Published online Jan 9, 2021. doi: 10.5409/wjcp.v10.i1.1
Peer-review started: October 13, 2020
First decision: December 11, 2020
Revised: December 15, 2020
Accepted: December 24, 2020
Article in press: December 24, 2020
Published online: January 9, 2021
Processing time: 89 Days and 4.3 Hours
Epistaxis can be an isolated finding or a manifestation of a systemic disease. Some of the potential etiologies are usage of anticoagulants, bleeding disorders, vascular aneurysms, nasal neoplasm, hypertension and nasal steroids. Hereditary hemorrhagic telangiectasia (HHT) as a cause of recurrent epistaxis is uncommon.
In this report, we describe an 18-year-old adolescent with recurrent epistaxis, mucocutaneous telangiectasia and family history of HHT, consistent with HHT.
Timely diagnosis is needed not only to treat the epistaxis but also to be vigilant for other serious manifestations of this condition.
Core Tip: In patients with recurrent spontaneous epistaxis, a thorough history, family history, physical examination and investigation is necessary to exclude hereditary hemorrhagic telangiectasia which can present with multi-system involvement along with epistaxis.
- Citation: Acharya R, Portwood K, Upadhyay K. Hereditary hemorrhagic telangiectasia presenting as a recurrent epistaxis in an adolescent: A case report. World J Clin Pediatr 2021; 10(1): 1-6
- URL: https://www.wjgnet.com/2219-2808/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v10.i1.1
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant vascular disorder. Epistaxis, gastrointestinal (GI) bleeding, iron deficiency anemia, and mucocutaneous telangiectasia are the most common manifestations of the disease[1]. Epistaxis is secondary to telangiectasia of the nasal mucosa. Arteriovenous malformations (AVM) in the visceral organs such as lungs, liver and brain can occur for which symptomatic patients should be screened, as they can be fatal. Intestinal polyps have been described in association with HHT (juvenile polyposis-HHT overlap syndrome), which is seen in 1 percent of HHT cases and is due to mutations in SMAD4. Pulmonary and cerebral AVMs are more common in HHT1 patients, while hepatic AVMs and pulmonary hypertension are more common in those with HHT2[2].
An 18-year-old male patient with a past medical history of renal calculi, asthma, environmental allergies, irritable bowel syndrome, and frequent nosebleeds presented to the pediatric clinic for evaluation of nosebleeds.
Patient has had recurrent nosebleeds throughout his life, which seemed to have worsened as a teenager. The bleeding would usually last for 15 min and resolve with nasal compression. There was no history of nose picking or trauma to the nose.
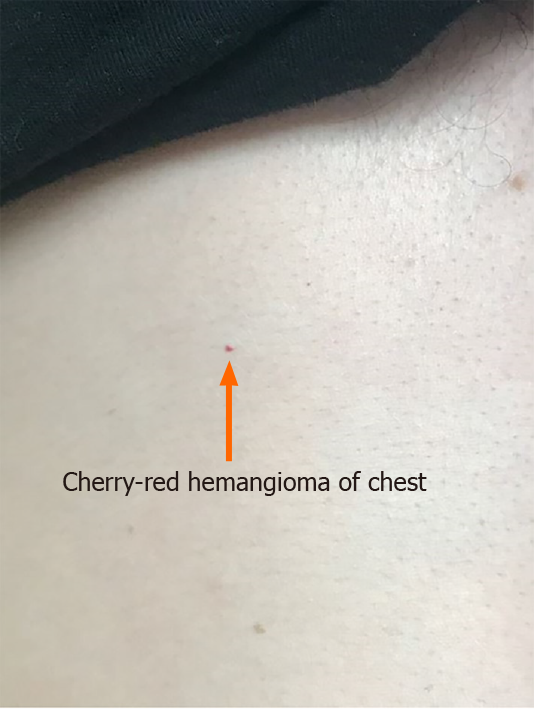
The patient denied bleeding while brushing his teeth, long duration of wound healing or swelling of joints. He denied bruising or petechiae but reported to have small red spots on his chest. Patient was known to have episodes of syncope during the episodes of nosebleeds. There was no history of black, tarry or grossly bloody stool, vomiting or blood in urine. There was no history of chest pain, shortness of breath, hemoptysis, hematemesis or seizures.
He had a history of mild intermittent asthma and dust mite allergy for which he was taking albuterol and montelukast. The family history was positive for primary biliary cholangitis, Hashimoto’s thyroiditis, psoriasis, asthma, arthritis along with pulmonary and liver AVMs and telangiectasias of the finger in the mother. Father had atrial fibrillation, hypertension and hyperlipidemia. Further family history revealed extensive bleeding history in the maternal side, including intestinal telangiectasias in maternal aunt and cousin and frequent nosebleeds in the maternal uncle. Maternal grandmother has had “bleeding problems in intestine and brain”.
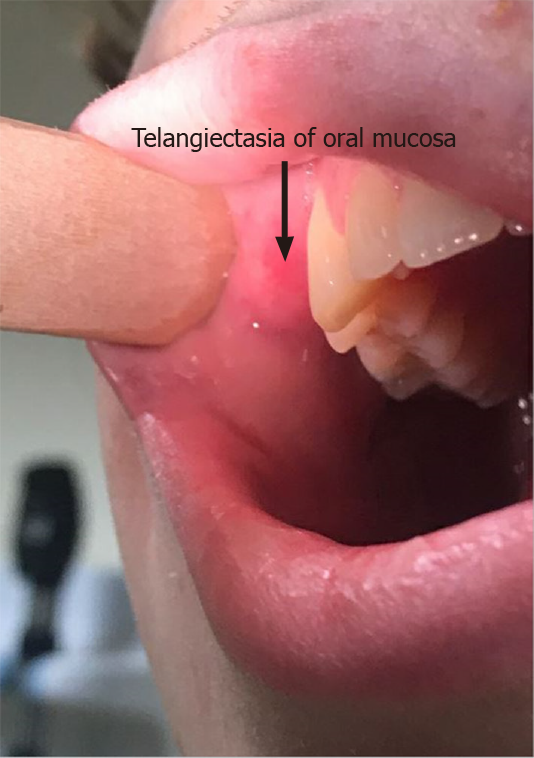
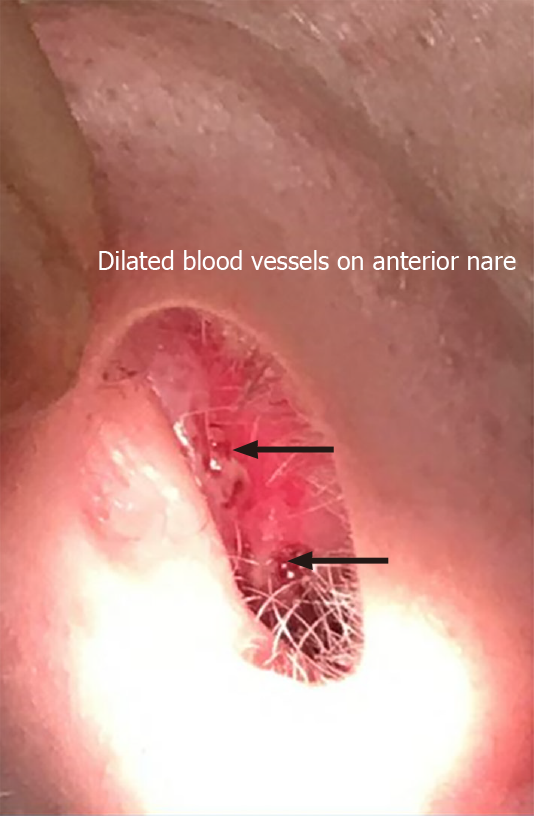
Physical examination revealed stable vital signs with blood pressure of 120/78 mmHg. Skin examination showed telangiectasias on gum line and inner lips along with cherry hemangiomas on the chest and back (Figures 1 and 2). There were no other skin rashes or lesions suggestive of autoimmune disease. Nasal examination showed erythematous mucus membranes with excoriation in bilateral nares, dilated blood vessels in anterior nares with no active bleeding, and no mass or polyps (Figure 3). Conjunctiva was injected. Rest of the physical examination was normal.
Investigations showed evidence of iron deficiency anemia (hemoglobin 9.5 gm/dL), which was thought to be secondary to long standing history of spontaneous recurrent epistaxis. Stool occult blood was normal. Liver function test was normal. Coagulation and bleeding profile were normal. Upper and/or lower GI endoscopies were not performed as there was no history suggestive of GI bleeding. Renal function panel showed serum creatinine of 0.7 mg/dL and stable electrolytes. Urinalysis was negative for proteinuria or hematuria. Thyroid function test was normal.
Chest X-ray was normal. Although he did not have clinical evidence of pulmonary or hepatic AVMs, screening for the latter with computed tomography (CT) of chest and abdomen showed no evidence of pulmonary or hepatic AVMs. Given absence of headache, seizures, and altered sensorium, a CT scan of the head and neck initially was not performed. The prior history of syncopal attacks during episodes of epistaxis was attributed to hypotension from significant blood loss. He was also referred to Genetics for genetic testing and was found to have mutation of ENG in chromosome 9, typical of HHT type 1. After genetic confirmation, a further screening with CT angiogram of head and neck did not reveal presence of AVMs.
Experts of Ear, Nose and Throat, Hematology, and Genetics.
Patient’s family history and current symptoms were consistent with HHT (Osler-Weber-Rendu syndrome). Patient’s mother and maternal cousin had also been diagnosed with this condition in the past. Since he met three out of four criteria for HHT (spontaneous and recurrent epistaxis, mucocutaneous telangiectasias, and first degree relative with HHT), a definite diagnosis of HHT was made.
Iron therapy was started for iron deficiency anemia. An ENT evaluation was also recommended who recommended supportive treatment given absence of bleeding at the time of evaluation.
Patient continues to have recurrent epistaxis with mild anemia, for which he continues to take oral iron therapy. Given absence of visceral involvement at this time, he is being followed up closely every few months in the outpatient clinic.
The presence of HHT may be suspected in patients with spontaneous and recurrent epistaxis, mucocutaneous telangiectasia in the fingertips, lips, oral mucosa or tongue, visceral involvement (such as GI, pulmonary, cerebral, spinal or hepatic AVM), and/ or a first-degree relative with HHT[2]. Three or more criteria indicate definite disease (Curacao criteria). The disease is suspected in patients meeting only two criteria. It is inherited as an autosomal dominant trait. Multiple variants of the three main genes can cause HHT. HHT1 is caused by mutations in the gene ENG which transcribes protein product, endoglin. HHT2 is due to sequence variants in ACVRL1, a gene than encodes protein product activin receptor-like kinase-1, or ALK-1. HTJP (HHT in association with juvenile polyposis) is due to mutation in SMAD4, which encodes the protein Smad4. Since genetic testing does not detect all mutations, the diagnosis of HHT does not rely on genetic testing. However, if genetic testing is done, identification of a pathogenic sequence variant in ENG, ACVRL1, or SMAD4 is typically seen[3]. Our patient had HHT1 sary to mutation of ENG in chromosome 9 (Invitae Hereditary Hemorrhagic Telangiectasia Panel, San Francisco, CA, United States).
The telangiectasias are generally not present at birth but develop with increasing age. Epistaxis is usually the earliest sign of disease, often occurring in childhood. Mucocutaneous and gastrointestinal telangiectasia develop with age and pulmonary AVM (PAVM) generally become apparent after puberty. Cerebral vascular malformations are also thought to develop during childhood and are clinically silent. Giordano et al[4] studied 44 children (mean age, 10.3 years; range, 1-18) with HHT1 and HHT2 and found that cerebrovascular AVMs were present in 7 of 44 cases, pulmonary AVMs in 20 of 44 cases, and liver AVMs in 23 of 44 cases. Large visceral AVMs were found in 27% children and were significantly more frequent in patients with HHT1. Only large AVMs were associated with symptoms and complications.
Over the age of 40, recurrent GI bleeding occurs in up to one-third patients with HHT, mostly occurring in the stomach or duodenum rather than the colon. In patients with severe anemia and/or overt GI bleeding, endoscopy is recommended to evaluate and visualize telangiectasias, which appear similar to their mucocutaneous counterparts and are surrounded by an anemic halo[5]. GI bleeding can present with iron deficiency anemia. Mucocutaneous telangiectasias are not used for diagnostic purposes but are frequently seen in lips, tongue, buccal mucosa and fingertips and present later in life[2].
PAVMs are abnormal thin-walled vessels that replace normal capillaries between the pulmonary arteries and veins. They often result in sac-like structures and provide a direct capillary-free communication creating a shunt. Arterial blood passing through these right-to-left shunts cannot be oxygenated, leading to hypoxemia resulting in polycythemia. PAVMs are mostly asymptomatic; however, one-third of affected patients may exhibit cyanosis, clubbing, and polycythemia. Patients are at risk for neurologic sequelae due to paradoxical embolism passing through the shunts. Cerebral events such as abscess or stroke along with transient ischemic attacks can occur in patients with asymptomatic PAVMs[6].
Patients with HHT may have cerebral or spinal cord involvement which can be clinically silent. Symptomatic patients with AVMs, or high-flow AV fistulae may present with seizures, ischemia of the surrounding tissue due to a steal effect, or hemorrhage. Thus, patients with symptoms suggestive of cerebral AVMs warrant further assessment with imaging. Medical management may be sufficient; however, some may need interventions such as neurosurgery, embolization, or stereotactic radiotherapy, alone or in combination[7].
Hepatic involvement may occurs in up to two-thirds of patients with HHT. Common manifestations are portal hypertension, biliary disease and heart failure. Hepatic AVMs places patients at risk for angina and heart failure secondary to shunts created between the hepatic artery and vein. Hepatic AVMs are suspected in patients with abnormal liver function test, hepatomegaly or liver bruit. Liver biopsy is not recommended due to risk of bleeding, but diagnosis can be confirmed with CT, magnetic resonance imaging, or sonogram[2].
Patients with HHT are at increased risk for venous thromboembolism for which treatment or prophylactic anticoagulation may be required[8]. Low serum iron level is associated with elevated factor VIII level which can lead to thromboembolic phenomenon. Hence, it is important, to identifying and treat iron deficiency anemia in these patients[2].
The second international HHT guidelines state that all children with recurrent bleeding and/or symptoms of anemia should be tested for iron deficiency anemia and started on oral or intravenous iron therapy, and blood transfusions for severe anemia[8]. Although, the genetic testing is not required for diagnosis of HHT, the testing is recommended for asymptomatic children of a parent with HHT. Also, screening for brain and pulmonary AVMs in asymptomatic children with HHT or at risk for HHT at the time of presentation/diagnosis is recommended. Brain AVMs with high risk features, large pulmonary AVMs and AVMs associated with reduced oxygen saturation should be treated in children, with a repeat screening for such at every five-year intervals[8].
The management of HHT is focused on reducing the symptoms arising from each organ system involvement. Recently, new therapeutic interventions targeting at vascular endothelial growth factor (VEGF) and the angiogenic pathway with anti-VEGF antibody (such as bevacizumab) and VEGF receptor 2 tyrosine kinase inhibitor (such as pazopanib) are being studied[9]. Tacrolimus and sirolimus have also shown promising results in some studies[9].
Patients with HHT are at risk for non-traumatic recurrent epistaxis and hemorrhages in the brain, liver, lungs, or other organs. A high index of suspicion for HHT is necessary in patients who present with recurrent epistaxis for timely evaluation and management.
Manuscript source: Unsolicited manuscript
Specialty type: Pediatrics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Govindarajan KK, Handra-Luca A, Kupeli S S-Editor: Gao CC L-Editor: A P-Editor: Li X
| 1. | Stuhrmann M, El-Harith el-HA. Hereditary hemorrhagic telangiectasia. Genetics, pathogenesis, clinical manifestation and management. Saudi Med J. 2007;28:11-21. [PubMed] |
| 2. | Govani FS, Shovlin CL. Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur J Hum Genet. 2009;17:860-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 3. | Fernandez-L A, Garrido-Martin EM, Sanz-Rodriguez F, Pericacho M, Rodriguez-Barbero A, Eleno N, Lopez-Novoa JM, Düwell A, Vega MA, Bernabeu C, Botella LM. Gene expression fingerprinting for human hereditary hemorrhagic telangiectasia. Hum Mol Genet. 2007;16:1515-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Giordano P, Lenato GM, Suppressa P, Lastella P, Dicuonzo F, Chiumarulo L, Sangerardi M, Piccarreta P, Valerio R, Scardapane A, Marano G, Resta N, Quaranta N, Sabbà C. Hereditary hemorrhagic telangiectasia: arteriovenous malformations in children. J Pediatr 2013; 163: 179-86. e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Grève E, Moussata D, Gaudin JL, Lapalus MG, Giraud S, Dupuis-Girod S, Calender A, Plauchu H, Saurin JC. High diagnostic and clinical impact of small-bowel capsule endoscopy in patients with hereditary hemorrhagic telangiectasia with overt digestive bleeding and/or severe anemia. Gastrointest Endosc. 2010;71:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet. 2006;43:97-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 265] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, Al-Shahi Salman R, Vicaut E, Young WL, Houdart E, Cordonnier C, Stefani MA, Hartmann A, von Kummer R, Biondi A, Berkefeld J, Klijn CJ, Harkness K, Libman R, Barreau X, Moskowitz AJ; international ARUBA investigators. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 788] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 8. | Faughnan ME, Mager JJ, Hetts SW, Palda VA, Lang-Robertson K, Buscarini E, Deslandres E, Kasthuri RS, Lausman A, Poetker D, Ratjen F, Chesnutt MS, Clancy M, Whitehead KJ, Al-Samkari H, Chakinala M, Conrad M, Cortes D, Crocione C, Darling J, de Gussem E, Derksen C, Dupuis-Girod S, Foy P, Geisthoff U, Gossage JR, Hammill A, Heimdal K, Henderson K, Iyer VN, Kjeldsen AD, Komiyama M, Korenblatt K, McDonald J, McMahon J, McWilliams J, Meek ME, Mei-Zahav M, Olitsky S, Palmer S, Pantalone R, Piccirillo JF, Plahn B, Porteous MEM, Post MC, Radovanovic I, Rochon PJ, Rodriguez-Lopez J, Sabba C, Serra M, Shovlin C, Sprecher D, White AJ, Winship I, Zarrabeitia R. Second International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. Ann Intern Med. 2020;173:989-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 9. | Robert F, Desroches-Castan A, Bailly S, Dupuis-Girod S, Feige JJ. Future treatments for hereditary hemorrhagic telangiectasia. Orphanet J Rare Dis. 2020;15:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |