Published online Jan 9, 2022. doi: 10.5409/wjcp.v11.i1.85
Peer-review started: January 9, 2021
First decision: May 6, 2021
Revised: June 20, 2021
Accepted: December 7, 2021
Article in press: December 7, 2021
Published online: January 9, 2022
Processing time: 362 Days and 16.3 Hours
Cardiac involvement in neonates with perinatal asphyxia not only complicates perinatal management but also contributes to increased mortality.
To assess cardiac troponin T (cTnT) levels in asphyxiated neonates and their correlation with echocardiography findings, inotrope requirement, hypoxic-ischemic encephalopathy (HIE) stages, and mortality.
cTnT levels, echocardiographic findings, the requirement of inotropes, HIE stages, and outcome were studied in neonates of gestational age ≥ 34 wk with perinatal asphyxia.
Among 57 neonates with perinatal asphyxia, male gender, cesarean section, forceps/vacuum-assisted vaginal delivery and late preterm included 33 (57.9%), 23 (40.4%), 3 (5.3%), and 12 (21.1%) respectively. The mean gestational age was 38.4 wk (1.6 wk). HIE stages I, II, and III were observed in 7 (12.3%), 37 (64.9%), and 9 (15.8%) neonates respectively. 26 (45.6%) neonates had echocardiographic changes and 19 (33.3%) required inotropes. cTnT levels were elevated in 41 (71.9%) neonates [median (IQR); 0.285 (0.211-0.422) ng/mL]. The Median cTnT level showed an increasing trend with increasing changes in echocardiography (P = 0.002). Two neonates with mitral regurgitation and global hypokinesia had the highest cTnT levels (1.99 and 0.651 ng/mL). Of 31 neonates with normal echocardiography, 18 (58.06%) showed elevated cTnT. cTnT levels were significantly higher in those who required inotropic support than those who did not (P = 0.007). Neonates with HIE stage III had significantly higher cTnT levels compared to those with HIE stage I/II (P = 0.013). Survivors had lower median cTnT levels [0.210 (0.122-0.316) ng/mL] than who succumbed [0.597 (0.356-1.146) ng/mL].
cTnT levels suggestive of cardiac involvement were observed in 71.9% of as
Core Tip: Cardiac involvement in perinatal asphyxia complicates the management and increases mortality. We assessed cardiac troponin T (cTnT) levels in asphyxiated neonates and their correlation with echocardiography findings, hypoxic-ischemic encephalopathy (HIE) stages, and mortality. Elevated cTnT levels suggestive of cardiac involvement were found in 71.9% of neonates and correlated with increasing grades of ischemic changes in echocardiography. cTnT levels were elevated in 58% of neonates in the absence of echocardiographic findings. Significantly higher cTnT levels in neonates with HIE stage III than those with HIE stage I and II as well as higher cTnT levels in non-survivors than survivors show its predictive role.
- Citation: Yellanthoor RB, Rajamanickam D. Correlation of cardiac troponin T levels with inotrope requirement, hypoxic-ischemic encephalopathy, and survival in asphyxiated neonates. World J Clin Pediatr 2022; 11(1): 85-92
- URL: https://www.wjgnet.com/2219-2808/full/v11/i1/85.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v11.i1.85
The myocardium is vulnerable to ischemic injury in asphyxiated neonates[1-5]. Perinatal asphyxia causes significant morbidity and mortality, especially in developing countries[6-9]. The cardiac involvement in perinatal asphyxia varies. The reported asphyxial cardiomyopathy in neonates ranges from 24%-78%[3-6].
Ischemic cardiac dysfunction results in decreased cardiovascular reserve. The affected neonates may present with myocardial failure, bradycardia, and hypotension along with morbidities related to other systems. The assessment of the extent of myocardial injury and appropriate management influence treatment and outcome. Clinical assessment alone is considered inadequate to guide management or predict the outcome. Serum creatinine kinase muscle-brain isoenzyme (CK-MB) lacks cardiac specificity in the neonate and the levels are affected by gestational age, mode of delivery, and birth weight. CK-MB levels were reported to be 2 to 5 times higher in neonates born by normal vaginal delivery as compared to those born by cesarean section[2]. Echocardiography helps in identifying the extent of cardiac dysfunction but needs expertise.
Cardiac troponin T (cTnT) has been explored as a more specific biomarker for the diagnosis of myocardial injury in asphyxiated neonates[10,11]. Troponin T can be detected at earlier stages than CK-MB and it also remains high for a longer period[10]. Further, cTnT levels are found to be elevated in 30%-50% of cases having normal CK-MB levels[2]. The levels also may correlate with mortality. In this context, we aimed to evaluate the role of cTnT in perinatally asphyxiated neonates as a marker of myo
Neonates of gestational age ≥ 34 wk with perinatal asphyxia were prospectively studied over two years in a neonatal intensive care unit of a University teaching hospital. The demographic and birth details, clinical examination data were collected. cTnT levels, echocardiographic findings, the requirement of inotropes, evidence of other system involvement, HIE in particular, and outcome were collected.
About 0.5 mL of blood collected using standard sampling tubes (BD red vac
Neonate was considered to have perinatal asphyxia if he/she met any of the following criteria: Need of bag and mask or bag and tube ventilation at birth with Apgar score of ≤ 6 at 5 min; hypoxic encephalopathy features (lethargy, seizures, hypotonia, coma or irritability); cord blood pH ≤ 7.0, or arterial pH in neonates ≤ 7.2. Neonates with congenital heart defects, major anomalies, and those who expired within the first hour of birth were excluded.
HIE was considered in asphyxiated neonates if they had neurologic manifestations (seizures, coma, hypotonia). HIE was divided into Sarnat stages 1, 2, and 3 based on standard clinical features[3,4,12]. Heart rate < 100/min was considered bradycardia. Systolic and or diastolic blood pressure (BP) equal to or lower than the 5th percentile for age and sex was considered hypotension. Capillary filling time (CFT) > 3 s was considered as increased CFT.
Echocardiograph was obtained with the "Philips CX-50" machine. Following echocardiographic findings were considered as suggestive of myocardial ischemia: Mitral regurgitation (MR) or right ventricular (RV)/left ventricular (LV)/global hypokinesia, tricuspid regurgitation (TR), and pulmonary artery hypertension (PAH) with TR[2]. Renal dysfunction was considered if creatinine level was more than the upper limit of the normal reference range for gestational age with or without urine output < 1 mL/kg/h[2]. Transaminase levels more than twice the normal levels (normal aspartate transaminase (AST) up to 40 U/L and alanine transaminase (ALT) up to 45 U/L) were considered as hepatic dysfunction.
The results are expressed as frequencies and percentages. Data were analyzed using SPSS v16.0 software. Differences in the median of quantitative data among different stages of HIE and echocardiographic changes were compared by Kruskal- Wallis test and proportions by Chi-Square tests. A P < 0.05 was considered statistically significant. Ethical approval was obtained from the Institutional Ethical Committee. Informed consent was obtained from the parents.
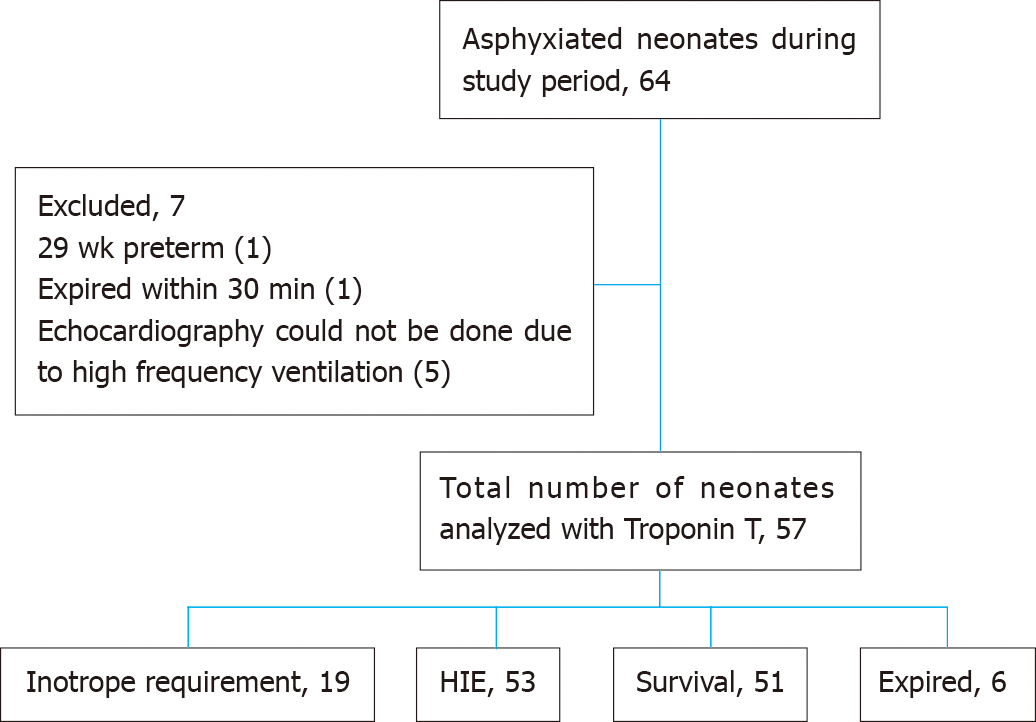
This study included 57 neonates with perinatal asphyxia (Figure 1). Male gender, lower segment cesarean section (LSCS), forceps or vacuum-assisted vaginal delivery, and late preterm included 33 (57.9%), 23 (40.4%), 3 (5.3%), and 12 (21.1%) respectively. The mean gestational age was 38.4 ± 1.6 wk.
Mode of resuscitation at birth included intubation in 26 (45.6%) and bag and mask ventilation in 15 (26.3%). Organ involvement in perinatal asphyxia included brain in 53 (93%), hepatic in 35 (61.4%), renal in 26 (45.6%), and cardiac in 30 (52.6%). Mechanical ventilation was needed in 20 (35.1%) neonates. HIE stage 1, 2 and 3 were observed in 7 (12.3%), 37 (64.9%) and 9 (15.8%) neonates respectively. Four neonates did not have HIE. Six neonates died.
Of 57 asphyxiated neonates, 26 (45.6%) neonates had echocardiographic changes. MR with global hypokinesia was observed in two (3.5%) neonates. Inotropic support was required in 19 (33.3%) neonates.
Elevated cTnT levels were observed in 41 (71.9%) neonates studied, with median (IQR) of 0.285 (0.211-0.422) ng/mL. The maximum value observed was 1.99 ng/mL (Table 1).
| cTnT levels (ng/mL) | n (%) | Median | IQR |
| Normal | 16 (28.1) | 0.084 | 0.052-0.114 |
| Elevated | 41 (71.9) | 0.285 | 0.211-0.422 |
The median cTnT level showed an increasing trend with the increase in changes in echocardiography (P = 0.002 Kruskal-Wallis Test) (Table 2). MR with global hypo
| Echocardiograph findings | n (%) | cTnT levels (ng/mL); median (IQR) |
| Normal | 31 (54.4) | 0.193 (0.085-0.282) |
| TR | 8 (14.03) | 0.223 (0.199-0.266) |
| PAH with TR | 16 (28.07) | 0.405 (0.228-0.557) |
| MR + Global hypokinesia | 2 (3.5) | 1.99, 0.6511 |
cTnT levels in those who required inotropic support were significantly higher when compared to those who did not require inotropic support (P = 0.007, Chi-Square test) (Table 3).
| Inotrope use | n (%) | cTnT levels (ng/mL); median (IQR) |
| Inotrope not required | 38 (66.6) | 0.192 (0.087-0.272) |
| Inotrope required | 19 (33.4) | 0.394 (0.269-0.543) |
Neonates who had HIE stage III had significantly higher levels of cTnT levels when compared to neonates with HIE stage I and II (P = 0.013 Kruskal-Wallis test) (Table 4). Six out of these 9 neonates with HIE stage III, having much higher cTnT levels succumbed.
| HIE stages | n (%) | cTnT levels (ng/mL); median (IQR) |
| Stage 1 | 7 (13.2) | 0.086 (0.047-0.271) |
| Stage 2 | 37 (69.8) | 0.255 (0.133-0.349) |
| Stage 3 | 9 (17.0) | 0.394 (0.239-0.758) |
Six neonates among 57 enrolled with perinatal asphyxia died. Median cTnT levels in those who survived were 0.210 (0.122-0.316) ng/mL which is comparatively lower than the median cTnT level in those who succumbed [0.597 (0.356-1.146) ng/mL] (Table 5).
| n (%) | cTnT levels (ng/mL); median (IQR) | |
| Survived | 51 (89.5) | 0.210 (0.122-0.316) |
| Succumbed | 6 (10.5) | 0.597 (0.356-1.146) |
Cardiac involvement secondary to tissue ischemia in neonates with perinatal asphyxia can occur as a part of a multi-organ involvement or isolated cardiac event[12-16]. Myocardium involvement not only complicates perinatal management but also increases mortality. The present study has identified the significant role of cTnT levels in identifying cardiac involvement, its correlation with echocardiography findings, inotrope requirement, HIE stages, and mortality.
In severely asphyxiated infants, cardiac dysfunction more commonly affects the right ventricle. The various manifestations related to cardiac dysfunction are respiratory distress, congestive cardiac failure, hypotension, delayed capillary refilling time, bradycardia, cardiogenic shock, and systolic murmur due to MR and TR[8,17]. Sinus bradycardia and lowered systemic BP are commonly seen in neonatal hypoxic ischemia. These features are observed in the present study. Costa et al[18] looked at BP in asphyxiated newborns. They observed significantly lower systolic and diastolic BPs in asphyxiated neonates compared to control newborns[18]. Hypotension is a late sign of under-perfusion. Hypotension is often due to peripheral vasodilatation or poor cardiac output. González de Dios et al[19] studied cardiac involvement (n = 31) in 156 asphyxiated term neonates. They categorized dysrhythmias and mild hypotension as minor cardiac involvement and TR, myocardial ischemia, cardiogenic shock, or hypovolemic shock as major cardiac involvement[19].
Echocardiography certainly could guide the management of these infants about fluid resuscitation and choice of inotropic support. Echocardiography is helpful in the early identification of tricuspid insufficiency, compromised LV output, and stroke volume in infants who suffer from perinatal asphyxia. Echocardiographic findings such as regional wall abnormalities, increased echogenicity of papillary muscle, compromised LV function, and tricuspid or mitral valve insufficiency resulting in reduced contractility, low cardiac output, decreased stroke volume, and elevated pressure of pulmonary artery suggest myocardial ischemia[17,19]. The mitral valve insufficiency and patent ductus arteriosus correlate with severe degrees of asphyxia injury. Tricuspid insufficiency was observed significantly at a higher rate in as
In asphyxiated neonates, despite preferential myocardial perfusion, hypoxia leads to myocardial damage. If ischemia progresses, especially beyond 20 min, over 60% of the cellular adenosine triphosphate will be used up, lactate in myocardial tissue increases about 12 times, glycogen and creatine phosphate reserves decrease resulting in dramatic structural changes[10,20,21]. This also causes damage to the cell membrane and releases CK-MB and cTnT into the bloodstream. CK-MB levels although significantly elevated in asphyxiated infants they do not appear to discriminate well those infants with cardiovascular compromise[10,21]. The highest levels occur at 12 h following birth and the levels decrease by 48 h of life[20].
The structure of troponin T is unique to the myocardium. Troponin concentration in the myocardium is much higher compared to CK-MB. In healthy humans, troponin levels in plasma are negligible. The troponin levels raise within a few hours after the acute ischemic episode and remain high for 10–14 d. This increases the diagnostic time range[2].
The present study has identified elevated cTnT levels in 71.9% of asphyxiated neonates and established a correlation of these levels with echocardiographic findings. Costa et al[18] reported significantly higher cTnT in neonates having echocardiograph signs of myocardial damage[18]. They also found that cTnT levels in neonates su
Myocardial dysfunction also impacts cerebral hemodynamics and decreases cerebral perfusion[22-25]. Cerebral hemodynamic disturbances such as a decreased rate of flow of blood including the velocity of highest systolic blood flow and velocity of blood flow at the end of diastole, raised index of pulsatility and resistance observed among neonates suffering from perinatal asphyxia are more frequent in infants who have cardiac dysfunction affecting the left ventricle. In the present study, neonates with HIE stage 3 had the highest cTnT levels. Higher cTnT levels in neonates with HIE and its correlation with mortality was also reported by Bhasin and Kohli[25] The limitation of the present study includes not analyzing the values of cTnT during therapeutic hypothermia and its changes as a prognostic marker of the final outcome.
Elevated cTnT levels suggestive of cardiac involvement in 71.9% of asphyxiated neonates establish its importance. The cTnT levels correlate with an increasing grade in echocardiography findings. Elevated cTnT levels in 58% of neonates with normal echocardiography findings suggest its biomarker role even in the absence of echocardiographic findings. Elevated cTnT levels in neonates with HIE stage III being significantly higher than those with HIE stage I and II show its predictive role. cTnT levels in non-survivors are likely to be much higher than those among survivors.
The myocardial ischemic injury in asphyxiated neonates complicates management and may lead to higher mortality. Cardiac troponin T (cTnT) levels are expected to rise early in myocardial ischemia and remain high for about two weeks.
cTnT levels are better markers than Serum creatinine kinase muscle-brain isoenzyme levels and could be predictive of mortality.
The present study determined cTnT levels in asphyxiated neonates and found out its relationship with echocardiography findings, inotrope requirement, hypoxic-ischemic encephalopathy (HIE) stages, and mortality.
cTnT levels are estimated in all asphyxiated neonates along with echocardiography evaluation.
Among asphyxiated neonates, cTnT levels were elevated in 71.9%. Further, the cTnT levels correlated with increasing grades of ischemic changes in echocardiography. Elevated cTnT levels in 58% of neonates with normal echocardiography findings suggested its role as a biomarker. cTnT levels in neonates with HIE stage III were significantly higher than those with HIE stage I and II. cTnT levels were higher in non-survivors than survivors.
cTnT could be a potential and clinically useful biomarker for asphyxia related myocardial injury in neonates.
Further studies to determine the exact cut of levels of cTnT predicting mortality are needed.
Authors thank the Head and staff members of the Department of Paediatrics for their kind help, suggestions, and cooperation for the study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Life Member of Indian Academy of Pediatrics; Life Member of National Neonatology Forum, Karnataka Branch, India.
Specialty type: Pediatrics
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Spasojevic SD S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Barberi I, Calabrò MP, Cordaro S, Gitto E, Sottile A, Prudente D, Bertuccio G, Consolo S. Myocardial ischaemia in neonates with perinatal asphyxia. Electrocardiographic, echocardiographic and enzymatic correlations. Eur J Pediatr. 1999;158:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Sweetman D, Armstrong K, Murphy JF, Molloy EJ. Cardiac biomarkers in neonatal hypoxic ischaemia. Acta Paediatr. 2012;101:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 3. | Adcock LM. Perinatal Asphyxia. In: Cloherty JP, editor. Manual of Neonatal Care, 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2011: 519-528. |
| 4. | Bernstein D. The fetal-to-neonatal circulatory transition. In: Behrman RE, Kliegman RM, Jenson HB, editors. Nelson textbook of pediatrics. 17th ed. Philadelphia: W.B. Saunders; 2004: 1479-1481. |
| 5. | Kanik E, Ozer EA, Bakiler AR, Aydinlioglu H, Dorak C, Dogrusoz B, Kanik A, Yaprak I. Assessment of myocardial dysfunction in neonates with hypoxic-ischemic encephalopathy: is it a significant predictor of mortality? J Matern Fetal Neonatal Med. 2009;22:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Lawn J, Shibuya K, Stein C. No cry at birth: global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths. Bull World Health Organ. 2005;83:409-417. [PubMed] |
| 7. | Agarwal R, Jain A, Deorari AK, Paul VK. Post-resuscitation management of asphyxiated neonates. Indian J Pediatr. 2008;75:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Ravi RNM, Gupta R, Kapoor AK. Evaluation of activity of creatine Phosphokinase (CPK) and its Isoenzyme CPK-MB in perinatal asphyxia and its implications for myocardial involvement. Bull NNF. 1999;13:2-7. |
| 9. | Rajakumar PS, Bhat BV, Sridhar MG, Balachander J, Konar BC, Narayanan P, Chetan G. Cardiac enzyme levels in myocardial dysfunction in newborns with perinatal asphyxia. Indian J Pediatr. 2008;75:1223-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Güneś T, Oztürk MA, Köklü SM, Narin N, Köklü E. Troponin-T levels in perinatally asphyxiated infants during the first 15 days of life. Acta Paediatr. 2005;94:1638-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Szymankiewicz M, Matuszczak-Wleklak M, Hodgman JE, Gadzinowski J. Usefulness of cardiac troponin T and echocardiography in the diagnosis of hypoxic myocardial injury of full-term neonates. Biol Neonate. 2005;88:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Kattwinkel J. American Academy of Pediatrics/American Heart Association: Textbook of Neonatal Resuscitation, 4th ed. Elk grove village, IL, American Academy of Pediatrics, American Heart Association, 2000.. |
| 13. | Walther FJ, Siassi B, Ramadan NA, Wu PY. Cardiac output in newborn infants with transient myocardial dysfunction. J Pediatr. 1985;107:781-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 73] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Clark SJ, Yoxall CW, Subhedar NV. Measurement of right ventricular volume in healthy term and preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2002;87:F89-F93; discussion F93-F94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Donnelly WH, Bucciarelli RL, Nelson RM. Ischemic papillary muscle necrosis in stressed newborn infants. J Pediatr. 1980;96:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 55] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Dattilo G, Tulino V, Tulino D, Lamari A, Falanga G, Marte F, Patanè S. Perinatal asphyxia and cardiac abnormalities. Int J Cardiol. 2011;147:e39-e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Herdy GV, Lopes VG, Aragão ML, Pinto CA, Tavares Júnior PA, Azeredo FB, Nascimento PM. [Perinatal asphyxia and heart problems]. Arq Bras Cardiol. 1998;71:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Costa S, Zecca E, De Rosa G, De Luca D, Barbato G, Pardeo M, Romagnoli C. Is serum troponin T a useful marker of myocardial damage in newborn infants with perinatal asphyxia? Acta Paediatr. 2007;96:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | González de Dios J, Moya M, Vioque J. [Risk factors predictive of neurological sequelae in term newborn infants with perinatal asphyxia]. Rev Neurol. 2001;32:210-216. [PubMed] |
| 20. | Boo NY, Hafidz H, Nawawi HM, Cheah FC, Fadzil YJ, Abdul-Aziz BB, Ismail Z. Comparison of serum cardiac troponin T and creatine kinase MB isoenzyme mass concentrations in asphyxiated term infants during the first 48 h of life. J Paediatr Child Health. 2005;41:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Möller JC, Thielsen B, Schaible TF, Reiss I, Kohl M, Welp T, Gortner L. Value of myocardial hypoxia markers (creatine kinase and its MB-fraction, troponin-T, QT-intervals) and serum creatinine for the retrospective diagnosis of perinatal asphyxia. Biol Neonate. 1998;73:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Perlman JM, Tack ED, Martin T, Shackelford G, Amon E. Acute systemic organ injury in term infants after asphyxia. Am J Dis Child. 1989;143:617-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Liu J, Li J, Gu M. The correlation between myocardial function and cerebral hemodynamics in term infants with hypoxic-ischemic encephalopathy. J Trop Pediatr. 2007;53:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Volpe JJ. Hypoxic-ischemic encephalopathy: Clinical aspects. In: Neurology of the Newborn, 5th ed, Saunders Elsevier, Philadelphia; 2008: 400. |
| 25. | Bhasin H, Kohli C. Myocardial dysfunction as a predictor of the severity and mortality of hypoxic ischaemic encephalopathy in severe perinatal asphyxia: a case-control study. Paediatr Int Child Health. 2019;39:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |