Published online Jul 9, 2021. doi: 10.5409/wjcp.v10.i4.72
Peer-review started: January 20, 2021
First decision: February 15, 2021
Revised: February 16, 2021
Accepted: April 20, 2021
Article in press: April 20, 2021
Published online: July 9, 2021
Processing time: 167 Days and 14.5 Hours
The indirect methods of reference intervals (RI) establishment based on data mining are utilized to overcome the ethical, practical challenges and the cost associated with the conventional direct approach.
To generate RIs for serum creatinine in children and adolescents using an indirect statistical tool.
Data mining of the laboratory information system was performed for serum creatinine analyzed from birth to 17 years for both genders. The timeline was set at six years from January 2013 to December 2018. Microsoft Excel 2010 and an indirect algorithm developed by the German Society of Clinical Chemistry and Laboratory Medicine’s Working Group on Guide Limits were used for the data analysis.
Data were extracted from 96104 samples and after excluding multiple samples for the same individual, we calculated RIs for 21920 males and 14846 females, with stratification into six discrete age groups.
Serum creatinine dynamics varied significantly across gender and age groups.
Core Tip: Good laboratory practices advocate the necessity for generation of population specific reference intervals (RIs). The indirect methods of RIs establishment based on data mining are utilized to overcome the ethical, practical challenges and the cost associated with the conventional direct approach. The population specific RIs generated for pediatric serum creatinine levels in this study will assist in more accurate comprehension of the variations in creatinine and facilitate patient care.
- Citation: Ahmed S, Zierk J, Siddiqui I, Khan AH. Indirect determination of serum creatinine reference intervals in a Pakistani pediatric population using big data analytics. World J Clin Pediatr 2021; 10(4): 72-78
- URL: https://www.wjgnet.com/2219-2808/full/v10/i4/72.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v10.i4.72
Reliable, accurate and population specific reference intervals (RIs) for laboratory analyses are pivotal for laboratory results interpretation and appropriate clinical decision-making. RIs for an analyte are based on the 2.5th and 97.5th centiles values from a set of pre-defined healthy individuals[1,2]. Furthermore, to improve the diagnostic efficiency of biomarkers, various partitioning criteria for RIs have been deployed, particularly aimed to evaluate the influence of increasing age and gender dependence[3,4]. In the pediatric population, this portioning becomes more essential as physiological developments after birth and during adolescence result in fluctuations in the levels of many biomarkers, especially serum creatinine (CREA)[5].
The most commonly utilized and recommended ‘direct approach’ for RIs generation follows a more robust strategy, with a pre-selected population, that undergoes sample collection, processing and analysis in a controlled environment[6]. However, to utilize this approach in pediatrics is a challenging task, owing to ethical, financial and practical issues. Whereas, the indirect approach can be more effectively and conveni
In most clinical settings, evaluation of kidney function is carried out by requisition of biochemical analysis of serum CREA and 24 h CREA clearance as an indirect measure for the estimation of glomerular filtration rate (GFR)[9]. However, the growth mediated changes in CREA, especially in infancy and during puberty, due notably to its renal tubular secretion and the influence of muscle mass and dietary intake, makes the interpretation even more challenging[10].
The majority of laboratories in LMIC, are unable to establish their population specific RIs and seldom rely on published literature or adopt the ones cited by the manufacturers in kit information sheets[11]. Whereas, some laboratories also imple
A team of investigators performed data mining of the laboratory information system at the Section of Clinical Chemistry, Aga Khan University. Ethical approval for the study was obtained from the Ethical review committee (ERC, #5348-Pat-ERC-18) of the university. All serum CREA measurements for both genders, including both in-house as well as ambulatory cases from birth to 17 years, were retrieved, regardless of the indication for test requisition. The timeline was set at six years from January 2013 to December 2018.
The biochemical analysis was carried out on a Siemens ADVIA 1800 platform. The precision of the assay was 3.8% at 1.8 mg/dL (159 μmol/L) and 3.7% at 8.4 mg/dL (743 μmol/L), and the method was linear from 0-25 mg/dL (0-2210 μmol/L). As most of the laboratories in Pakistan are well versed with the conventional system of units, the levels of CREA are expressed in mg/dL. The laboratory is accredited by the College of American Pathologist and internal quality assurance is practiced in light of the Clinical & Laboratory Standards Institute standards.
The statistical analysis was performed using Microsoft Excel 2010 and the indirect algorithm proposed and pre-validated the German Society of Clinical Chemistry and Laboratory Medicine’s Working Group freely available online as a software pack
To calculate the respective 2.5th and 97.5th percentiles, the data were split into six age groups, for each gender, ranging from birth i.e., 0 d- < 2 years, 2- < 5 years, 5- < 9 years, 9- < 12 years, 12- < 15 years and 15- < 17 years, respectively, as defined previously by Tahmasebi et al[11] in the CALIPER cohort of healthy children and adolescents[11].
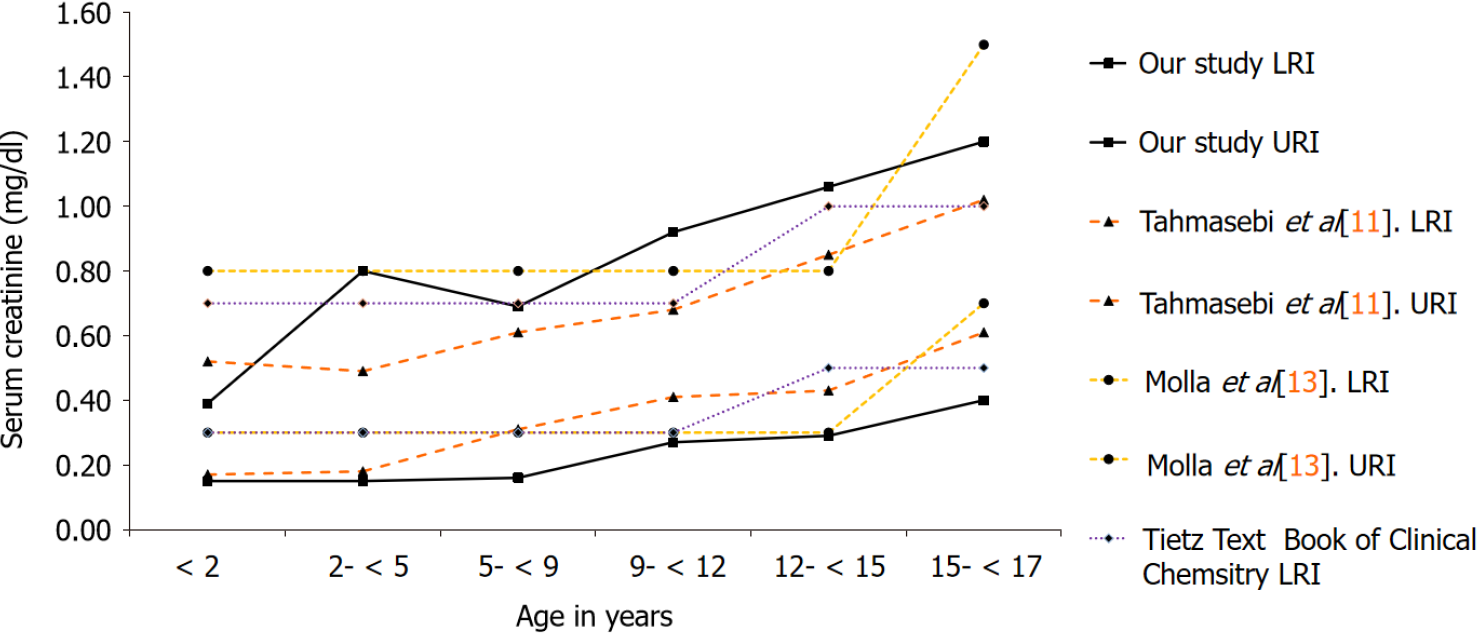
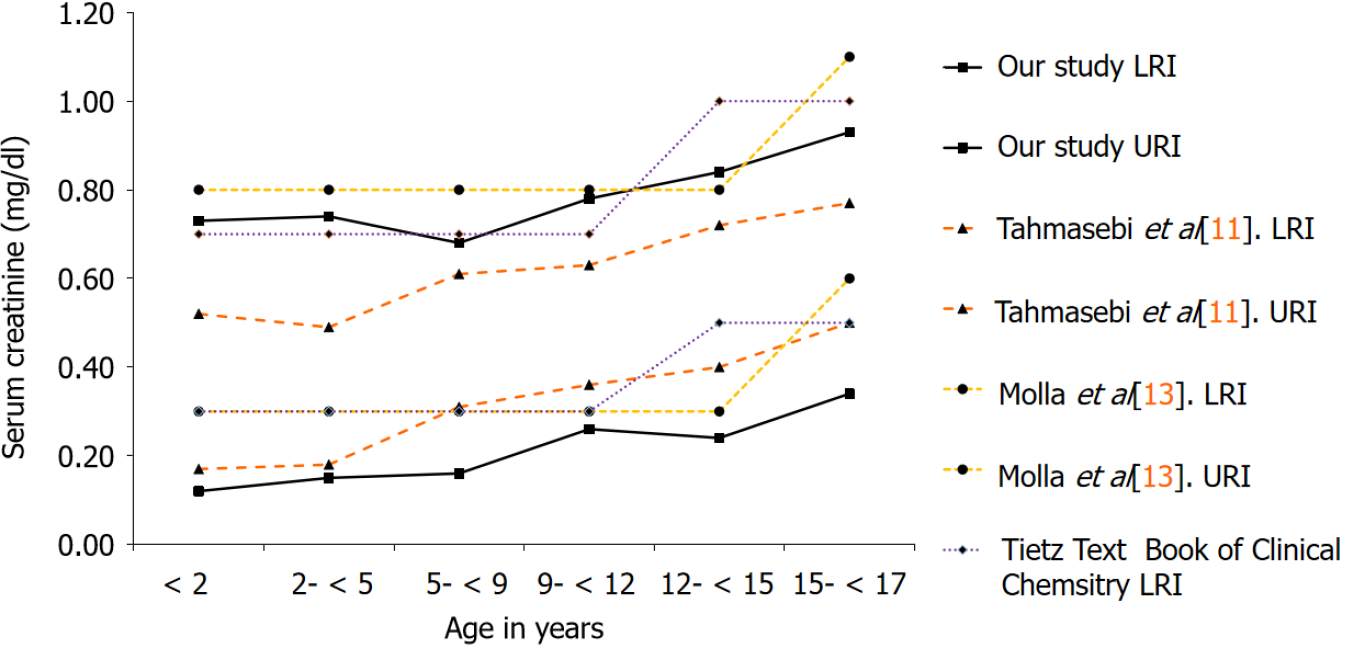
For the evaluation of calculated RIs, we performed a comparison of our results with Tahmasebi et al[11] that has established pediatric RIs for CREA on the Siemens ADVIA 1800[11]. Additionally, we also compared our findings with a local study by Molla et al[13] that has established direct RIs for CREA in an apparently healthy Pakistani population, for the combined 0-14 and 15 years onwards age groups, respectively, without partitioning into fine grained age clusters[13]. Lastly, the RIs currently in use by our laboratory for children and adolescents adopted from the Tietz textbook of clinical chemistry and molecular diagnostics were also evaluated[14].
From a total of 96104 samples analyzed for CREA over the study timeline, patients with multiple samples were further scrutinized and only the first sample analyzed was included in the final analysis. The lower and upper RIs were calculated based on 36766 CREA results obtained, including 21920 males and 14846 females as depicted in Tables 1 and 2. The complex age-related dynamics were more pronounced in the pre-pubertal group as represented by a significant proportion of samples in this age range.
| Age (yr) | n | Our study, LRI | Our study, URI | Tahmasebi et al[11], LRI | Tahmasebi et al[11], URI |
| < 2 | 9658 | 0.15 mg/dL (13 μmol/L) | 0.39 mg/dL (34 μmol/L) | 0.17 mg/dL (15 μmol/L) | 0.52 mg/dL (46 μmol/L) |
| 2- < 5 | 2964 | 0.15 mg/dL (13 μmol/L) | 0.80 mg/dL (71 μmol/L) | 0.18 mg/dL (16 μmol/L) | 0.49 mg/dL (43 μmol/L) |
| 5- < 9 | 2833 | 0.16 mg/dL (14 μmol/L) | 0.69 mg/dL (61 μmol/L) | 0.31 mg/dL (27 μmol/L) | 0.61 mg/dL (54 μmol/L) |
| 9- < 12 | 1796 | 0.27 mg/dL (24 μmol/L) | 0.92 mg/dL (81 μmol/L) | 0.41 mg/dL (36 μmol/L) | 0.68 mg/dL (60 μmol/L) |
| 12- < 15 | 2291 | 0.29 mg/dL (26 μmol/L) | 1.06 mg/dL (94 μmol/L) | 0.43 mg/dL (38 μmol/L) | 0.85 mg/dL (75 μmol/L) |
| 15- < 17 | 2378 | 0.40 mg/dL (35 μmol/L) | 1.26 mg/dL (111 μmol/L) | 0.61 mg/dL (54 μmol/L) | 1.02 mg/dL (90 μmol/L) |
| Age (yr) | n | Our study, LRI | Our study, URI | Tahmasebi et al[11], LRI | Tahmasebi et al[11], URI |
| < 2 | 6323 | 0.12 mg/dL (11 μmol/L) | 0.73 mg/dL (65 μmol/L) | 0.17 mg/dL (15 μmol/L) | 0.52 mg/dL (46 μmol/L) |
| 2- < 5 | 2012 | 0.15 mg/dL (13 μmol/L) | 0.74 mg/dL (65 μmol/L) | 0.18 mg/dL (16 μmol/L) | 0.49 mg/dL (43 μmol/L) |
| 5- < 9 | 1997 | 0.16 mg/dL (14 μmol/L) | 0.68 mg/dL (60 μmol/L) | 0.31 mg/dL (27 μmol/L) | 0.61 mg/dL (54 μmol/L) |
| 9- < 12 | 1204 | 0.26 mg/dL (23 μmol/L) | 0.78 mg/dL (69 μmol/L) | 0.36 mg/dL (32 μmol/L) | 0.63 mg/dL (56 μmol/L) |
| 12- < 15 | 1573 | 0.24 mg/dL (21 μmol/L) | 0.84 mg/dL (74 μmol/L) | 0.40 mg/dL (35 μmol/L) | 0.72 mg/dL (64 μmol/L) |
| 15- < 17 | 1737 | 0.34 mg/dL (35 μmol/L) | 0.93 mg/dL (82 μmol/L) | 0.50 mg/dL (44 μmol/L) | 0.77 mg/dL (68 μmol/L) |
Figures 1 and 2 illustrate the comparison of our results with RIs established using the direct method as reported by Tahmasebi et al[11], Molla et al[13] and the current RIs being used for reporting by our laboratory adopted from the Tietz textbook of clinical chemistry and molecular diagnostics.
Due to the lack of standardized data formats and experience in dealing with big data analytics, the majority of laboratories in LMIC as well as a few developed countries, considerably lag behind in evaluating the transformative potential of the big data they have in store. The methodology employed was based on big data analytics and extraction of data from the laboratory information system of a tertiary care hospital’s laboratory that receives specimens from the entire country in order to ensure participation from all the ethnic groups existing in Pakistan.
Compared to the study by Molla et al[13] and RIs reported in the Tietz textbook of clinical chemistry and molecular diagnostics, a notable strength of this study is that it demonstrates a strong influence of age on CREA activity with the age-wise par
A literature review revealed that most of the reported RIs for CREA, have been established using healthy population-based approaches i.e. direct methods. While this approach is undoubtedly considered the gold standard, it has certain limitations including those specifically pertaining to expenses for conducting these large-scale prospective studies especially for a LMIC. Additionally, attainment of a minimally acceptable sample size for the different age groups in pediatrics is also a concern. The indirect method not only made it possible to statistically analyze big data (n = 36766), acquired as part of routine care, which further minimized the ethical and practical concerns. However, this approach, requires significant refinement of the specimen selection alongside validated and robust statistical analysis. In this context, we utilized an established algorithm that had already been extensively evaluated and validated by large scale multicenter studies[4,15]. Notably, a literature review revealed that RIs in children established using direct methods do not correctly account for the extensive changes with age as most of them lack age-based partitioning. Moreover, in instances of non-normal distribution, the direct method often generates unacceptably broad confidence intervals (CIs) limiting their widespread adoption[16].
Next to, the RIs reported in the CALIPER cohort, our proposed RIs for CREA seem to differ. In particular, our lower reference limits (LRIs) are considerably lower than the CALIPER cohort, indicating that Pakistanis tend to have a different genetic structure with significantly lower lean tissue mass and a lower GFR compared to the CALIPER cohort. The LRIs and upper reference limits from the CALIPER cohort and the study by Molla et al[13] remain continuous up to five years of age, on the contrary, this study demonstrates pronounced age-related fluctuations in this age group for both genders. The maximum values were attained at 12 years in all the studies evaluated, trailed by an incline, having a probable association with the increase in muscle mass with age and attainment of puberty. On gender stratification, our study demonstrated that the peak levels of CREA attained in males i.e., 1.26 mg/dL (111 μmol/L) significantly differed from females i.e. 0.93 mg/dL (82 μmol/L). The need for fine grained age- and gender-based RIs for CREA is also supported by another study by Pottel et al[17] that has established age- and gender-specific CREA RIs from hospital laboratory data based on different statistical methods, and has shown pronounced age-based fluctuation in CREA for both genders[17]. This phenomenon is in accor
Considering the scarcity of literature on fine grained age group-based pediatric RIs for CREA in Pakistan, one of the highly densely populated countries reportedly with a high burden of kidney disease, the data mining approach can serve as the missing link[21,22]. Furthermore, the deployment of indirect approaches using “big data” solu
In addition to the merits of this real-world big-data approach in laboratory medicine, there is a notable limitation of this indirect algorithm, that any potential differences cannot be analyzed between the groups formulated; hence, individual results have to been complemented with clinical judgement and correlation. Moreover, the CIs with the established RIs were not calculated, as the used algorithm does not contain a provision for CI generation.
Good laboratory practices advocate the necessity for generation of population specific RIs, which is widely lacking, particularly in LMIC owing to the various challenges of the conventional direct method. This study has highlighted and further substantiated the utility of an alternative validated indirect algorithm by data mining in a clinical laboratory in Pakistan. This approach can be easily adopted by laboratories in resource constrained regions and the RIs generated will provide more accurate comprehension of laboratory reports in order to facilitate clinical care.
Population specific reference intervals (RIs) are pivotal for laboratory results interpre
The indirect methods of RIs establishment based on big data analytics overcome the challenges and the cost associated with the conventional direct approach.
To establish RIs for serum creatinine (CREA) levels in Pakistani children using an indirect data mining approach.
RIs were calculated using a previously validated indirect algorithm developed by the German Society of Clinical Chemistry and Laboratory Medicine’s Working Group on Guide Limits.
The lower and upper RIs were calculated based on 36766 CREA results obtained from 21920 males and 14846 females.
These RIs generated for serum CREA demonstrate the complex age- and gender-related dynamics occurring with physiological development.
This indirect approach can be easily adopted by laboratories in resource constrained regions and the RIs generated will provide more accurate comprehension of laboratory reports in order to facilitate clinical care.
Manuscript source: Unsolicited manuscript
Specialty type: Pediatrics
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aksionchyk M S-Editor: Fan JR L-Editor: Webster JR P-Editor: Yuan YY
| 1. | Adeli K, Higgins V, Seccombe D, Collier CP, Balion CM, Cembrowski G, Venner AA, Shaw J; CSCC Reference Interval Harmonization (hRI) Working Group. National Survey of Adult and Pediatric Reference Intervals in Clinical Laboratories across Canada: A Report of the CSCC Working Group on Reference Interval Harmonization. Clin Biochem. 2017;50:925-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Clinical and Laboratory Standards Institute (CLSI). Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. CLSI document EP28-A3. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. |
| 3. | Higgins V, Nieuwesteeg M, Adeli K. Reference intervals: theory and practice. In Contemporary Practice in Clinical Chemistry. Academic Press, 2020: 37-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Arzideh F, Wosniok W, Haeckel R. Reference limits of plasma and serum creatinine concentrations from intra-laboratory data bases of several German and Italian medical centres: Comparison between direct and indirect procedures. Clin Chim Acta. 2010;411:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Bohn MK, Higgins V, Adeli K. CALIPER paediatric reference intervals for the urea creatinine ratio in healthy children & adolescents. Clin Biochem. 2020;76:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Jones GRD, Haeckel R, Loh TP, Sikaris K, Streichert T, Katayev A, Barth JH, Ozarda Y; IFCC Committee on Reference Intervals and Decision Limits. Indirect methods for reference interval determination - review and recommendations. Clin Chem Lab Med. 2018;57:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 7. | Ahmed S, Zierk J, Khan AH. Establishment of Reference Intervals for Alkaline Phosphatase in Pakistani Children Using a Data Mining Approach. Lab Med. 2020;51:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 8. | Ichihara K, Boyd JC; IFCC Committee on Reference Intervals and Decision Limits (C-RIDL). An appraisal of statistical procedures used in derivation of reference intervals. Clin Chem Lab Med. 2010;48:1537-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Ahmed S, Jafri L, Khan AH. Evaluation of 'CKD-EPI Pakistan' Equation for estimated Glomerular Filtration Rate (eGFR): AComparison of eGFR Prediction Equations in Pakistani Population. J Coll Physicians Surg Pak. 2017;27:414-418. [PubMed] |
| 10. | Delanaye P, Cavalier E, Pottel H. Serum Creatinine: Not So Simple! Nephron. 2017;136:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 11. | Tahmasebi H, Higgins V, Woroch A, Asgari S, Adeli K. Pediatric reference intervals for clinical chemistry assays on Siemens ADVIA XPT/1800 and Dimension EXL in the CALIPER cohort of healthy children and adolescents. Clin Chim Acta. 2019;490:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Arzideh F, Brandhorst G, Gurr E, Hinsch W, Hoff T, Roggenbuck L, Rothe G, Schumann G, Wolters B, Wosniok W, Haeckel R. An improved indirect approach for determining reference limits from intra-laboratory data bases exemplified by concentrations of electrolytes. Laboratoriums Medizin. 2009;33:52-66. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Molla A, Khurshid M, Manser WT, Lalani R, Alam A, Mohammad Z. Suggested reference ranges in clinical chemistry for apparently healthy males and females of Pakistan. J Pak Med Assoc. 1993;43:113-115. [PubMed] |
| 14. | Burtis CA, Ashwood ER, Bruns DE. Reference intervals. Tietz textbook of clinical chemistry and molecular diagnostics-e-book. 2012; 4: 2264. |
| 15. | Arzideh F, Wosniok W, Haeckel R. Indirect reference intervals of plasma and serum thyrotropin (TSH) concentrations from intra-laboratory data bases from several German and Italian medical centres. Clin Chem Lab Med. 2011;49:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Siest G, Henny J, Gräsbeck R, Wilding P, Petitclerc C, Queraltó JM, Hyltoft Petersen P. The theory of reference values: an unfinished symphony. Clin Chem Lab Med. 2013;51:47-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Pottel H, Vrydags N, Mahieu B, Vandewynckele E, Croes K, Martens F. Establishing age/sex related serum creatinine reference intervals from hospital laboratory data based on different statistical methods. Clin Chim Acta. 2008;396:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | O'Leary JG, Wong F, Reddy KR, Garcia-Tsao G, Kamath PS, Biggins SW, Fallon MB, Subramanian RM, Maliakkal B, Thacker L, Bajaj JS. Gender-Specific Differences in Baseline, Peak, and Delta Serum Creatinine: The NACSELD Experience. Dig Dis Sci. 2017;62:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 19. | Cirillo M, Anastasio P, De Santo NG. Relationship of gender, age, and body mass index to errors in predicted kidney function. Nephrol Dial Transplant. 2005;20:1791-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Lo Sasso B, Vidali M, Scazzone C, Agnello L, Ciaccio M. Reference interval by the indirect approach of serum thyrotropin (TSH) in a Mediterranean adult population and the association with age and gender. Clin Chem Lab Med. 2019;57:1587-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | World Health Organization. WHO country cooperation strategy at a glance: Pakistan. [cited 10 January 2021]. Available from: https://apps.who.int/iris/bitstream/handle/10665/136607/ccsbrief_pak_en.pdf. |
| 22. | Jafar TH. The growing burden of chronic kidney disease in Pakistan. N Engl J Med. 2006;354:995-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Jooma R, Jalal S. Designing the first ever health insurance for the poor in Pakistan--a pilot project. J Pak Med Assoc. 2012;62:56-58. [PubMed] |
| 24. | Hoq M, Karlaftis V, Mathews S, Burgess J, Donath SM, Carlin J, Monagle P, Ignjatovic V. A prospective, cross-sectional study to establish age-specific reference intervals for neonates and children in the setting of clinical biochemistry, immunology and haematology: the HAPPI Kids study protocol. BMJ Open. 2019;9:e025897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |