Published online Mar 9, 2022. doi: 10.5409/wjcp.v11.i2.151
Peer-review started: April 13, 2021
First decision: July 27, 2021
Revised: August 2, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: March 9, 2022
Processing time: 329 Days and 14.2 Hours
Anaphylaxis is a life-threatening condition that develops as a reaction to exposure to an allergen which can be found in common foods such as cow's milk, egg, fish, and nuts in children. The use of an intramuscular adrenaline auto-injector (AAI) is considered the most essential treatment in these situations and parents and caregivers are always encouraged to carry this device for use in an emergency which commonly takes place in public places such as restaurants, schools, and parks, where medical staff are not guaranteed to be available. However, previous studies, in different settings, have reported underuse of the AAI by parents.
To explore the reasons for underutilisation of the AAI in our community.
A cohort of parents attending the paediatric allergy clinic at Al Ain Hospital in the United Arab Emirates completed a questionnaire survey aimed at assessing their understanding and knowledge of their child's allergy management, including their aptitude with the use of the AAI, as well as their competence and comfort in providing this treatment in an emergency.
Of 47 parents participating in the study, 39 were Emirati parents (83% and most parents who completed the survey were mothers (66%). As expected, food was the main cause of allergic reactions requiring prescription of the auto-injector device. Tree nuts and peanuts were noted to be the most common offending food in these children (62% and 38%, respectively). A doctor provided demonstrations and training on using the auto-injector device to 94% of the parents. More than two-thirds of the parents and caregivers (79%) were deemed knowledgeable on the indication for use of the device. Reluctance to administer the device was expressed by many of the parents, despite their satisfaction with the coaching they received on using the device in the study.
Ongoing coaching and teaching of parents on use of the AAI is paramount. However, this should be carried out together with psychological support to aid the parents to eliminate their hesitancy and acquire sufficient confidence in using the device when needed. Group teaching and sharing experiences is an excellent educational technique in a non-formal setting. Paediatric clinic play therapists can also have a role in needle phobia desensitisation for parents and children. More research is needed to explore the lack of empowerment and other reasons behind their fear and anxiety in using the device to plan effective interventions.
Core Tip: This is a retrospective study evaluating parents’ knowledge of the indications and use of adrenaline auto-injectors in children with anaphylaxis. The state of mind of parents towards the use of the device during anaphylactic episodes in terms of stress, anxiety, comfort, and confidence with the use of the adrenaline auto-injector (AAI) were also evaluated. The study concluded that training and education on how to use the AAI are important, but the psychological status of these parents should not be overlooked, and that sufficient psychological support should be provided in order to assist them to overcome stress and anxiety.
- Citation: Narchi H, Elghoudi A, Al Dhaheri K. Barriers and challenges affecting parents’ use of adrenaline auto-injector in children with anaphylaxis. World J Clin Pediatr 2022; 11(2): 151-159
- URL: https://www.wjgnet.com/2219-2808/full/v11/i2/151.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v11.i2.151
Anaphylaxis is a potentially severe and deadly condition that affects multiple body systems with a rapid onset allergic reaction[1]. Adrenaline is the only and immediate choice of drug in the community and the healthcare system, considered for all ages. Moreover, in children, the leading cause of anaphylaxis is food allergy that commonly occurs in public settings such as shopping centres, public parks, restaurants, and schools by triggering food allergens such as peanuts, tree nuts, shrimp, fish, sesame, cow's milk, and egg. In addition, the main food allergens may vary according to ethnicity. For patients with other underlying allergic conditions such as asthma, the risk of anaphylaxis can be higher. Age is also considered a risk factor, with teenagers being the highest risk group. Within our local community in Al Ain city, the prevalence of food allergy has been reported to be up to 8% in children, mainly caused by fish, fruit, and egg[2]. Additionally, information from a systematic review shows that in children, the incidence of food-induced anaphylaxis globally ranges between 1-77 per 100000 people and about 1-761 per 100000 people may face anaphylaxis from any cause[3].
Following the effective and efficient diagnosis and treatment of the initial food allergic reaction, children with anaphylaxis are mostly referred to their local child allergy service, where age-appropriate advice is delivered to these children and their parents. The allergy action plan focuses on avoiding the offending food and promptly administering the adrenaline auto-injector (AAI) when and as needed. Food remains the main trigger of anaphylaxis in public places[4]. To overcome this condition, it is mandatory for parents of children with food allergies, caregivers, and school nurses to remain vigilant and detect and treat food-induced anaphylaxis in children using the AAI. Potential or manifested anaphylaxis with hives along with respiratory, cardiovascular symptoms or neurological symptoms such as a reduced level of consciousness, is ideally treated by lying the patient flat and raising the legs to help restore the circulation along with immediate administration of the AAI into the mid outer thigh with the preferred route being an intramuscular injection[4]. According to the European Academy of Allergy and Clinical Immunology (EAACI), the prescribing indications for AAI are either relative or absolute indications. The relative signs include previous mild or moderate reactions to traces of food, mild or moderate reaction to a food with asthma, medical help remoteness, and teenagers as a risk-taker group. The absolute indications exclusively include allergy to venom in adults, food allergy with moderate to severe asthma, a disorder of mast cells with high baseline serum tryptase, previous idiopathic anaphylaxis, previous exercise-induced anaphylaxis, and previous anaphylaxis to food allergens[4]. Our practice is that we usually prescribe two AAI devices, one for school and one for the home. In some exceptional circumstances, the number may change; for example, as per family conditions such as divorce or separation where the child may stay and spend a long time in two different places; or obesity as in these situations the child sometimes needs more than two AAI devices. At each clinic visit, the parents are provided with repeated technical and medical information and a demonstration on use of the AAI device.
We always prescribe AAI to all the children who need it; it was observed that the parents sometimes do not pay attention to the expiry date and renewal of their children's AAI[5]. Moreover, in an actual anaphylaxis situation, some do not appreciate the urgent need for AAI administration. Instead, they prefer to rush to the nearest emergency department. The delay in providing lifesaving treatment may potentially result in life-threatening events. Some do not carry the device all the time, and they do not even know how to use it properly[6]. According to recent studies, mortality has been linked with anaphylaxis cases; it was primarily related to AAI delay, underuse, and faulty administration techniques[7], and more than 50% of anaphylaxis patients did not receive the required amount of AAI.
However, the findings of previous reports from other populations with different traditions, nationalities, and cultures are different to our current population. As a result, we decided to explore our local population's current practice and compliance in Al Ain city using the AAI to treat children with anaphylaxis.
A previous clinic audit revealed that, despite prior training, approximately 3% of parents remained unsure how and when to use the AAI. Therefore, we calculated that a representative sample size of 45 families in our clinic population would be required to give the study an adequate power of 80% with 5% error and 95% confidence. In addition, groups were reported as percentages and numbers, and to quantify the qualitative information, a Likert scale was used. The statistical analysis also compared the univariate association between the outcomes of interest and the explanatory variables using ANOVA (analysis of variance) for more than three groups and an unpaired Students t-test for two variables. Moreover, an ordered logistic model tested the association between explanatory variables and outcomes while correcting for potential cofounders. Therefore, the statistical analysis was performed with the two-tailed P value < 0.05 as significant and STATA 15.0 software (StataCorp, Texas, United States).
The results were obtained after conducting 47 questionnaires that were mainly (83%, n=39) completed by Emirati families, with mothers accounting for 66% of the respondents. Furthermore, after performing the analysis, it was found that (45%, n = 21) of the children experienced anaphylaxis. 89% of the children prescribed with the AAI were under five (Table 1). On answering the question of their willingness to use the AAI in unintentional allergic response causing anaphylaxis, 79% (n = 37) declared that they would use the AAI. In the questions intended to assess knowledge of the AAI indication, approximately 19% of the respondents stated that they would use the AAI device in a situation of rashes that affected the lips and caused swelling. About 2% of the respondents admitted a complete lack of knowledge of AAI usage. The allergy doctors trained 94% of the parents, and 36% of the parents felt confident and competent to use the AAI after experiencing AAI usage at least once in an actual situation. Most of the children were prescribed two AAI devices, one for school and one for the home.
| Informant | ||
| Father | 16 (34) | |
| Mother | 31 (66) | |
| Child’s nationality | ||
| Emirati | 39 (83) | |
| Foreign | 8 (17) | |
| Child’s age group | ||
| < 5 yr | 21 (45) | |
| 5-10 yr | 20 (42) | |
| > 10 yr | 6 (13) | |
| Child’s sex | ||
| Male | 25 (53) | |
| Female | 22 (47) | |
| Indication for AAI | ||
| Food allergy | 42 (89) | |
| Idiopathic anaphylaxis | 2 (4) | |
| Insect/venom-induced allergy | 3 (7) | |
| Number of AAI prescribed | ||
| 1 | 10 (21) | |
| 2 | 33 (70) | |
| 3 | 3 (7) | |
| 4 | 1 (2) | |
| Parents’ awareness of when to use the AAI | ||
| Rash with breathing difficulty | 37 (79) | |
| Rash with swollen lips | 9 (19) | |
| Unsure | 1 (2) | |
| Training provided by | ||
| Doctor | 44 (94) | |
| Nurse | 1 (2) | |
| Pharmacist | 1 (2) | |
| Do not remember | 1 (2) | |
| Has used an AAI before | 17 (36) | |
As shown in Table 2, the most common food allergens reported were tree nuts (62%) followed by peanuts (38.5%). The data showed that sesame was a typical offending food which was not the case in the region previously.
| Allergen | n | % |
| Tree nuts | 29 | 62 |
| Peanuts | 18 | 38.5 |
| Egg | 9 | 19 |
| Cow’s milk | 8 | 17 |
| Sesame | 7 | 15 |
| Shrimp | 4 | 8.5 |
| Strawberry | 4 | 8.5 |
| Wheat | 3 | 6.5 |
| Lentil | 3 | 6.5 |
| Others | 11 | 23 |
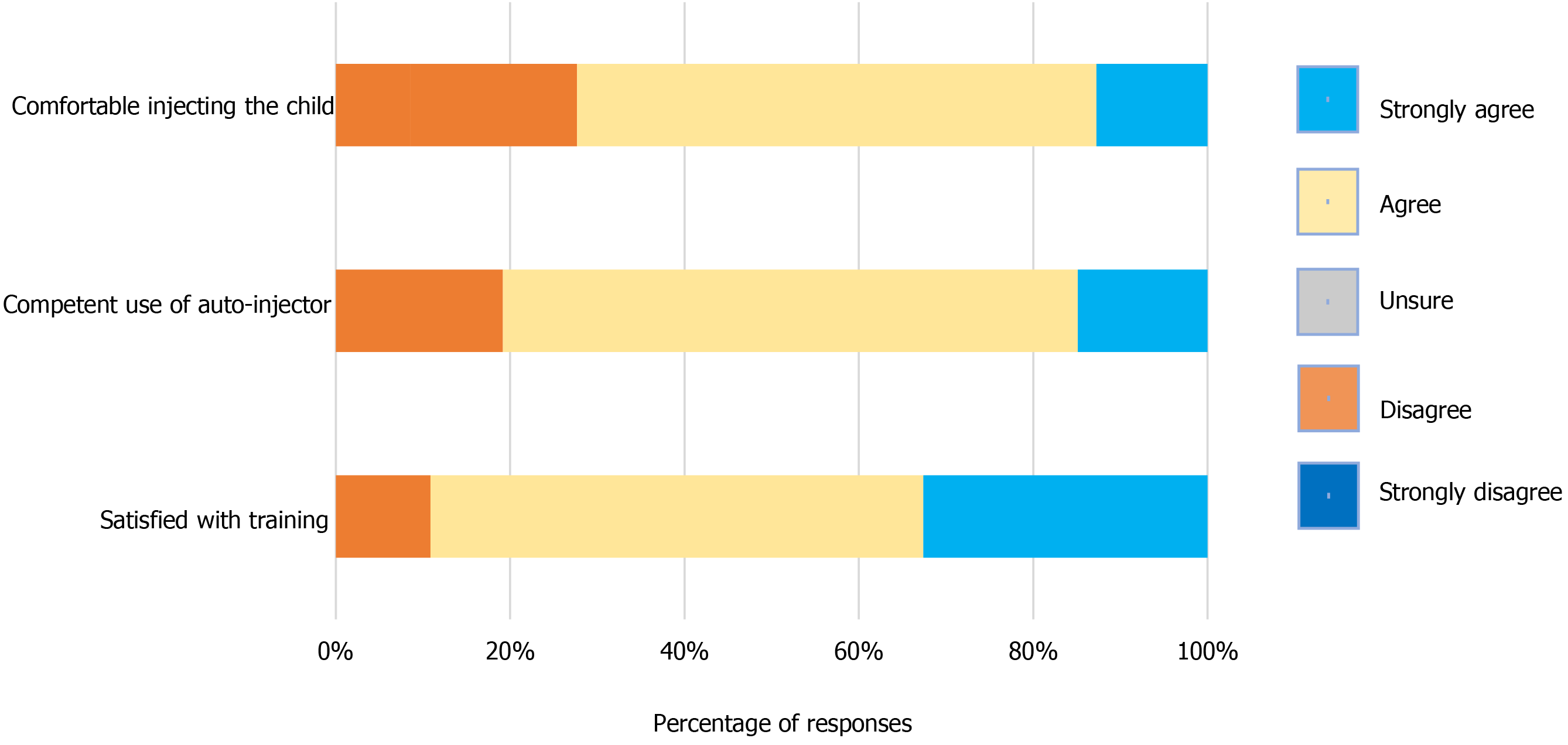
Figure 1 and Table 3 detail parental perceptions and insights of their satisfaction, capability, and comfort with the training sessions on use of the AAI device. About 72% of the respondents strongly admitted being comfortable in using the AAI device, and 59.6% moderately agreed. In comparison, 12.8% felt neutral, 19% were unsure about usage, and 8.5% did not show interest in using the device. However, approximately 80.8% of the respondents demonstrated their overall competency in using the AAI device, 14.9% showed competency, while 66% were moderately competent, and 19% were unsure about their competence. Other results showed that 89% of the respondents were satisfied with the AAI device training, about 55% were moderately satisfied, and 31.9% were delighted. However, 10.6% were not satisfied with the training received and usage, and only 2% were unsatisfied.
| Satisfaction with the training received | Competency in using the AAI | Comfortable and not scared to use the AAI | ||
| Child’s gender | Male | 3.3 ± 1.0 | 3.5 ± 0.6 | 3.5 ± 0.7 |
| Female | 3.2 ± 1.1 | 3.7 ± 0.8 | 3.3 ± 0.9 | |
| P value1 | 0.7 | 0.2 | 0.5 | |
| Age group (yr) | < 5 | 3.3 ± 1.1 | 3.6 ± 0.7 | 3.5 ± 0.8 |
| 5-10 | 3.3 ± 1.0 | 3.7 ± 0.8 | 3.4 ± 0.8 | |
| > 10 | 2.8 ± 1.1 | 3.6 ± 0.8 | 3.1 ± 0.9 | |
| P value2 | 0.6 | 0.8 | 0.7 | |
| Diagnosis | Food allergy | 3.2 ± 1.1 | 3.6 ± 0.7 | 3.4 ± 0.8 |
| Idiopathic anaphylaxis | 2.5 ± 0.7 | 3.0 ± 0 | 2.5 ± 0.7 | |
| Insect/venom induced allergy | 3.6 ± 0.6 | 3.6 ± 0.6 | 3.3 ± 0.6 | |
| P value2 | 0.5 | 0.4 | 0.2 |
Therefore, no significant difference was found after performing the univariate analysis, with minor differences in using the AAI device by parents and their level of satisfaction while training, their level of competency in its use, and the comparison between the past and the current usage of the device. The relationship between the training by non-physicians and physician trainers is shown in Table 3.
However, in the ordered logistic regression model, adjusting for potential cofounders, the baseline characteristics associated with the parental comfort level for use of the AAI were evaluated and only their previous use of AAI was significantly associated with their competency in using the device (Table 4).
| Satisfaction with the training received | Competence in using the AAI | Comfortable and not scared to use the AAI | |
| Informant | 0.7 (-5.6,2.1); 0.2 | -0.9 (-2.2, 0.4); 0.2 | -0.4 (-1.7, 0.8); 0.4 |
| Nationality | -0.5 (-2.1, 1.0); 0.5 | 0.5 (-1.1, 2.1); 0.5 | -0.1 (-1.6, 1.3); 0.8 |
| Age group | -0.5 (-1.4, 0.4), 0.3 | 0.1 (-0.8, 1.1); 0.7 | -0.3 (-1.2, 0.6); 0.5 |
| Gender | -0.2 ((-1.4, 1.0); 0.7 | -0.2 (-1.4, 1.0); 0.7 | 0.7 (-0.4, 1.9); 0.2 |
| Diagnosis | 0.2 (-0.9, 1.4); 0.6 | -0.4 (-1.5, 0.8); 0.5 | -0.4 (-1.6, 0.7); 0.5 |
| Training provider | 0.4 (-2.2, 3.0); 0.7 | -16.4 (-4320, 4287); 0.9 | -1.2 (-4.0, 1.5); 0.3 |
| Has used AAI before | 0.3 (-0.9, 1.6); 0.6 | 1.6 (0.2, 2.9); 0.02 | 0.1 (-1.1, 1.4); 0.8 |
The present study highlights the risk of anaphylaxis and its factors, which significantly influence the parental usage of AAI devices in the local population in Al Ain city, UAE.
Cases of anaphylaxis are commonly triggered in children by food allergens such as nuts. Furthermore, in local studies, about 15% of anaphylaxis cases (unreported previously) were induced by sesame[2], an uncommon food allergen in the Middle East and North Africa region as it is widely used and almost all children would have been exposed to it or its oil very early in life.
Our results show that the parents' comfort level and self-perceived practical capability in using the AAI were less than expected; even with parents' education and training sessions, we observed persistent doubts in administration of the AAI. Moreover, regardless of training from the allergy specialist, and as the majority expressed insufficient awareness on when to administer, which probably reflected the level of anxiety in thinking any allergic reaction is anaphylaxis, or it could have been due to inadequate information provided when the AAI was prescribed.
The number of parents who admitted being uncomfortable or unsure about using the AAI is concerning and may harm their use of the device when needed. According to the findings of a previous study, a similar number of parents of children aged from 0 to 18 years experiencing anaphylaxis and food allergies used an AAI device. Furthermore, 75% of participants displayed proper technique and abilities for using the auto-injector device[9].
Considering the anxiety and psychological effect of having a child with a food allergy, and as many of the respondents were mothers, it is possible that the psychological effect of anxiety and stress encountered by these mothers affected mental function reflected in the lack of confidence in using the AAI despite satisfaction with the training received. The effect of having a child with a food allergy and potential anaphylaxis in limiting the mental capacity in making sound decisions regarding the child's care has been well documented in the literature[10], and it may even outweigh the stress associated with having a child experiencing diabetes mellitus type I and other chronic diseases[9].
The highlighted difficulties associated with training demonstrate that more effort is needed to sufficiently train parents in anaphylaxis management of their children by providing them with a quality-training session in a calm and friendly atmosphere aided by audio-visual and tactile experiences using the trainer AAI device in the training sessions. Although parents did not admit to suffering undefined psychosomatic factors undermining their use of the device, it has been shown that it is not information or earlier anaphylaxis experience that increases the ease of parents in using the AAI. We deduce that authorisation together with continuous education on the use of the device and preventing anxiety correlate with parents' level of comfort[10]. Therefore, to address these issues, caregivers and parents of these children should be encouraged to practice with an outdated AAI on objects such as an orange, apple, and cardboard instead of discarding them as this type of practice may help improve their level of confidence.
The variety and number of psychological stresses that parents face appear to have been underestimated as they influence the success of any parental empowerment and training, and must be addressed by providing psychological support[11,12]. Group meetings of these parents would provide an opportunity to share experiences of fear, worries, and anxieties using the AAI, which might be beneficial in overcoming the psychological barrier[11].
Our study included a self-administered questionnaire, which could have been skewed by parental recall bias or a desire to satisfy the treating physician. A face-to-face interview with the help of a simulation manikin might have overcome these restrictions and would provide a more objective assessment of the parents' confidence and technical ability than just recalling or interpreting occurrences. Our standard practice is to use an AAI trainer to educate and review previous knowledge and skills in using the device at every clinic visit. We delayed the training until parents completed the surveys to reduce bias.
The single-centre and small sample size are other limitations, which majorly prevented the generalizability of the findings to our local community in our city or the UAE. More extensive multicentre research involving other local paediatric clinics would have been beneficial to address such constraints.
Qualitative research, including face-to-face interviews, will be required to better understand the parents' attitudes and beliefs in controlling allergic reactions in their children. The goal would be to elicit, document, and analyse their reactions, opinions, and sentiments concerning the psychological impact of their child's allergy on them and their mental process. Clinicians and psychologists might use this knowledge to design suitable treatments to aid the family.
There is a requirement to advance and recover the psychological barrier and consolidate educational provision for parents whose children have an anaphylaxis risk. Furthermore, AAI trainer devices must be present in schools and the home for periodical practice, which helps to generate and maintain their confidence. The AAI trainer devices are helpful as a visual and tactual tool to overcome the fear of the lack of skills in treating anaphylaxis and ideally should be made available to all parents. In addition, it is practical to use outdated AAI devices on objects such as oranges, apples, and so on. Group teaching and sharing experiences are an excellent way to enhance learning and reduce stress. Psychology input unfortunately remains a luxury and is unavailable in most health providers. Play therapists could also have a role as they are capable of explaining needle phobia desensitisation to children and their parents.
Food allergy is common in the paediatric age group and food allergic reactions commonly occur in the community. The adrenaline auto-injector (AAI), issued to groups of children at risk of anaphylaxis, remains the first and only drug of choice for treating anaphylaxis. However, data from different parts of the world demonstrate that AAI is underused by parents and caregivers. The rationale behind this attitude is multilateral and could be attributed to issues such as poor training on the use of the auto-injector device, not understanding when it should be used, and both fear and anxiety of using it.
From our daily observations in the local paediatric allergy clinic, we found many cases of anaphylaxis that occurred at home due to the ingestion of offending food, and parents opted to call 999 or bring the child to the emergency department rather than using the AAI prescribed at the scene. Obviously, underuse of the AAI can put the affected child at risk of severe morbidity or mortality. In every clinic, we reviewed the indication for use of the AAI by parents and provided a visual demonstration on how to use it.
To study the attitude of parents of children at risk of anaphylaxis with regard to the use of AAI in an attempt to identify what prevents these parents from using the AAI when needed. The results of this research would help professionals to be more focused on certain issues when providing counselling and training on the use of the AAI to this cohort of parents.
Parents of children with previous or potential anaphylaxis who have been issued with an AAI were requested to complete a paper questionnaire on their understanding of the indications for use of the AAI, competence in using the device, confidence and empowerment in using it in stressful emergency situations.
The vast majority of parents admitted receiving good and informative training on using the device and demonstrated good knowledge on its indications. However, that was not enough to provide them with the confidence and courage to use the device due to other factors such as anxiety, fear, or not wanting to hurt the child with the AAI needle. Psychological uneasiness in using the device can limit parents’ ability to use it.
In addition to routine training in these groups of parents on the indication of using the AAI and the technique on how to use it, health professionals need to pay attention to the psychological factor which could prevent these parents from underusing the device when needed. Psychological, behavioural therapy and needle phobia desensitisation would help to overcome the barriers of phobia and anxiety which could interfere with sound decision-making in the treatment of their children in emergency situations.
Parent training on the use of AAI should be structured and focused. Audio-visual tools should be available in the clinic to help with training. However, the fear factor and the psychological status of these parents should not be overlooked. A routine referral or referral of selected cases to the local psychological service should be accessible to these parents. Play therapists can also have an important role in both children and parents by reducing needle phobia when present.
We are indebted to the parents who participated in the study by sharing their experiences and feelings.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: United Arab Emirates
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nag DS S-Editor: Wang LL L-Editor: Webster JR P-Editor: Wang LL
| 1. | Leung DS, HA; Geha, RS; Szefler, SJ. Pediatric Allergy: Principles and Practice. St. Louis: Mosby, 2003. |
| 2. | Al-Hammadi S, Al-Maskari F, Bernsen R. Prevalence of food allergy among children in Al-Ain city, United Arab Emirates. Int Arch Allergy Immunol. 2010;151:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Wang C, Lin W, Wang Y, Fu L. Suppression of Hippo Pathway by Food Allergen Exacerbates Intestinal Epithelia Instability and Facilitates Hypersensitivity. Mol Nutr Food Res. 2021;65:e2000593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Muraro A, Roberts G, Worm M, Bilò MB, Brockow K, Fernández Rivas M, Santos AF, Zolkipli ZQ, Bellou A, Beyer K, Bindslev-Jensen C, Cardona V, Clark AT, Demoly P, Dubois AE, DunnGalvin A, Eigenmann P, Halken S, Harada L, Lack G, Jutel M, Niggemann B, Ruëff F, Timmermans F, Vlieg-Boerstra BJ, Werfel T, Dhami S, Panesar S, Akdis CA, Sheikh A; EAACI Food Allergy and Anaphylaxis Guidelines Group. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 633] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 5. | Simons FE, Peterson S, Black CD. Epinephrine dispensing patterns for an out-of-hospital population: a novel approach to studying the epidemiology of anaphylaxis. J Allergy Clin Immunol. 2002;110:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Curtis C, Stukus D, Scherzer R. Epinephrine preparedness in pediatric patients with food allergy: an ideal time for change. Ann Allergy Asthma Immunol. 2014;112:560-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Simons FE. First-aid treatment of anaphylaxis to food: focus on epinephrine. J Allergy Clin Immunol. 2004;113:837-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Hourihane JO, Kilburn SA, Dean P, Warner JO. Clinical characteristics of peanut allergy. Clin Exp Allergy. 1997;27:634-639. [PubMed] |
| 9. | Avery NJ, King RM, Knight S, Hourihane JO. Assessment of quality of life in children with peanut allergy. Pediatr Allergy Immunol. 2003;14:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 313] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Esenboga S, Ocak M, Cetinkaya PG, Sahiner UM, Soyer O, Buyuktiryaki B, Sekerel BE. Physicians prescribe adrenaline autoinjectors, do parents use them when needed? Allergol Immunopathol (Madr). 2020;48:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Kim JS, Sinacore JM, Pongracic JA. Parental use of EpiPen for children with food allergies. J Allergy Clin Immunol. 2005;116:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Knibb R, Halsey M, James P, du Toit G, Young J. Psychological services for food allergy: The unmet need for patients and families in the United Kingdom. Clin Exp Allergy. 2019;49:1390-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |