Published online May 9, 2021. doi: 10.5409/wjcp.v10.i3.29
Peer-review started: December 25, 2020
First decision: January 18, 2021
Revised: January 25, 2021
Accepted: March 7, 2021
Article in press: March 7, 2021
Published online: May 9, 2021
Processing time: 118 Days and 22.9 Hours
Data regarding the most suitable diagnostic method for the diagnosis of glucose impairment in asymptomatic children and adolescents are inconclusive. Furthermore, limited data are available on the reproducibility of the oral glucose tolerance test (OGTT) in children and adolescents who are obese (OB).
To investigate the usefulness of the OGTT as a screening method for glucose dysregulation in children and adolescents.
Eighty-one children and adolescents, 41 females, either overweight (OW), OB or normal weight (NW) but with a strong positive family history of type 2 diabetes mellitus (T2DM), were enrolled in the present observational study from the Outpatient Clinic of Paediatric Endocrinology of the University Hospital of Patras in Greece. One or two 3-h OGTTs were performed and glucose, insulin and C-peptide concentrations were measured at several time points (t = 0 min, t = 15 min, t = 30 min, t = 60 min, t = 90 min, t = 120 min, t = 180 min).
Good repetitiveness was observed in the OGTT response with regard to T2DM, while low repetitiveness was noted in the OGTT response with regard to impaired glucose tolerance (IGT) and no repetitiveness with regard to impaired fasting glucose (IFG). In addition, no concordance was observed between IFG and IGT. During the 1st and 2nd OGTTs, no significant difference was found in the glucose concentrations between NW, OW and OB patients, whereas insulin and C-peptide concentrations were higher in OW and OB compared to NW patients at several time points during the OGTTs. Also, OW and OB patients showed a worsening insulin and C-peptide response during the 2nd OGTT as compared to the 1st OGTT.
In mild or moderate disorders of glucose metabolism, such as IFG and IGT, a diagnosis may not be reached using only one OGTT, and a second test or additional investigations may be needed. When glucose metabolism is profoundly impaired, as in T2DM, one OGTT is probably more reliable and adequate for establishing the diagnosis. Excessive weight and/or a positive family history of T2DM possibly affect the insulin and C-peptide response in the OGTT from a young age.
Core Tip: In mild or moderate disorders of glucose metabolism, such as impaired fasting glucose and impaired glucose tolerance, a diagnosis may not be reached using only one oral glucose tolerance test (OGTT), and a second test or additional investi
- Citation: Kostopoulou E, Skiadopoulos S, Partsalaki I, Rojas Gil AP, Spiliotis BE. Repetitiveness of the oral glucose tolerance test in children and adolescents. World J Clin Pediatr 2021; 10(3): 29-39
- URL: https://www.wjgnet.com/2219-2808/full/v10/i3/29.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v10.i3.29
The prevalence of obesity during childhood and adolescence shows an alarmingly increasing trend worldwide. Obesity is highly correlated with a constellation of disorders, including impaired glucose metabolism manifesting as impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and type 2 diabetes mellitus (T2DM)[1].
With regard to the diagnosis of T2DM, recommendations of the American Diabetes Association (ADA) involve measurement of fasting plasma glucose as a screening method because of its availability and ease of performance[2]. Rocchini[3] has sugges
In adults, the OGTT is considered to be superior to fasting glucose (FG) for the identification of subjects at increased risk for cardiovascular disease; however, the ADA recommends a second OGTT to confirm the diagnosis of T2DM, due to its low reproducibility[5]. Limited data are available on the reproducibility of the OGTT in obese (OB) children.
The objective of the present study was to investigate the possible repetitiveness of the response to repeat OGTTs and assess its diagnostic and prognostic value in children and adolescents with excess weight or a strong family history of T2DM.
A total of 81 children and adolescents, 41 females, who were determined to be overweight (OW), OB or with a strong positive family history of T2DM (defined as more than 3 individuals within three generations), were enrolled in the study from the Outpatient Clinic of Paediatric Endocrinology and Diabetes of the University Hospital of Patras in Greece, during a period of 5 years. Fifty-five out of the 81 patients (67.9%) were OB, 17 (21%) were OW and 9 (11.1%) had a normal weight (NW) but a positive family history of T2DM. The participants were randomly selected. The research was approved by the Research Ethics Committee of the University Hospital of Patras (IRB number: 348/9.5.2017) and informed consent was obtained from the parents of the children involved in the study. OW was defined as a body mass index (BMI) of 85%-95% and OB as a BMI of > 95%. The mean age of the studied subjects was 12.27 + 2.96 years (min: 4.91 years, max: 21.25 years).
A 3-h OGTT was performed in all patients. After the administration of oral glucose at a dose of 1.75 g/kg (max: 75 g), blood samples were obtained at t = 0 min, t = 15 min, t = 30 min, t = 60 min, t = 90 min, t = 120 min and t = 180 min, and glucose, insulin and C-peptide concentrations were measured. C-peptide concentrations were measured for 28 subjects, due to cost restrictions, 23 of the participants underwent 2 OGTTs (6 with NW, 4 OW and 13 OB). Three-hour OGTTs were performed in the studied population as it has been our experience that the 3-h OGTT can identify the children who have a delayed insulin response and are able to normalize their glucose levels at the 3-h time-point if they have an abnormal glucose response at the 2-h point. Other groups have also found that 3 h is a better duration for an OGTT to capture the full spectrum of glucose and insulin excursions in youth[6,7].
The OGTT was repeated in order to assess the glucose, insulin and C-peptide response in OB and OW patients, who did not manage to achieve a significant weight loss and agreed to undergo a second OGTT during the study period.
IFG was defined as plasma glucose between 100 mg/dL and 125 mg/dL at time 0 min (t = 0 min) and IGT as plasma glucose between 140 mg/dL and 199 mg/dL at 120 min (t = 120 min). T2DM was defined as plasma glucose at time 0 min > 125 mg/dL and plasma glucose at 120 min ≥ 200 mg/dL[8].
Weight, height and pubertal development were assessed in all the participants. Tanner stages in the boys were correlated with testicular volume as follows: (1) Tanner I: < 4 cm3; (2) Tanner II: 4-8 cm3; (3) Tanner III: 10-12 cm3; (4) Tanner IV: 15-20 cm3; and (5) Tanner V: 20-25 cm3.
Fasting plasma glucose was assessed by the hexokinase method with the use of a biochemical analyzer (Olympus AU600). Insulin concentrations were measured with the Electro-Chemiluminescence immunoassay method and the E170 Immunology Analyzer by Roche was used for the process. C-peptide was determined by radio
Data are presented as mean ± SD for normally distributed continuous variables. Comparisons of the glucose, insulin and C-peptide concentrations between the OGTTs were made using the Mann Wilcoxon test for unpaired data and the Wilcoxon Signed Rank test for paired data. Correlations between glucose, insulin or C-peptide concentrations and BMI or age were assessed with Spearman’s rho correlation coefficient. The threshold for statistical significance was defined as P ≤ 0.05. All analyses were performed with the SPSS Statistical Software Package (IBM SPSS Statistics, version 24, Chicago, IL, United States).
Of the 23 patients who underwent 2 OGTTs, the mean age during the 1st OGTT was 12.46 years and during the 2nd OGTT it was 14.55 years.
Of the entire population, during the 1st OGTT there were 2 patients (2.4%) who had IFG (1 NW and 1 OW), 5 patients (6.1%) who had IGT (2 NW and 3 OB) and 2 patients (2.5%) who had T2DM (1 OB patient who had T2DM based on glucose concentrations of > 125 mg/dL at t = 0 min, and 1 NW patient who had T2DM based on glucose concentrations of > 125 mg/dL at t = 0 min and > 200 mg/dL at t = 120 min) (Table 1).
| Number of patients | IFG | IGT | T2DM |
| 1st OGTT | 2 | 5 | 2 |
| 2nd OGTT | 0 | 1 | 2 |
| Repetitiveness (%) | 0/2 (0) | 1/5 (20) | 2/2 (100) |
The 2 patients who had IFG during the 1st OGTT underwent 2 OGTTs in total and had normal FG during the 2nd OGTT. Of the 5 patients with IGT during the 1st OGTT, only 1 OB patient also had IGT during the 2nd OGTT (repetitiveness: 20%). The OB patient who had a glucose concentration of > 125 mg/dL during the 1st OGTT also had a glucose concentration of > 125 mg/dL during the 2nd OGTT. The NW patient who had a glucose concentration of > 125 mg/dL at t = 0 min and > 200 mg/dL at t = 120 min during the 1st OGTT, had IFG and glucose concentrations of > 200 mg/dL at t = 120 min during the 2nd OGTT (Table 1).
Only one of the 2 patients who fulfilled the criteria for T2DM based on the t = 0 min criterion, also fulfilled the criteria for T2DM based on the t = 120 min criterion. No concordance was observed between IFG and IGT, as of the 5 patients with IGT, none had IFG.
As previously mentioned, 4 of the 5 subjects who had IGT during the 1st OGTT, had normal glucose concentrations at t = 120 min during the 2nd OGTT.
In the 2 patients with IFG, IFG was not present in both OGTTs. In contrast, FG remained abnormal in the patients with T2DM.
The number of patients with IFG, IGT or T2DM during the 1st or 2nd OGTT in whom the glucose disorder was confirmed in both OGTTs is shown in Table 2.
| Number of patients | IFG | IGT | T2DM |
| 1st or 2nd OGTT | 3 | 8 | 2 |
| Confirmation of results in both OGTTs | 0 | 2 | 2 |
| Repetitiveness (%) | 0/3 (0) | 2/8 (25) | 2/2 (100) |
NW patients: These patients increased their BMI-SDS (no statistically significant difference, P > 0.05) by 0.37, but retained a normal weight.
During the 1st OGTT, one patient had IFG (106 mg/dL), two patients had IGT (142 mg/dL and 146 mg/dL, respectively), and one patient fulfilled the criteria for T2DM based on both FG [Glu (t = 0 min): 128 mg/dL] and glucose at t = 120 min (Glu: 211 mg/dL). The patient with IFG did not have IGT or T2DM.
During the 2nd OGTT, one patient had IFG (Glu: 122 mg/dL), the same one who had T2DM during the 1st OGTT. The patient who had IFG during the 1st OGTT did not have IFG or T2DM during the 2nd OGTT. Also, one patient had T2DM (t = 120 min, Glu: 244 mg/dL). This is the same patient who had T2DM during the 1st OGTT. No patients had IGT.
OW patients: The OW patients increased their BMI-SDS by 0.23, but remained overweight.
During the 1st OGTT, one patient had IFG, with a borderline glucose concentration at t = 0 min of 100 mg/dL. None of the patients had IGT or T2DM. During the 2nd OGTT, one patient had IGT. This patient did not have IFG, IGT or T2DM during the 1st OGTT. None of the OW patients had IFG or IGT.
OB patients: The OB patients showed no statistically significant changes in the BMI-SDS between the 1st and 2nd OGTT (P > 0.05).
During the 1st OGTT, one patient had T2DM based on glucose at t = 0 min (Glu: 142 mg/dL) and 3 patients had IGT (Glu: 185 mg/dL, Glu: 157 mg/dL, Glu: 168 mg/dL). During the 2nd OGTT (n = 19), two patients had IGT, one of whom also had IGT during the 1st OGTT, and no patients had IFG and 1 patient had T2DM (the same one with T2DM during the 1st OGTT).
Of the 2 patients with T2DM, one was of normal weight, but with a positive family history of T2DM (Tanner III, 14 years old), and the second was OB (Tanner II and 7.75 years old).
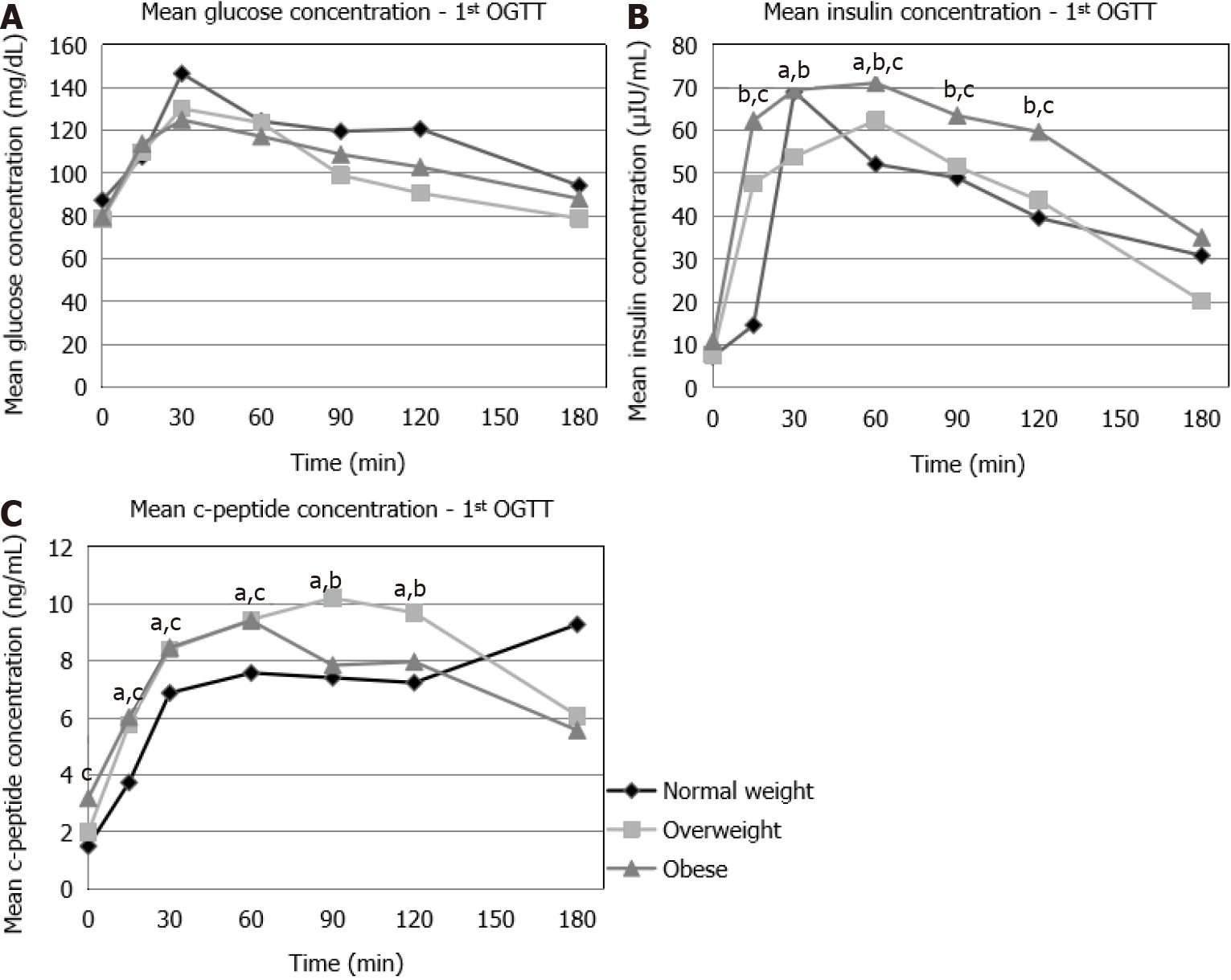
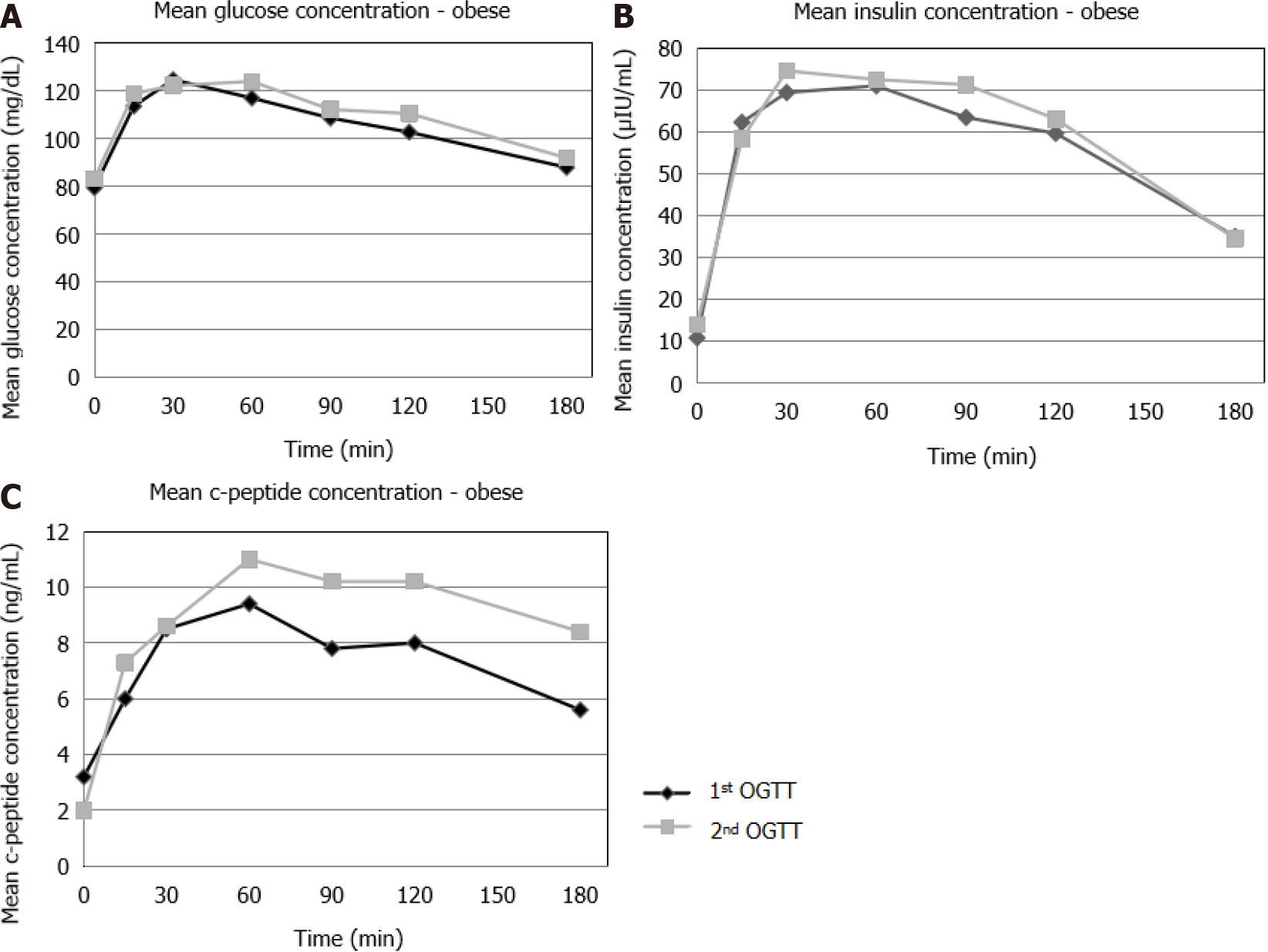
During the first OGTT, no statistically significant difference was found in the glucose concentrations between the NW, OW and OB patients (Figure 1A). Insulin concentrations were significantly higher in the OW compared to the NW patients at t = 15 min, t = 60 min, as well as in OB compared to NW patients at t = 15 min, t = 60 min, t = 90 min, t = 120 min and in OB compared to OW patients at t = 15 min, t = 30 min, t = 60 min, t = 90 min, t = 120 min (Figure 1B). C-peptide concentrations were signi
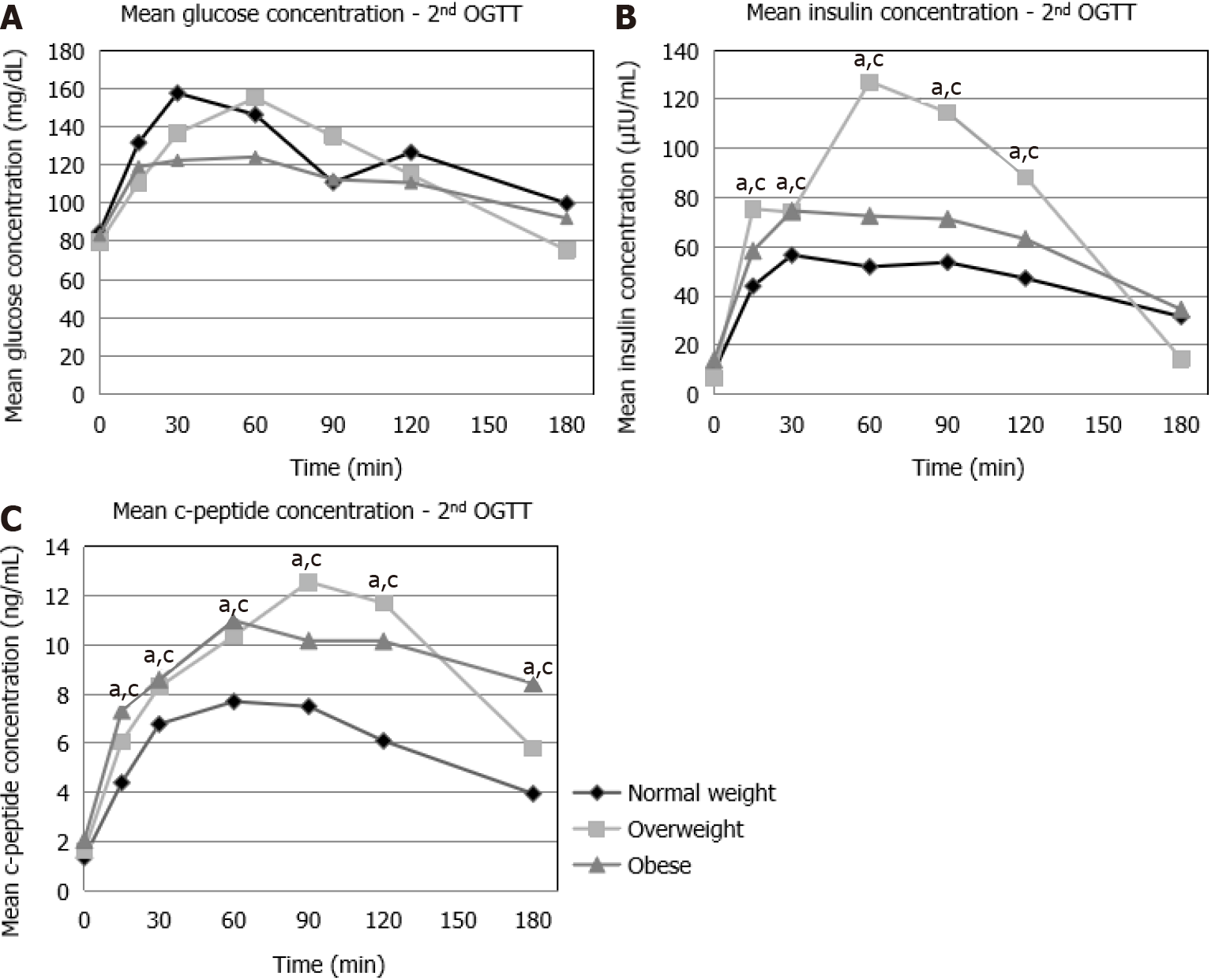
During the 2nd OGTT, no statistically significant differences were observed in the glucose concentrations between the NW, OW and OB patients (Figure 2A). Insulin concentrations were significantly higher in OW (t = 15 min, t = 30 min, t = 60 min, t = 90 min, t = 120 min) and in OB (t = 15 min, t = 30 min, t = 60 min, t = 90 min, t = 120 min) compared to NW patients (Figure 2B). C-peptide concentrations were also significantly higher in OW and in OB (t = 15 min, t = 30 min, t = 60 min, t = 90 min, t = 120 min, t = 180 min) compared to NW patients (Figure 2C).
When glucose, insulin and C-peptide concentrations were compared for each BMI category between the different OGTTs, the results were as follows:
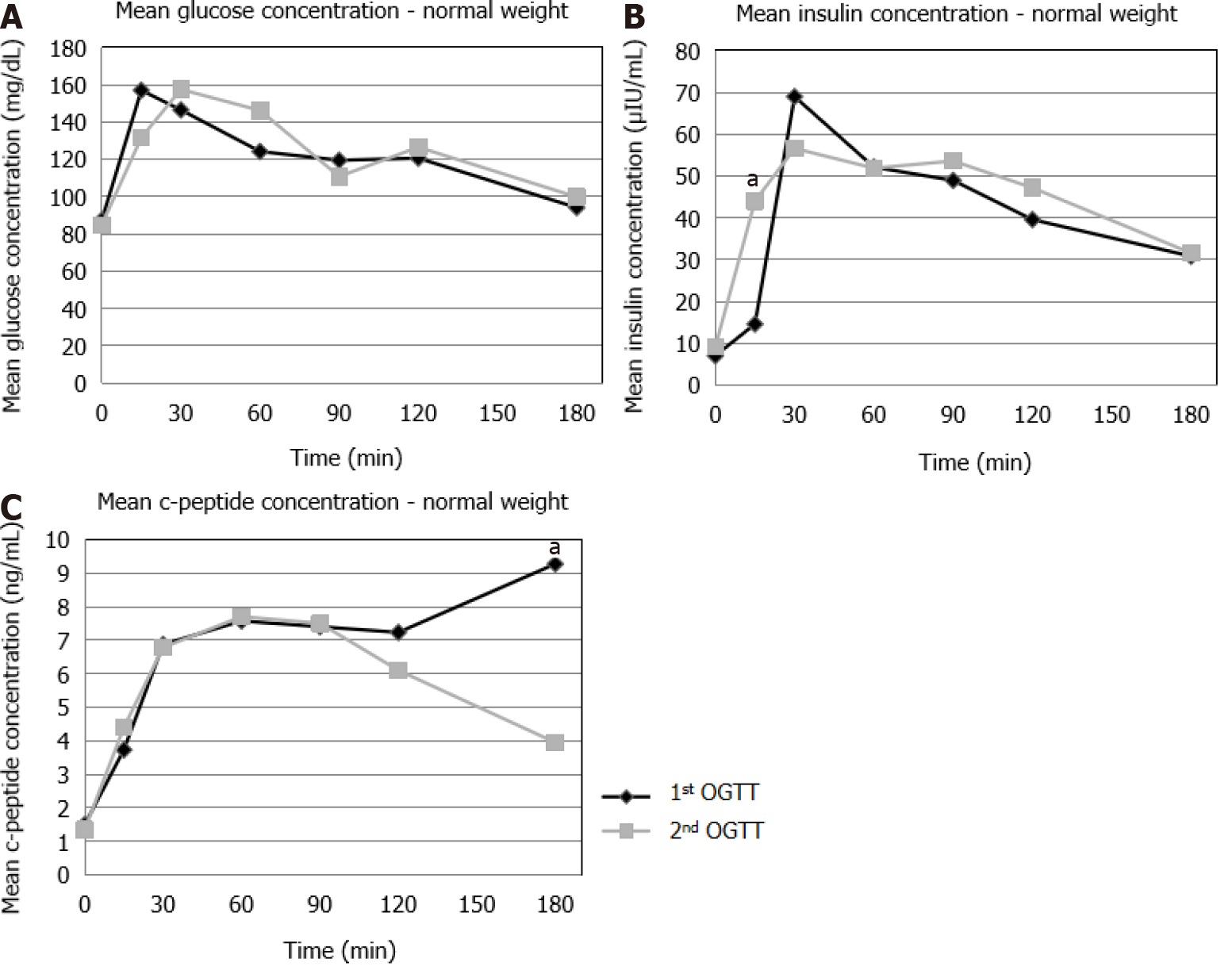
NW: No statistically significant differences were observed in the glucose concentra
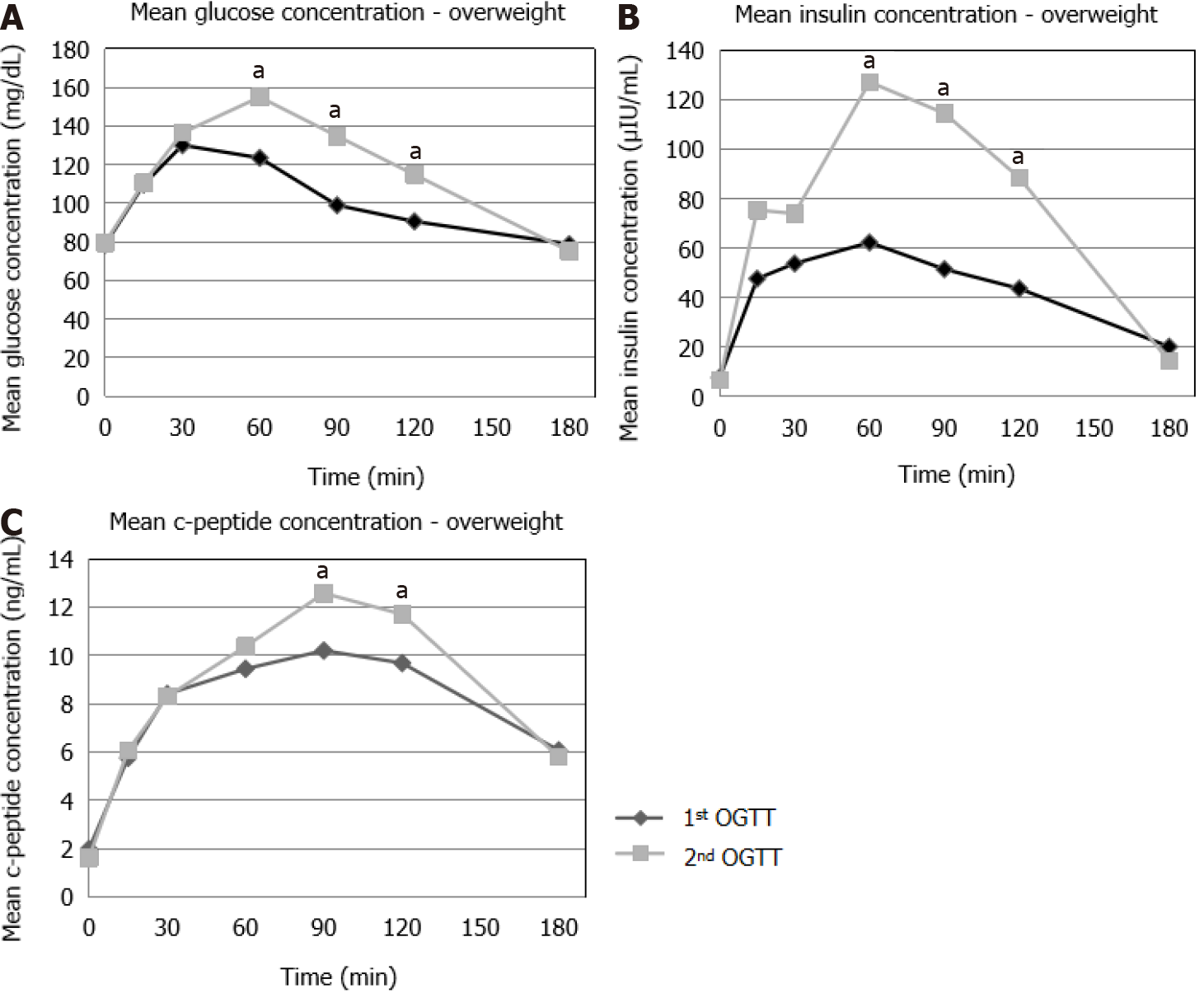
OW: A significant increase was seen in the glucose (t = 60 min, t = 90 min and t = 120 min), insulin (t = 60 min, t = 90 min and t = 120 min) and C-peptide (t = 90 min, t = 120 min) concentrations in the 2nd OGTT compared to the 1st OGTT (Figure 4).
OB: No statistically significant differences were observed in the glucose concen
Our study suggests that a reliable diagnosis of disorders of glucose metabolism, such as IFG and IGT, may not be possible using only one OGTT; hence, a second test or additional investigations may be needed to confirm the diagnosis. It may be that in the case of patients with profoundly impaired glucose metabolism, as in the case of T2DM, one OGTT is probably more reliable and adequate for establishing the diagnosis, whereas in mild or moderate disorders of glucose metabolism, a second OGTT is possibly needed for confirmation. This is in agreement with the ADA recommendation for performing a second OGTT in order to confirm the diagnosis of diabetes in adults[5]. Importantly, it should be taken into consideration that, despite the controlled research conditions, reproducibility of the OGTT is not always ideal, particularly in the case of mild or moderate disorders of glucose metabolism, due to procedural variability and intra-individual variation.
In addition, no repetitiveness in the OGTT response was observed with regard to IFG in the total population. Low repetitiveness was noted in IGT, as among the 6 patients who underwent 2 OGTTs and had IGT during the 1st or the 2nd OGTT, only 1 (16.7%) had IGT in both OGTTs. Of note, of the 4 patients who had IGT during the 1st OGTT, none had IFG. All these patients would not have been identified as being at increased risk for T2DM if only a FG had been performed.
In contrast, the normal weight patient who met the criteria for T2DM during the 1st OGTT at t = 0 min and t = 120 min, also exhibited repetitiveness in the glucose response at t = 0 min and t = 120 min during the 2nd OGTT. Similarly, the OB patient who met the criteria for T2DM during the 1st OGTT at t = 0 min, also exhibited repetitiveness in the glucose response at t = 0 min during the 2nd OGTT. These data may suggest that repetitiveness between different OGTTs is better in the context of more severe abnormalities in glucose regulation. The fact that FG remained abnormal in the second patient may also suggest that IFG is a reliable marker of abnormal glucose regulation when the dysregulation is significant. Of course, the sample was very small (only 1 patient had T2DM).
The poor correlation we observed between IFG and IGT has also been reported in the literature[8,9]. The percentage of IGT during the 1st OGTT in our study was 13.7%, whereas the percentage of IFG was 3.4%. In the 2nd OGTT, the percentage of IGT was 10.3%, whereas that of IFG was 3.4%. This is in agreement with reports in adult populations, which show that patients with IGT are not identified by a FG test[9]. This observation is also in agreement with reports in the literature stating that the repro
One interesting finding of the present study is that of the studied population, only a small percentage exhibited disorders of glucose metabolism such as IFG, IGT or T2DM. Obesity in children is a predisposing factor for glucose dysregulation; however, a prolonged period is possibly needed before the effects of excessive weight become manifest, possibly due to the existence of reserves or protective mechanisms in children and adolescents. Our study also supports the notion that there seems to be a predisposition for impaired glucose metabolism in subjects with a strong family history of T2DM, independent of their weight status.
Furthermore, during the 1st OGTT and the 2nd OGTT, a positive correlation was observed between BMI and insulin, as well as between BMI and C-peptide concentrations (Figure 1B, 1C, 2B and 2C). Also, OW and OB patients showed a worsening insulin and C-peptide response during the 2nd OGTT (Figure 4B, 4C, 5B and 5C). Since the weight status did not worsen between the two OGTTs, this may be explained by progressed puberty since it is well known that there is a “physiologic insulin resistance” seen normally in pubertal children[13]. Also, it may suggest that children with excessive weight gain are prone to metabolic disturbances with the progression of age compared to their normal-weight peers.
The present study has some strengths and limitations. The strong points include the fact that the study population consisted of a quite large and wide age-range sample of children and adolescents. Also, the present study is, to our knowledge, the first to assess the beneficial role of the OGTT in identifying disorders of glucose metabolism in children and adolescents, as compared to single measurements, such as FG. In addition, although studies comparing two OGTTs have been performed in children and adolescents with an interval of 1 d to 25 d in order to assess the reproducibility of the OGTT, no studies of longer intervals have been performed, thus far, in order to investigate the repetitiveness of the OGTT in the paediatric population. On the other hand, the small sample of patients with impaired glucose metabolism and the small sample of patients who repeated the OGTT, represent limitations of the study. Also, C-peptide concentrations were measured in only 28 patients and the number of patients with NW and a positive family history of T2DM was limited. Therefore, further studies on larger populations are needed to verify these findings.
Our study of the glucose response during repeated OGTTs, adds to the existing knowledge pertaining to glucose regulation in children and adolescents with excess weight. It also highlights that a family history of abnormal glucose metabolism may place children and adolescents at a higher risk for glucose dysregulation. More importantly, the findings of this study infer that the OGTT is superior to single measurements, such as FG, in diagnosing disorders of glucose metabolism, parti
Fasting plasma glucose is used as a screening tool for the diagnosis of disorders of glucose metabolism due to its ease of performance. The oral glucose tolerance test (OGTT) has been proposed as a possibly useful screening method for the diagnosis of impaired glucose metabolism and increased risk for diabetes in children. Data regarding the most appropriate screening method to diagnose disordered glucose metabolism are inconclusive.
Additional information is needed in order to determine the usefulness of the OGTT in diagnosing impaired glucose metabolism.
To investigate the pattern of glucose, insulin and C-peptide responses in repeated OGTTs and to determine the diagnostic and prognostic value of the OGTT regarding the development of disorders of glucose metabolism.
A 3-h OGTT was performed in 81 children and adolescents with excess weight or a strong positive family history of type 2 diabetes mellitus (T2DM), and the glucose, insulin and C-peptide responses were evaluated at multiple time points. The OGTT was repeated in a proportion of the patients and comparisons were made between the responses of glucose, insulin and C-peptide. The glucose, insulin and C-peptide concentrations between the two OGTTs were compared using the Mann Wilcoxon Test for unpaired data and the Wilcoxon Signed Rank test for paired data. Correlations between the body mass index or the age and the glucose, insulin or C-peptide concentrations during the OGTTs were assessed using Spearman’s rho correlation coefficient.
None of the patients with impaired fasting glucose exhibited repetitiveness of the finding in both OGTTs. Eighty percent of the subjects with impaired glucose tolerance during the 1st OGTT, had normal glucose concentrations at t = 120 min during the 2nd OGTT. Repetitiveness was observed for the diagnosis of T2DM in both OGTTs.
In patients with profoundly impaired glucose metabolism, as in the case of T2DM, one OGTT is probably adequate for diagnosing the disorder. In patients with milder disorders of glucose metabolism, a second OGTT is possibly needed for confirmation. The OGTT seems to be superior to single measurements, such as fasting glucose, in diagnosing disorders of glucose metabolism, particularly mild glucose dysregulation, i.e., impaired fasting glucose and impaired glucose tolerance. Disorders of glucose metabolism are uncommon in overweight or obese children and adolescents.
Further studies are needed in order to determine the possible repetitiveness of the OGTT in children and adolescents with risk factors for T2DM, such as increased weight or a positive family history. Further studies are needed in order to confirm the diagnostic and prognostic superiority of the OGTT with regard to glucose dysregu
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Paediatric Endocrinology; Endocrine Society; and Patras Medical Association.
Specialty type: Pediatrics
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Skrypnik D S-Editor: Gao CC L-Editor: Webster JR P-Editor: Yuan YY
| 1. | Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2013;4:270-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 201] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (3)] |
| 2. | American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 740] [Article Influence: 29.6] [Reference Citation Analysis (1)] |
| 3. | Rocchini AP. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346:854-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 148] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Alberti G, Zimmet P, Shaw J, Bloomgarden Z, Kaufman F, Silink M; Consensus Workshop Group. Type 2 diabetes in the young: the evolving epidemic: the international diabetes federation consensus workshop. Diabetes Care. 2004;27:1798-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 287] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 5. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30 Suppl 1:S42-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 488] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 6. | Bacha F, Gungor N, Arslanian SA. Measures of beta-cell function during the oral glucose tolerance test, liquid mixed-meal test, and hyperglycemic clamp test. J Pediatr. 2008;152:618-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Galderisi A, Tricò D, Dalla Man C, Santoro N, Pierpont B, Groop L, Cobelli C, Caprio S. Metabolic and Genetic Determinants of Glucose Shape After Oral Challenge in Obese Youths: A Longitudinal Study. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissén M, Isomaa B, Forsen B, Homström N, Saloranta C, Taskinen MR, Groop L, Tuomi T; Botnia study group. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes. 2005;54:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 263] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Tuomilehto J. Point: a glucose tolerance test is important for clinical practice. Diabetes Care. 2002;25:1880-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab. 2008;93:4231-4237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin RS, Caprio S. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1149] [Cited by in RCA: 1060] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 12. | Invitti C, Guzzaloni G, Gilardini L, Morabito F, Viberti G. Prevalence and concomitants of glucose intolerance in European obese children and adolescents. Diabetes Care. 2003;26:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res. 2006;60:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 237] [Article Influence: 12.5] [Reference Citation Analysis (0)] |