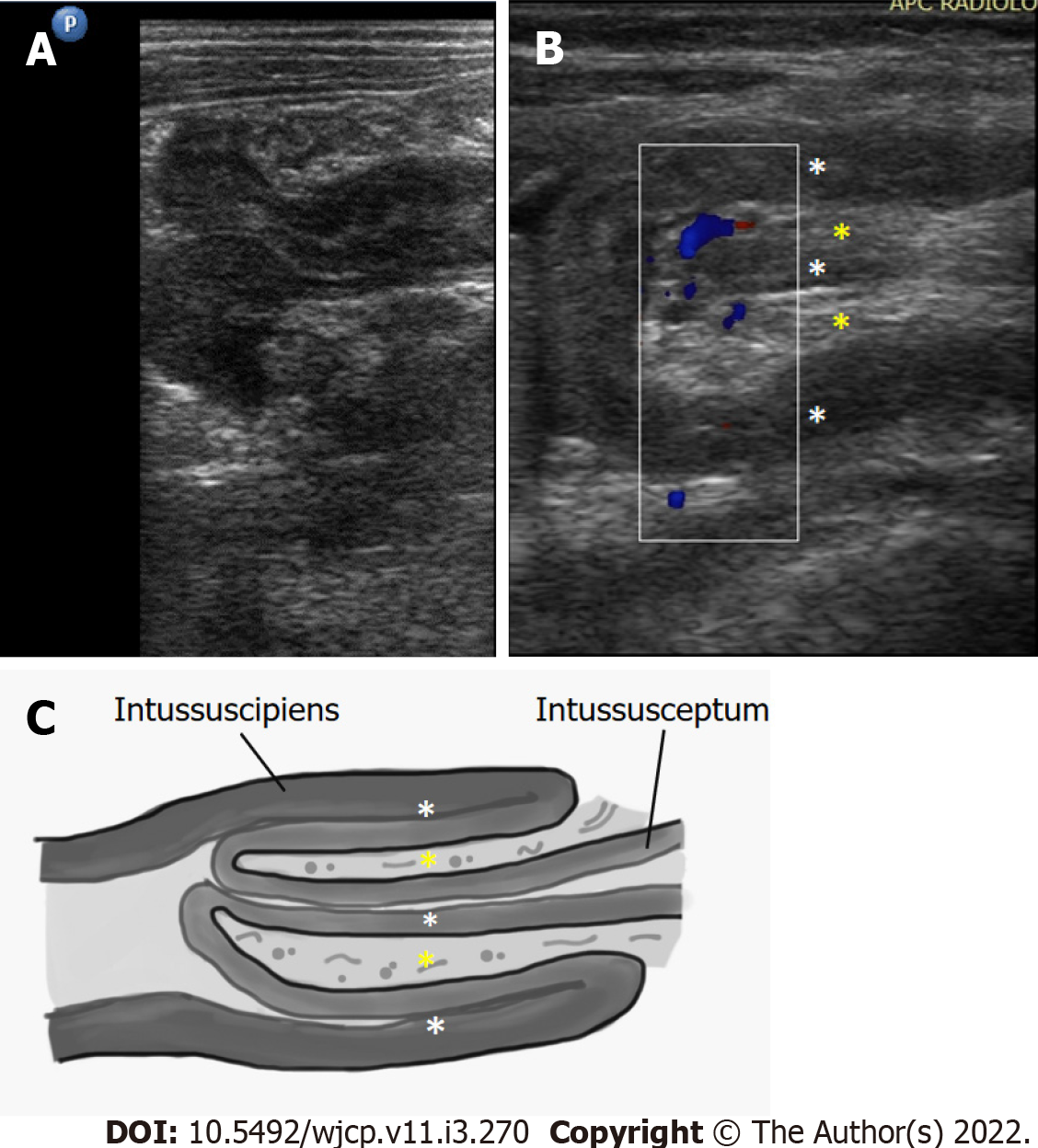
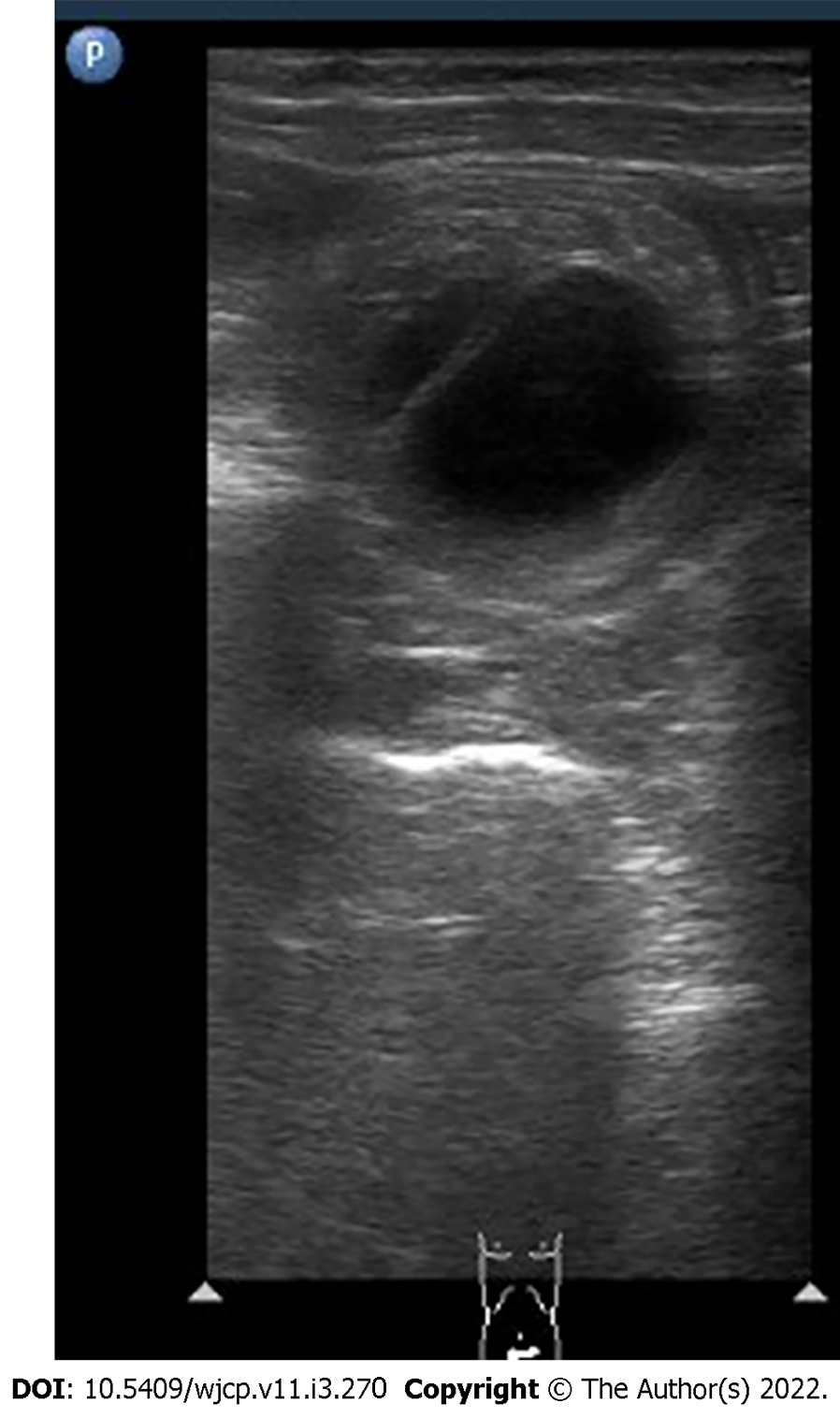
Ultrasound has a high sensitivity (98%-100%) and specificity (88%-100%) for diagnosis of intussusception. Multiple concentric ring sign and crescent in doughnut sign are seen on axial scans (Figure 19). On longitudinal scans, sandwich and hayfork signs are explained[65](Figure 20). The intussuscipiens contains the entering limb and the returning limb of the intussusceptum, along with the mesentery. This gives variable ultrasound features depending on the length of the involved segment.
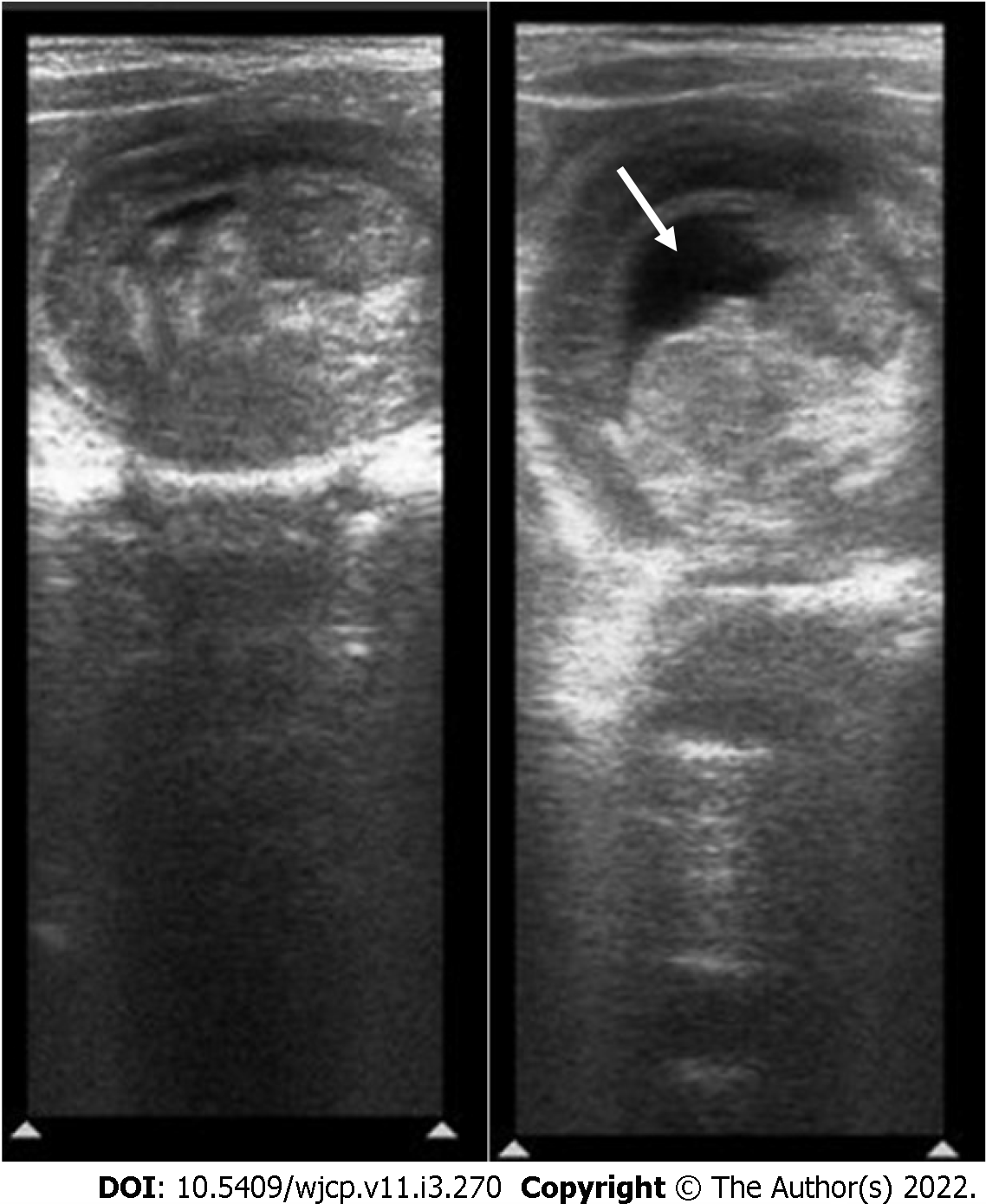
Most cases of intussusception are idiopathic. However, duplication cyst, polyps, tumor, or Meckel’s diverticulum can act as a lead point and are more common in neonates or older children. USG is very sensitive in picking up and characterization of lead points[62, 65](Figure 21).
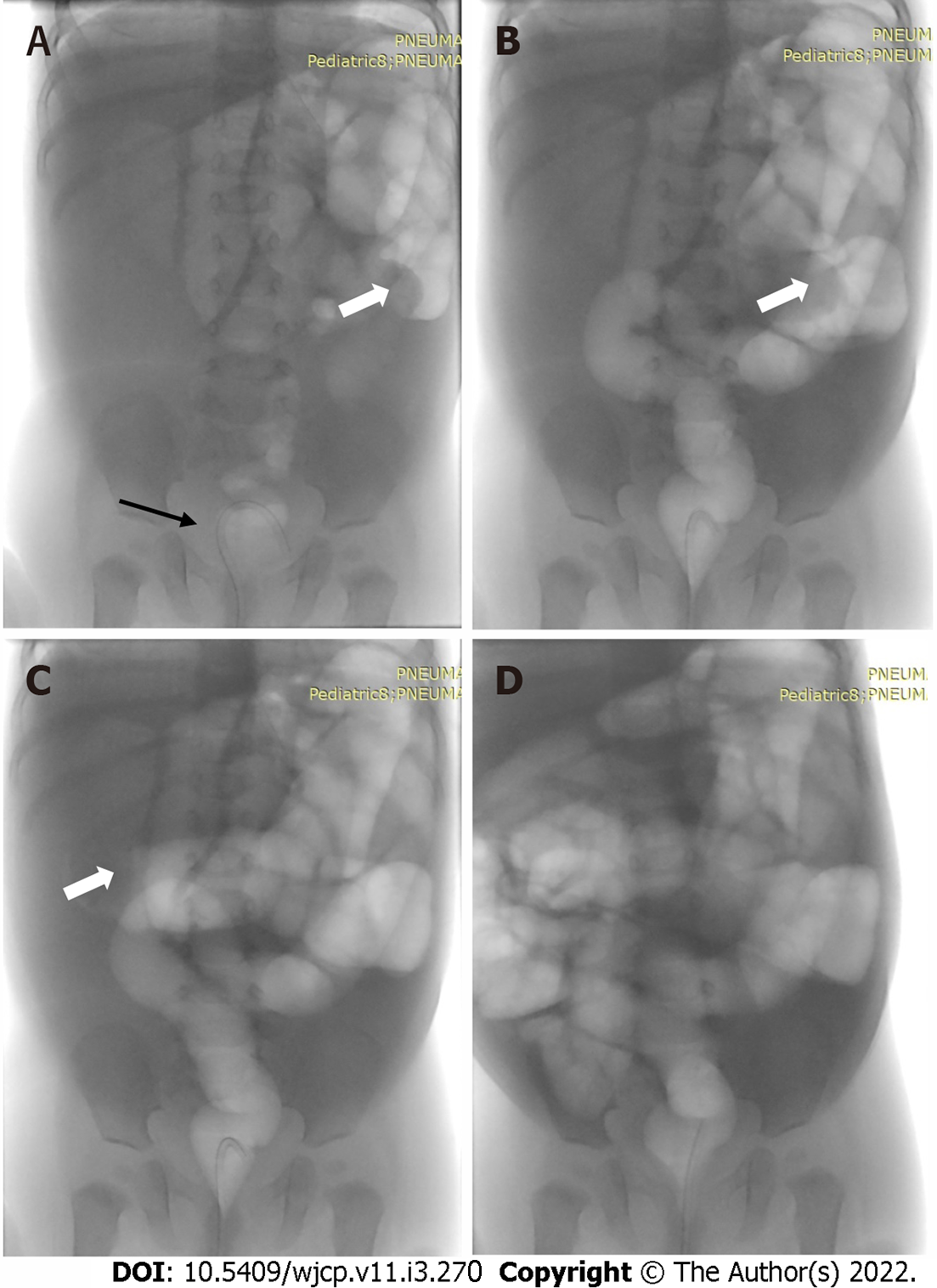
Under real time fluoroscopy, uncomplicated ileocolonic and colocolonic intussusceptions can be reduced using barium enema, water-soluble contrast agents, and pneumatic reduction[62] (Figure 22).
To conclude, bleeding per rectum in children can occur due to a variety of medical and surgical causes across the different age groups. The referring clinicians as well as the radiologists must be aware of the various radiological findings of common and uncommon causes of bleeding per rectum in children, which may require medical and surgical management at the time of presentation.
We thank Dr. Bhujade H, Assistant Professor, Department of Radiodiagnosis and Imaging, Postgraduate Institute of Medical Education and Research, Sector-12, Chandigarh, 160012, India for designing the diagrammatic image for Figure 20.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Govindarajan KK, India S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Qi WW
| 1. | Stoll BJ. Epidemiology of necrotizing enterocolitis. Clin Perinatol. 1994;21:205-218. [PubMed] |
| 2. | Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr. 1990;117:S6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 180] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Rowe MI, Reblock KK, Kurkchubasche AG, Healey PJ. Necrotizing enterocolitis in the extremely low birth weight infant. J Pediatr Surg. 1994;29:987-90; discussion 990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Martinez-Tallo E, Claure N, Bancalari E. Necrotizing enterocolitis in full-term or near-term infants: risk factors. Biol Neonate. 1997;71:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Bolisetty S, Lui K, Oei J, Wojtulewicz J. A regional study of underlying congenital diseases in term neonates with necrotizing enterocolitis. Acta Paediatr. 2000;89:1226-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Lee JS, Polin RA. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol. 2003;8:449-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Epelman M, Daneman A, Navarro OM, Morag I, Moore AM, Kim JH, Faingold R, Taylor G, Gerstle JT. Necrotizing Enterocolitis: Review of State-of-the-Art Imaging Findings with Pathologic Correlation. RadioGraphics March. 2007;27:285-305. [RCA] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Leonidas JC, Hall RT, Amoury RA. Critical evaluation of the roentgen signs of neonatal necrotizing enterocolitis. Ann Radiol (Paris). 1976;19:123-132. [PubMed] |
| 9. | Pochaczevsky R, Kassner EG. Necrotizing enterocolitis of infancy. Am J Roentgenol Radium Ther Nucl Med. 1971;113:283-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Robinson AE, Grossman H, Brumley GW. Pneumatosis intestinals in the neonate. Am J Roentgenol Radium TherNucl Med. 1974;120:333-341. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 11. | Bell RS, Graham CB, Stevenson JK. Roentgenologic and clinical manifestations of neonatal necrotizing enterocolitis. Experience with 43 cases. Am J Roentgenol Radium TherNucl Med. 1971;112:123-134. [RCA] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Kirks DR, O’Byrne SA. The value of the lateral abdominal roentgenogram in the diagnosis of neonatal hepatic portal venous gas (HPVG). Am J Roentgenol Radium TherNucl Med. 1974;122:153-158. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Buonomo C. The radiology of necrotizing enterocolitis. Radiologic clinics of North America. 1999;37:1187-1198. [RCA] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Faingold R, Daneman A, Tomlinson G, Babyn PS, Manson DE, Mohanta A, Moore AM, Hellmann J, Smith C, Gerstle T, Kim JH. Necrotizing enterocolitis: assessment of bowel viability with color doppler US. Radiology. 2005;235:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Kim WY, Kim WS, Kim IO, Kwon TH, Chang W, Lee EK. Sonographic evaluation of neonates with early-stage necrotizing enterocolitis. Pediatr Radiol. 2005;35:1056-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Levy AD, Hobbs CM. From the archives of the AFIP. Meckel diverticulum: radiologic features with pathologic Correlation. Radiographics. 2004;24:565-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Cullen JJ, Kelly KA, Moir CR, Hodge DO, Zinsmeister AR, Melton 3rd LJ. Surgical management of Meckel's diverticulum. An epidemiologic, population-based study. Annals of surgery. 1994;220:564. [RCA] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 205] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Sagar J, Kumar V, Shah DK. Meckel's diverticulum: a systematic review. J R Soc Med. 2006;99:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Cooney DR, Duszynski DO, Camboa E, Karp MP, Jewett TC Jr. The abdominal technetium scan (a decade of experience). J Pediatr Surg. 1982;17:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Sfakianakis GN, Conway JJ. Detection of ectopic gastric mucosa in Meckel's diverticulum and in other aberrations by scintigraphy: ii. indications and methods-a 10-year experience. J Nucl Med. 1981;22:732-738. [PubMed] |
| 21. | Paulsen SR, Huprich JE, Fletcher JG, Booya F, Young BM, Fidler JL, Johnson CD, Barlow JM, Earnest F 4th. CT enterography as a diagnostic tool in evaluating small bowel disorders: review of clinical experience with over 700 cases. Radiographics. 2006;26:641-57; discussion 657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 281] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 22. | Thakkar K, Fishman DS, Gilger MA. Colorectal polyps in childhood. Curr Opin Pediatr. 2012;24:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Thakkar K, Alsarraj A, Fong E, Holub JL, Gilger MA, El Serag HB. Prevalence of colorectal polyps in pediatric colonoscopy. Dig Dis Sci. 2012;57:1050-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Adolph VR, Bernabe K. Polyps in children. Clin Colon Rectal Surg. 2008;21:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Nugent KP, Talbot IC, Hodgson SV, Phillips RK. Solitary juvenile polyps: not a marker for subsequent malignancy. Gastroenterology. 1993;105:698-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Campbell AM, Sugarman I. Does painless rectal bleeding equate to a colonic polyp? Arch Dis Child. 2017;102:1049-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Lee BG, Shin SH, Lee YA, Wi JH, Lee YJ, Park JH. Juvenile polyp and colonoscopic polypectomy in childhood. Pediatr Gastroenterol Hepatol Nutr. 2012;15:250-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Laufer I, Levine MS. Principles of double contrast diagnosis. Double contrast gastrointestinal radiology, Philadelphia: Saunders, 1992: 9-54. [DOI] [Full Text] |
| 29. | Miller WT, Levine MS, Rubesin SE, Laufer I. Bowler-hat sign: a simple principle for differentiating polyps from diverticula. Radiology. 1989;173:615-617. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Vining DJ, Gelfand DW. Noninvasive colonoscopy using helical CT scanning, 3D reconstruction, and virtual reality. Presented at the 23rd Annual Meeting and Postgraduate Course of the Society of Gastrointestinal Radiologists. Maui HI. 1994;127:310-316. [DOI] [Full Text] |
| 31. | Luboldt W, Steiner P, Bauerfeind P, Pelkonen P, Debatin JF. Detection of mass lesions with MR colonography: preliminary report. Radiology. 1998;207:59-65. [RCA] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Lauenstein TC, Ajaj W, Kuehle CA, Goehde SC, Schlosser TW, Ruehm SG. Magnetic resonance colonography: comparison of contrast-enhanced three-dimensional vibe with two-dimensional FISP sequences: preliminary experience. Invest Radiol. 2005;40:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Mekhjian HS, Switz DM, Melnyk CS, Rankin GB, Brooks RK. Clinical features and natural history of Crohn's disease. Gastroenterology. 1979;77:898-906. [RCA] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 234] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Motil KJ, Grand RJ, Davis-Kraft L, Ferlic LL, Smith EO. Growth failure in children with inflammatory bowel disease: a prospective study. Gastroenterology. 1993;105:681-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 190] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Fraquelli M, Colli A, Casazza G, Paggi S, Colucci A, Massironi S, Duca P, Conte D. Role of US in detection of Crohn disease: meta-analysis. Radiology. 2005;236:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 36. | Booya F, Fletcher JG, Huprich JE, Barlow JM, Johnson CD, Fidler JL, Solem CA, Sandborn WJ, Loftus EV Jr, Harmsen WS. Active Crohn Disease: CT Findings and Interobserver Agreement for Enteric Phase CT Enterography. Radiology. 2006;241:787-795. [RCA] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Choi D, Jin Lee S, Ah Cho Y, Lim HK, Hoon Kim S, Jae Lee W, Hoon Lim J, Park H, Rae Lee Y. Bowel wall thickening in patients with Crohn's disease: CT patterns and correlation with inflammatory activity. Clin Radiol. 2003;58:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Goldberg HI, Gore RM, Margulis AR, Moss AA, Baker EL. Computed tomography in the evaluation of Crohn disease. AJR Am J Roentgenol. 1983;140:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 98] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Wallihan DB, Towbin AJ, Denson LA, Salisbury S, Podberesky DJ. Inflammatory bowel disease in children and adolescents: assessing the diagnostic performance and interreader agreement of magnetic resonance enterography compared to histopathology. Acad Radiol. 2012;19:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Lee SS, Ha HK, Yang SK, Kim AY, Kim TK, Kim PN, Lee MG, Myung SJ, Jung HY, Kim JH, Min YI. CT of prominent pericolic or perienteric vasculature in patients with Crohn's disease: correlation with clinical disease activity and findings on barium studies. AJR Am J Roentgenol. 2002;179:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Bodily KD, Fletcher JG, Solem CA, Johnson CD, Fidler JL, Barlow JM, Bruesewitz MR, McCollough CH, Sandborn WJ, Loftus EV Jr, Harmsen WS, Crownhart BS. Crohn Disease: mural attenuation and thickness at contrast-enhanced CT Enterography--correlation with endoscopic and histologic findings of inflammation. Radiology. 2006;238:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 42. | Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn's disease. Acad Radiol. 2009;16:597-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 43. | Koelbel G, Schmiedl U, Majer MC, Weber P, Jenss H, Kueper K, Hess CF. Diagnosis of fistulae and sinus tracts in patients with Crohn disease: value of MR imaging. AJR Am J Roentgenol. 1989;152:999-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Essary B, Kim J, Anupindi S, Katz JA, Nimkin K. Pelvic MRI in children with Crohn disease and suspected perianal involvement. Pediatr Radiol. 2007;37:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Dunn EA, Olsen ØE, Huisman TA. The Pediatric Gastrointestinal Tract: What Every Radiologist Needs to Know. In Diseases of the Abdomen and Pelvis Springer, Cham, 2018: 157-166. [DOI] [Full Text] |
| 46. | Shimanuki Y, Aihara T, Takano H, Moritani T, Oguma E, Kuroki H, Shibata A, Nozawa K, Ohkawara K, Hirata A, Imaizumi S. Clockwise whirlpool sign at color Doppler US: an objective and definite sign of midgut volvulus. Radiology. 1996;199:261-264. [RCA] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 105] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Peterson CM, Anderson JS, Hara AK, Carenza JW, Menias CO. Volvulus of the gastrointestinal tract: appearances at multimodality imaging. Radiographics. 2009;29:1281-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 48. | Applegate KE, Anderson JM, Klatte EC. Intestinal malrotation in children: a problem-solving approach to the upper gastrointestinal series. Radiographics. 2006;26:1485-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 49. | Fisher JK. Computed tomographic diagnosis of volvulus in intestinal malrotation. Radiology. 1981;140:145-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 157] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Arora A, Sarin SK. Multimodality imaging of primary extrahepatic portal vein obstruction (EHPVO): what every radiologist should know. Br J Radiol. 2015;88:20150008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Weiss B, Shteyer E, Vivante A, Berkowitz D, Reif S, Weizman Z, Bujanover Y, Shapiro R. Etiology and long-term outcome of extrahepatic portal vein obstruction in children. World J Gastro 2010; 16: 4968. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Pargewar SS, Desai SN, Rajesh S, Singh VP, Arora A, Mukund A. Imaging and radiological interventions in extra-hepatic portal vein obstruction. World J Radiol. 2016;8:556-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Khanna R, Sarin SK. Non-cirrhotic portal hypertension-diagnosis and management. J hepat. 2014;60:421-441. [RCA] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 262] [Article Influence: 23.8] [Reference Citation Analysis (2)] |
| 54. | Prasad GR, Billa S, Bhandari P, Hussain A. Isolated inferior mesenteric portal hypertension with giant inferior mesenteric vein and anomalous inferior mesenteric vein insertion. Journal of Indian Association of Pediatric Surgeons. 2013;18:84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Liu R, Adler DG. Duplication cysts: Diagnosis, management, and the role of endoscopic ultrasound. Endosc Ultrasound. 2014;3:152-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 56. | Tong SC, Pitman M, Anupindi SA. Best cases from the AFIP. Ileocecal enteric duplication cyst: radiologic-pathologic correlation. Radiographics. 2002;22:1217-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Sharma S, Yadav AK, Mandal AK, Zaheer S, Yadav DK, Samie A. Enteric duplication cysts in children: A clinicopathological dilemma. J Clin Diagnostic Res. 2015;9:EC08-EC11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Flynn-O'Brien KT, Rice-Townsend S, Ledbetter DJ. Structural anomalies of the gastrointestinal tract. In: Avery's Diseases of the Newborn Elsevier, Pjiladelphia, PA, 2018: 1039-1053. [DOI] [Full Text] |
| 59. | Dachman H, Buck L, Shekitka KM, Olmsted W, Hinton CB. Colorectal Hemangloma: Radiologic Findings. Radiology. 1988;167:31-34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Yoo S. GI-Associated Hemangiomas and Vascular Malformations. Clin Colon Rectal Surg. 2011;24:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Han EC, Kim SH, Kim HY, Jung SE, Park KW. Gastrointestinal hemangioma in childhood: a rare cause of gastrointestinal bleeding. Korean J Pediatr. 2014;57:245-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | del-Pozo G, Albillos JC, Tejedor D, Calero R, Rasero M, de-la-Calle U, López-Pacheco U. Intussusception in children: current concepts in diagnosis and enema reduction. Radiographics. 1999;19:299-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 150] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception: a literature review. PLoS One. 2013;8:e68482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 64. | Lioubashevsky N, Hiller N, Rozovsky K, Segev L, Simanovsky N. Ileocolic vs small-bowel intussusception in children: can US enable reliable differentiation? Radiology. 2013;269:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 65. | Verschelden P, Filiatrault D, Garel L, Grignon A, Perreault G, Boisvert J, Dubois J. Intussusception in children: reliability of US in diagnosis--a prospective study. Radiology. 1992;184:741-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 132] [Article Influence: 4.0] [Reference Citation Analysis (0)] |