Published online Mar 9, 2021. doi: 10.5409/wjcp.v10.i2.7
Peer-review started: December 22, 2020
First decision: January 7, 2021
Revised: January 22, 2021
Accepted: February 12, 2021
Article in press: February 12, 2021
Published online: March 9, 2021
Processing time: 75 Days and 21.7 Hours
McCune–Albright syndrome (MAS) is caused by postzygotic somatic mutations of the GNAS gene. It is characterized by the clinical triad of fibrous dysplasia, café-au-lait skin spots, and endocrinological dysfunction. Myriad complications in MAS, including hepatobiliary manifestations, are also reported.
This is a case of a 4-year-old boy who presented with MAS with neonatal cholestasis. He was suspected to have Alagille syndrome due to neonatal cholestasis with intrahepatic bile duct paucity in liver biopsy, peripheral pulmonary artery stenosis, and renal tubular dysfunction. By the age of 2 years, his cholestatic liver injury gradually improved, but he had repeated left femoral fractures. He did not exhibit endocrinological abnormality or café-au-lait skin spots. However, MAS was suspected due to fibrous dysplasia at the age of 4 years. No mutation was identified in the GNAS gene in the DNA isolated from the peripheral blood, but an activating point mutation (c.601C>T, p.Arg201Cys) was observed in the DNA extracted from the affected bone tissue and that extracted from the formalin-fixed paraffin-embedded liver tissue, which was obtained at the age of 1 mo.
MAS should be considered as a differential diagnosis for transient cholestasis in infancy.
Core Tip: McCune–Albright syndrome (MAS) is caused by postzygotic somatic mutations of the GNAS gene. It is characterized by the clinical triad of fibrous dysplasia, café-au-lait skin spots, and endocrinological dysfunction. MAS complications other than the triad are also reported. This is the case of a boy with MAS diagnosed with Alagille syndrome in his infancy based on intrahepatic bile duct paucity in liver biopsy, neonatal cholestasis, cardiac manifestation, and renal tubular dysfunction. MAS should be considered as a differential diagnosis for transient cholestasis in infancy.
- Citation: Satomura Y, Bessho K, Kitaoka T, Takeyari S, Ohata Y, Kubota T, Ozono K. Neonatal cholestasis can be the first symptom of McCune–Albright syndrome: A case report. World J Clin Pediatr 2021; 10(2): 7-14
- URL: https://www.wjgnet.com/2219-2808/full/v10/i2/7.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v10.i2.7
McCune–Albright syndrome (MAS) is a rare sporadic disease characterized by the clinical triad of fibrous dysplasia, café-au-lait skin spots, and endocrinological dysfunction[1,2]. Its estimated prevalence ranges from 1/100000 to 1/1000000[3]. MAS is caused by postzygotic somatic mutations of the GNAS gene, which encodes the G protein stimulatory α subunit[4]. MAS complications other than the clinical triad, including hepatobiliary dysfunction, are reported[4-6].
Alagille syndrome (ALGS) is an autosomal dominant disorder with a wide spectrum of clinical variability. The main clinical features and malformations are chronic cholestasis due to intrahepatic bile duct paucity (decreased bile duct-to-portal tract ratio: < 0.4), cardiac disease (particularly peripheral pulmonary artery stenosis), skeletal deformity (particularly butterfly vertebrae), ocular abnormalities (particularly posterior embryotoxon), and characteristic facial features. Additional features include intracranial bleeding, dysplastic kidneys, and bone fractures[7,8]. The majority of cases are caused by JAG1 gene haploinsufficiency, encoding a ligand jagged1 in the Notch signaling pathway[9,10]. Mutations in NOTCH2, a receptor in the same signaling pathway, are identified in some ALGS patients who do not have mutations in JAG1[11].
This is a case of a boy who was diagnosed with ALGS in his infancy based on intrahepatic bile duct paucity in liver biopsy, peripheral pulmonary artery stenosis, and renal tubular dysfunction and later with MAS based on radiographic findings of fibrous dysplasia.
A 4-year-old boy complained of repeated left femoral fractures.
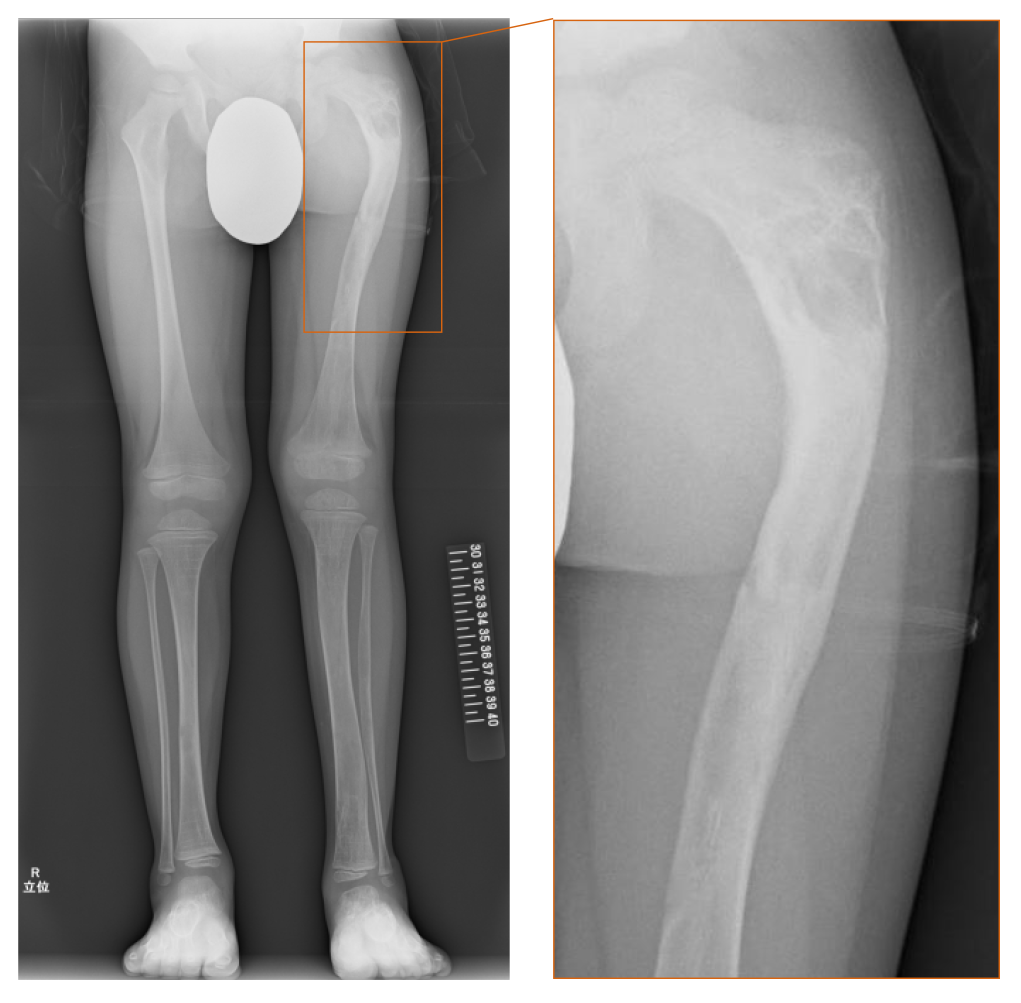
The patient had repeated left femoral fractures for four times (at 1 year and 3 mo, 1 year and 11 mo, 2 years and 10 mo, and 4 years and 3 mo old), and the difference in the length of his lower limbs gradually became apparent by the age of 2 years. While repeated femoral fractures were initially considered as bone metabolic disorders associated with ALGS, the serum phosphate levels had remained at the lower limit of the standard for age, and the level of fibroblast growth factor 23 (FGF23) was high as 117 pg/mL (reference range: 15-49 pg/mL[12]). At the age of 4 years and 8 mo, radiographic findings revealed a “ground-glass” appearance in his left femur and tibia and “shepherd’s crook deformity” in his left thigh bone, which were characteristic features of fibrous dysplasia (Figure 1).
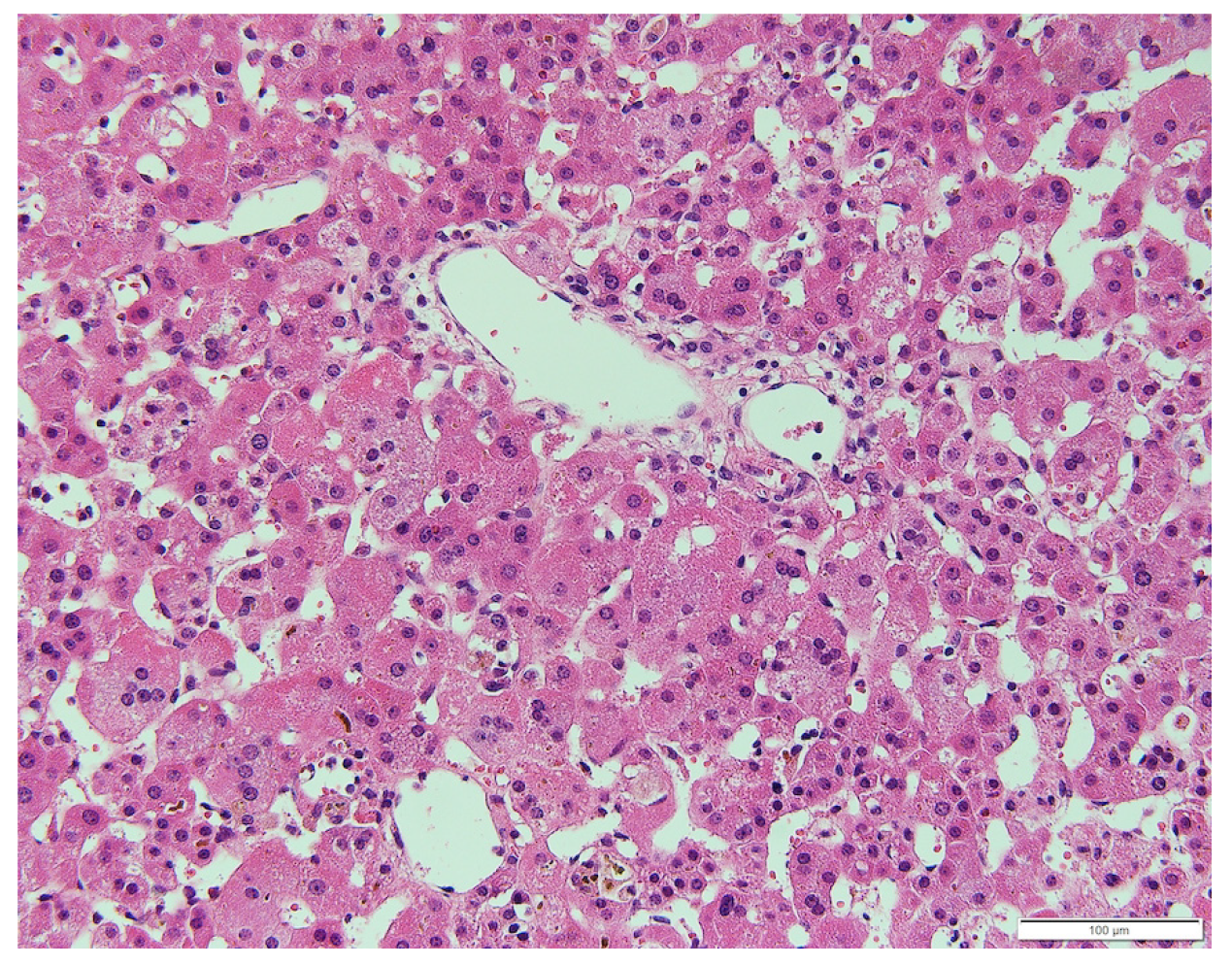
The patient was born at 40 wk and 6 d’ gestation; with a birth weight of 2726 g. Failure to thrive was noted at 18 d following birth. Further evaluation of this concern revealed hepatomegaly, elevated liver transaminase level [aspartate aminotransferase (AST) 193 U/L, alanine aminotransferase (ALT) 424 U/L], and hyperbilirubinemia (T-Bil 8.0 mg/dL, D-Bil 6.6 mg/dL). Liver biopsy was performed at the age of 1 mo, which revealed bile duct paucity (the ratio of the bile duct to the portal tract was 0.1) (Figure 2). Other than cholestasis, peripheral pulmonary artery stenosis, hypokalemia, and metabolic acidosis due to renal tubular dysfunction were observed. No butterfly vertebrae or ocular abnormalities were found. Although any large deletion and duplication were not observed in the JAG1 gene by the fluorescence in situ hybridization analysis, the patient was clinically suspected to have ALGS and was listed for liver transplantation. Cholestatic liver injury was gradually normalized by the age of 2 years under oral ursodeoxycholic acid and glycyrrhizic acid treatment and did not deteriorate even after both medications were tapered. His DNA was further subjected to a targeted next-generation sequencing that covers 14 genes responsible for cholestatic liver diseases[13], and no pathogenic variants were found in his genes including JAG1 and NOTCH2.
The patient was born to non-consanguineous Japanese parents. The pregnancy had been uncomplicated, and his family history was unremarkable.
At the age of 4 years and 9 mo, his height was 101.7cm ( -0.81 SD); body weight, 15.2kg ( -0.82 SD); and arm span, 104 cm. The difference in the length of the lower limbs was 1 cm (right, 53 cm; left, 52 cm). He did not exhibit jaundice or hepatosplenomegaly. He was noted to have a grade 2/6 systolic heart murmur. He did not have café-au-lait skin spots. His testicular capacity was 2 mL, pubic hair had not yet grown, and no precocious puberty was observed.
Laboratory examination at the age of 4 years revealed elevated levels of serum alkaline phosphatase (2506 U/L, reference range: 430-1200 U/L), bone alkaline phosphatase (216 U/L, reference range: 59-107 U/L[14]), FGF23 (86 pg/mL), and serum type I collagen cross-linked N-telopeptide (171 nmolBCE/L, reference range: 14-57 nmolBCE/L[15]). No endocrinological abnormalities were found. The transaminase and bilirubin levels were within the reference ranges (AST 28 U/L, ALT 25 U/L, T-Bil 0.6 mg/dL, and D-Bil 0.2 mg/dL).
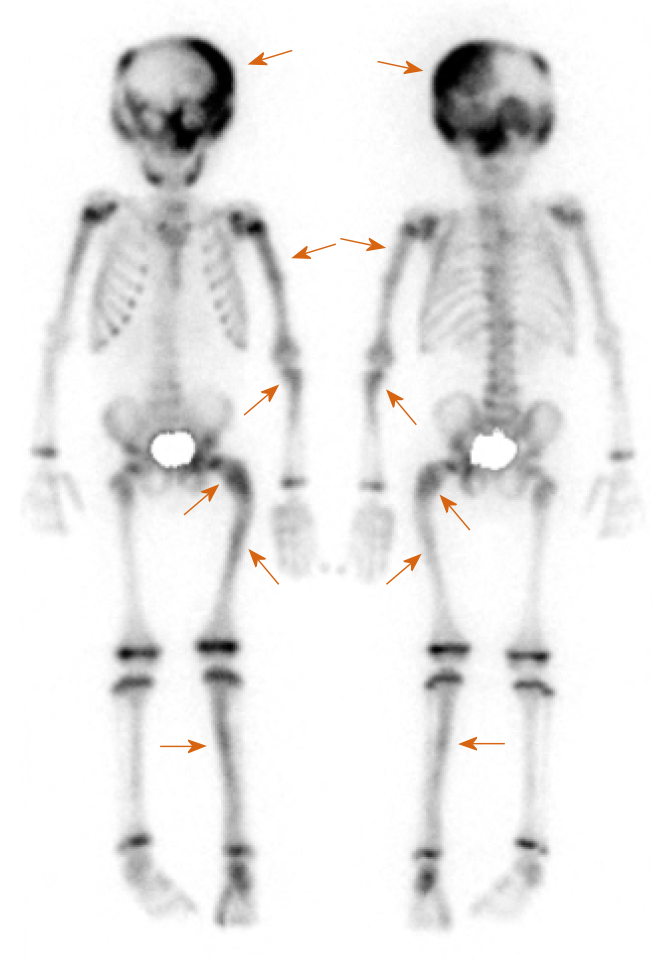
Bone scintigraphy with 99 mTc-hydroxymethylene diphosphonate, which was employed to detect lesions with enhanced bone metabolism, revealed multiple lesions with increased uptake in the left skull and upper left limb in addition to the left femur and left tibia (Figure 3).
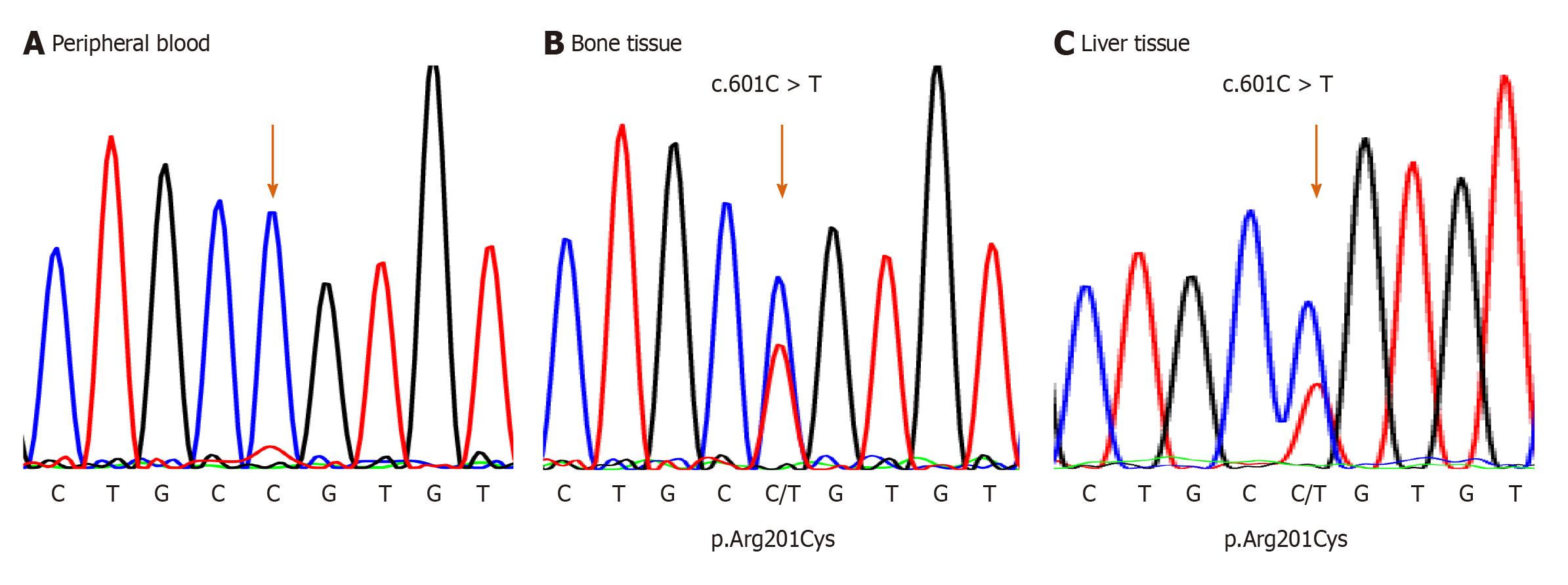
For the mutational analysis of the GNAS gene, genomic DNA from the peripheral blood was extracted using magLEAD Consumable Kit® (Precision System Science Co., Ltd., Chiba, Japan). In addition, it was polymerase chain reaction (PCR)-amplified for exons 7 to 10 and their splice sites of the GNAS gene, where mutation hotspots for MAS were reported. PCRs were conducted using the 5′-TCACTTCCG TTGAGCCTGAC-3′ and 5′-CTTGCACGGGGTTCTTCTCT-3′ primer set designed for detecting the mutation; however, sequencing after PCR did not reveal any mutations (Figure 4A).
Therefore, mutation analysis of the GNAS gene was also conducted from bone tissue samples, which were obtained from fibrous dysplastic lesions during a fracture surgery at the age of 5 years and 6 mo. The dissected bone sample was immediately snap-frozen using liquid nitrogen and crushed using 6700 Freezer/Mill (SPEX SamplePrep, NJ, United States). Genomic DNA from the bone tissue was extracted using DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany) and was PCR-amplified and sequenced similar to that of the peripheral blood. As a result, an activation point mutation (c.601C>T, p.Arg201Cys)[4] was detected in genomic DNA, and the patient was diagnosed with MAS (Figure 4B).
Furthermore, when he was 6 years old, DNA was extracted from a formalin-fixed paraffin-embedded (FFPE) liver tissue that was collected during the biopsy performed at the age of 1 mo. To isolate genomic DNA from the FFPE liver tissue, Agencourt FormaPure XL Total kit (Agencourt Bioscience Corporation, Beverly, MA, United States) was used. Genomic DNA from the liver tissue was PCR-amplified and sequenced for the corresponding site to the peripheral blood and bone tissue. PCRs and sequencing were conducted using the 5′-TTCGGTTGGCTTTGGTGAGA-3′ and 5′-CACGTCAAACATGCTGGTGG-3′ primer set designed for detecting the mutation. The same mutation from the bone tissue samples was observed (Figure 4C).
The final diagnosis of the presented case is MAS.
When he was 7 years old, an osteotomy was performed to correct the curvature of the left femur.
The patient was followed up for endocrine abnormalities, such as premature puberty and compression optic neuropathy, since bone scintigraphy revealed increased uptake in his skull. Furthermore, although his liver dysfunction did not persist, follow-up was continued with semiannual to annual abdominal ultrasonography for neoplasm in the liver.
MAS is caused by activating somatic mutations within the GNAS gene. These mutations occur in the early postzygotic period. The patient’s somatic cells are mosaic for the mutation; hence, the clinical features are determined by the distribution of the affected cells[4,16,17].
In MAS, hepatobiliary dysfunction is relatively rare, with a frequency of 5%-10%[18,19], and usually develops in the early stage of life as neonatal cholestasis[5,6,16,20,21]. Although cholestasis can be the first symptom of MAS and is sometimes followed by persistent elevation of the levels of serum liver enzymes, natural history has been reported as benign in most patients[5,6], and only a few cases required liver transplantation[20].
The histological findings of the patient in this report revealed intrahepatic bile duct paucity, which suggested ALGS along with characteristic features, such as neonatal cholestasis, peripheral pulmonary artery stenosis, renal tubular dysfunction, and recurrent bone fractures. Giant cell transformation has been the most common finding in the liver histology of MAS[5,22,23]. However, intrahepatic bile duct paucity was also reported in cases with MAS. In such cases, distinguishing MAS from ALGS based on clinical symptoms and pathological features is difficult as in our case, in which the difference in the length of the patient’s legs prompted us to suspect enhanced bone metabolism[6]. MAS should be considered among the differential diagnoses of ALGS when the liver tissue demonstrates intrahepatic bile duct paucity. A recent manuscript reported that combined sequencing of JAG1 and NOTCH2 along with copy number variant analysis of JAG1 did not identify pathogenic variants in 3.2% of patients who met the diagnostic criteria for ALGS[24]. Regarding renal tubular dysfunction and peripheral pulmonary artery stenosis in our case, we did not extract and sequence genomic DNA from renal tubular epithelial cells and pulmonary artery to detect the mutation in the tissues. Although our patient did not meet the classical diagnostic criteria of AGLS which is based on the presence of intrahepatic bile duct paucity on liver biopsy in association with at least three of the major clinical features: chronic cholestasis, cardiac disease, skeletal abnormalities, ocular abnormalities, and characteristic facial features, it is still possible that some other genes than GNAS or mutations in JAG1/NOTCH2 genes that cannot be detected with current methods are involved in AGLS-like renal and pulmonary features in our case.
Due to the somatic mosaic nature of the disease, a negative result of mutation analysis from the peripheral blood does not exclude the possibility of MAS[3,19], and DNA should be isolated from the affected tissues. In this case, GNAS gene mutation was detected from the surgical bone specimen and FFPE liver biopsy tissue, which was collected 6 years ago. As in this report, GNAS mutations have been detected in the liver tissue obtained from patients with neonatal cholestasis in previous reports[5,16,19,20]. The occurrence and severity of the hepatic phenotype depend on the number and location of the cells with the mutation[5,16]. Whether the patients still keep hepatic cells with the mutation in the GNAS gene following amelioration of their hepatic symptoms is unknown.
In most cases, neonatal cholestasis in patients with MAS resolves spontaneously. However, liver dysfunction may persist, and subsequent hepatic lesions may develop and exhibit malignant potential, such as hepatoblastoma and hepatocellular adenomas[6,21]. In this case, liver dysfunction did not persist, and liver lesions were not identified, but we continued to follow-up the patient for serum tumor markers with semiannual to annual abdominal ultrasonography.
We presented a case of a patient with MAS who was suspected of ALGS due to neonatal cholestasis and histological findings that revealed intrahepatic bile duct paucity. No pathogenic variants were noted in the JAG1 and NOTCH2 genes, and MAS was suspected from repeated fractures and radiographic findings. The mutation in the GNAS gene was detected in the bone and liver tissues, and the patient was diagnosed with MAS. MAS should be considered as a differential diagnosis for cholestasis in infancy.
Hepatobiliary dysfunction is relatively rare in MAS, but MAS should be considered as a part of the differential diagnosis of neonatal cholestasis with unknown causes, and genetic diagnosis using liver tissue is possible.
We would like to thank Dr. Fujitake Y at the Medical Department of Pediatrics of Kitasato University Hospital, who previously treated this patient in infancy and providing us the formalin-fixed paraffin-embedded liver tissue.
Manuscript source: Unsolicited manuscript
Specialty type: Pediatrics
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jafar-Nejad H S-Editor: Zhang H L-Editor: A P-Editor: Wang LYT
| 1. |
McCune DJ.
Osteitis fibrosa cystica: the case of a nine-year old girl who also exhibits precocious puberty, multiple pigmentation of the skin and hyperthyroidism |
| 2. | Albright F, Butler AM, Hampton AO, Smith P. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females. N Engl J Med. 1937;216:727-746. [DOI] [Full Text] |
| 3. | Dumitrescu CE, Collins MT. McCune-Albright syndrome. Orphanet J Rare Dis. 2008;3:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 257] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 4. | Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1002] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 5. | Silva ES, Lumbroso S, Medina M, Gillerot Y, Sultan C, Sokal EM. Demonstration of McCune-Albright mutations in the liver of children with high gammaGT progressive cholestasis. J Hepatol. 2000;32:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Johansen L, Haller W, Thyagarajan M, Kelly D, McKiernan P. Hepatic Lesions Associated With McCune Albright Syndrome. J Pediatr Gastroenterol Nutr. 2019;68:e54-e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Alagille D, Estrada A, Hadchouel M, Gautier M, Odièvre M, Dommergues JP. Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J Pediatr. 1987;110:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 385] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Turnpenny PD, Ellard S. Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2012;20:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 272] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 9. | Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, Costa T, Pierpont ME, Rand EB, Piccoli DA, Hood L, Spinner NB. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 877] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 10. | Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 765] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 11. | McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 502] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 12. | Bacchetta J, Dubourg L, Harambat J, Ranchin B, Abou-Jaoude P, Arnaud S, Carlier MC, Richard M, Cochat P. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95:1741-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Togawa T, Sugiura T, Ito K, Endo T, Aoyama K, Ohashi K, Negishi Y, Kudo T, Ito R, Kikuchi A, Arai-Ichinoi N, Kure S, Saitoh S. Molecular Genetic Dissection and Neonatal/Infantile Intrahepatic Cholestasis Using Targeted Next-Generation Sequencing. J Pediatr 2016; 171: 171-7. e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Yang L, Grey V. Pediatric reference intervals for bone markers. Clin Biochem. 2006;39:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | van der Sluis IM, Hop WC, van Leeuwen JP, Pols HA, de Muinck Keizer-Schrama SM. A cross-sectional study on biochemical parameters of bone turnover and vitamin d metabolites in healthy dutch children and young adults. Horm Res. 2002;57:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Shenker A, Weinstein LS, Moran A, Pescovitz OH, Charest NJ, Boney CM, Van Wyk JJ, Merino MJ, Feuillan PP, Spiegel AM. Severe endocrine and nonendocrine manifestations of the McCune-Albright syndrome associated with activating mutations of stimulatory G protein GS. J Pediatr. 1993;123:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 172] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Völkl TM, Dörr HG. McCune-Albright syndrome: clinical picture and natural history in children and adolescents. J Pediatr Endocrinol Metab. 2006;19 Suppl 2:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Ringel MD, Schwindinger WF, Levine MA. Clinical implications of genetic defects in G proteins. The molecular basis of McCune-Albright syndrome and Albright hereditary osteodystrophy. Medicine (Baltimore). 1996;75:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Lumbroso S, Paris F, Sultan C; European Collaborative Study. Activating Gsalpha mutations: analysis of 113 patients with signs of McCune-Albright syndrome--a European Collaborative Study. J Clin Endocrinol Metab. 2004;89:2107-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. |
Coles N, Comeau I, Munoz T, Harrington J, Mendoza-Londono R, Schulze A, Kives S, Kamath BM, Hamilton J.
Severe Neonatal Cholestasis as an Early Presentation of McCune-Albright Syndrome |
| 21. | Gaujoux S, Salenave S, Ronot M, Rangheard AS, Cros J, Belghiti J, Sauvanet A, Ruszniewski P, Chanson P. Hepatobiliary and Pancreatic neoplasms in patients with McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99:E97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Ikawa Y, Yachi Y, Inoue N, Kato A, Okajima M, Yachie A. Neonatal McCune-Albright Syndrome with Giant Cell Hepatitis. J Pediatr. 2016;178:298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Corsi A, Cherman N, Donaldson DL, Robey PG, Collins MT, Riminucci M. Neonatal McCune-Albright Syndrome: A Unique Syndromic Profile With an Unfavorable Outcome. JBMR Plus. 2019;3:e10134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Gilbert MA, Bauer RC, Rajagopalan R, Grochowski CM, Chao G, McEldrew D, Nassur JA, Rand EB, Krock BL, Kamath BM, Krantz ID, Piccoli DA, Loomes KM, Spinner NB. Alagille syndrome mutation update: Comprehensive overview of JAG1 and NOTCH2 mutation frequencies and insight into missense variant classification. Hum Mutat. 2019;40:2197-2220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |