Published online Dec 8, 2012. doi: 10.5409/wjcp.v1.i4.24
Revised: September 13, 2012
Accepted: December 5, 2012
Published online: December 8, 2012
Profound congenital sensorineural hearing loss (SNHL) is not so infrequent, affecting 1 to 2 of every 1000 newborns in western countries. Nevertheless, universal hearing screening programs have not been widely applied, although such programs are already established for metabolic diseases. The acquisition of spoken language is a time-dependent process, and some form linguistic input should be present before the first 6 mo of life for a child to become linguistically competent. Therefore, profoundly deaf children should be detected early, and referred timely for the process of auditory rehabilitation to be initiated. Hearing assessment methods should reflect the behavioural audiogram in an accurate manner. Additional disabilities also need to be taken into account. Profound congenital SNHL is managed by a multidisciplinary team. Affected infants should be bilaterally fitted with hearing aids, no later than 3 mo after birth. They should be monitored until the first year of age. If they are not progressing linguistically, cochlear implantation can be considered after thorough preoperative assessment. Prelingually deaf children develop significant speech perception and production abilities, and speech intelligibility over time, following cochlear implantation. Age at intervention and oral communication, are the most important determinants of outcomes. Realistic parental expectations are also essential. Cochlear implant programs deserve the strong support of community members, professional bodies, and political authorities in order to be successful, and maximize the future earnings of pediatric cochlear implantation for human societies.
- Citation: Vlastarakos PV. Profound deafness and the acquisition of spoken language in children. World J Clin Pediatr 2012; 1(4): 24-28
- URL: https://www.wjgnet.com/2219-2808/full/v1/i4/24.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v1.i4.24
The development of spoken language is one of the most spectacular accomplishments of a child. Language is central to most aspects of the child’s life, and plays a role in the acquisition of a sense of self, and the achievement of social identity[1]. In addition, the ability to share information about intentions, ideas and feelings plays a vital role in human interaction[2], and finally results in social integration.
It is widely accepted that if listening is not developed during the critical language learning years, the acquisition of spoken language is severely compromised[1]. Profound congenital sensorineural hearing loss (SNHL) is not so infrequent, as it is estimated to affect 1 to 2 of every 1000 newborns in western countries. More than 50% of cases of congenital SNHL are thought to be of genetic origin. Up to 80% of cases are inherited in a recessive manner, and up to 70% are non-syndromic. A mutation in the gap junction β-2 (GJB2) gene, which controls protein connexin 26, an important regulator of potassium flow in the inner ear, is responsible for 30%-50% of congenital non-syndromic SNHL. On the other hand, syndromes associated with congenital SNHL include: (1) Pendred’s syndrome (accompanying feature: goiter); (2) Alport’s syndrome (accompanying feature: renal failure); (3) Usher’s syndrome (accompanying feature: retinitis pigmentosa); (4) Waardenburg syndrome (accompanying feature: white tuft in the area of the forehead); (5) CHARGE syndrome (accompanying features: coloboma, heart defects, choanal atresia, retardation, genitourinary abnormalities); and (6) Jervell and Lange-Nielsen syndromes (accompanying features: long Q-T on ECG, arrythmias). Despite the relatively high incidence of congenital SNHL, however, universal hearing screening programs have not been widely applied, and most countries have only established screening programs for high-risk infants. By contrast, metabolic diseases such as phenylketonuria, with an incidence of approximately 1 in 15 000 births, are routinely detected through newborn screening.
Early identification, referral, and diagnosis of children with hearing loss are necessary to initiate the process of auditory rehabilitation, which can ensure in turn that the hearing impaired child will receive the maximum amount of auditory information during the critical periods for spoken language development, thus reducing the effects of auditory deprivation.
Indeed, the shear basis of our evolutionary advantage is the ability to increase our knowledge through our use of language. Language, however, does not just happen in an instance, but is a time-dependent process[3].
Three general conditions are widely accepted in the area of the acquisition of a first language in children: (1) babies are exposed to language from birth onwards. This occurs in conversational settings, and is provided by those close to them; (2) a first language is acquired by infants through communicative interaction with competent users of that language; and (3) the language addressed to the child displays some characteristics which make it especially helpful for the young language learner, who is an immature conversational partner. These characteristics do not only include the structural and semantic features of the language, but also particular communicative behaviours, such as the management of the child’s attention by the adult, who is trying to communicate with the child[1].
In fact, with regard to the latter condition, there seems to be a triangular scheme of communication leading to vocal development. The child’s and caregiver’s lines of visual regard form two sides of the triangle, and the language input from the caregiver, which is received by the child through audition, forms the third side. A communication link is formed, as the caregiver communicates with the child, while the child is looking at something, making their interaction meaningful. When a baby is profoundly deaf, the third side of the triangle is practically absent[4].
Given the three aforementioned conditions, which are almost always present in the early lives of normally developing infants in all cultures, receiving enough, and good enough language input for successful language development is rarely a problem for them. Language acquisition is a robust process for normally developing children, which fails only in cases of extreme deprivation[5].
The development of language appears to follow a hierarchical progression. It includes first the sound of words-phonology. It is then followed by the meaning of words-semantics, and finally by the rules of grammar-syntax. Semantics and syntax are, therefore, dependent on appropriate and timely phonological input. They can, however, develop further in the years to come. Both intrinsic (hearing, processing, neuroplasticity) and extrinsic mechanisms (linguistic input, social and cultural influences) affect the development of spoken language. Language acquisition seems, in fact, to be a product of both nature and nurture[3].
The CNS has an ability to adapt to sensory changes, which appears to be inversely proportional to age. It seems that for a child to become linguistically competent, some form linguistic input should be present before the first 6 mo of life. In addition, the acquisition of a normal language is guaranteed for children up to the age of six, is steadily compromised from then until shortly after puberty, and is rare thereafter[6].
Many studies have shown that hearing-impaired children use excessively high pitches[7,8] and inappropriate variations in the fundamental frequency of their voice[9]. Reduced sound repertoires, containing multiple errors, are also characteristic of profoundly hearing-impaired children. Substitutions of one sound for another, omissions, and distortions frequently occur[10]. Syllabic structures are adversely influenced, and fail to show the variety of features associated with normal-hearing speakers. Consonant and vowel productions also are replete with errors, and contribute to reductions in overall speech intelligibility[11]. Visible consonants produced in the front of the mouth are used more frequently, than less visible consonants produced in the back of the mouth[12,13]. Front vowels appear to be produced with more errors than back vowels, thus suggesting that profoundly hearing-impaired children may have difficulty with the position of the tongue[13].
With regard to vocabulary growth, although there is not a universal agreement as to the extent of normal variation between hearing children, estimates range from 2000 to 10 000 words for a 5-year-old. Most children encounter new words by the tens of thousands per year, and learn thousands of them. By comparing these numbers to those of a deaf child, some indication is given of the ensuing handicap. Di Carlo[14] estimated that a “typical five-year-old deaf child” has approximately 25 words! In fact, in the absence of any rehabilitation, congenitally deaf children will have little concept of the existence of verbal language, and effectively no experience of it, by the time they reach school age[15]. By the time these children reach the end of their school career, and again in the absence of appropriate rehabilitation, their English vocabulary may not actually exceed that of a 6-year-old hearing child.
For people who are deaf, the chances of misunderstandings occurring during everyday interactions are far greater than for normal-hearing people. Conversations between the deaf and normal hearing can be fraught with difficulty. However, society tends to categorize people, and subsequently decide upon what is normal for each person in these categories[16]. In effect, we make assumptions about people and how we expect them to be, and if someone doesn’t fit in with these expectations, he/she is then demoted to a “not-so-good-as-us” level, and may be stigmatised accordingly. As peers constitute a significant part in a child’s life, there is a strong possibility that hearing-impaired children will begin to perceive themselves as “different” from an early age, and run the risk of becoming stigmatized.
Early referral, timely diagnosis and appropriate management of infants with profound SNHL are now considered of paramount importance in the developed world. It is, therefore, essential that the related methods accurately reflect the behavioural audiogram[17].
Behavioural observation techniques based on the presentation of a loud sound, and the observation of the baby’s response, have been largely superseded by more objective hearing tests[18]. Nevertheless, they may still prove useful to General Pediatricians and Practitioners, albeit needing some experience in interpreting the variety of possible responses. The related tests include: (1) induction of the sound blinking reflex, with the baby quickly blinking his/her eyelids, or shutting them more tightly, when stimulated by a sudden sound of 105-115 decibel (dB); (2) the startle reflex (Moro reflex), a rapid movement of the infant’s head, with symmetrical extension of his/her extremities, while forming a C shape with the thumb and forefinger, when the infant is stimulated by a sudden noise of 80-85 dB in intensity. This is followed by a return to a flexed position with the extremities against the body; and (3) Rattle or bell tests, with the baby turning towards the sound, or widening his/her eyes with sound. The obtained response is not reflexive, and sometimes even normally-hearing infants are unable to make reliable direct head turn responses towards sound sources[19].
The progress of clinical audiology during the last decades has been remarkable, nevertheless, none of the three objective tests typically performed in most specialist centers (otoacoustic emissions-OAEs, auditory brainstem responses-ABRs, and auditory steady state responses-ASSRs) are perfect[20]. Additional disabilities (i.e., autism), which may not be able to be detected early in life[21], also need to be taken into account.
Apart from scientific dilemmas, reliable diagnosis is also very important to parents and family. Parents may indeed experience significant emotional stress during and following hearing assessment. Hence, both the diagnostic process and the certainty of the diagnosis are considered central for them, to accept the problem, and participate in future management[22]. In addition, parental and family bonding and behaviour towards the infant, along with their trust to physicians, may be disturbed, when the diagnosis is inaccurate or doubtful[23].
Hearing loss in the profoundly deaf can range, by definition, from a low of 90 dB to a high in the region of 120 dB. People with 120 dB hearing losses are probably totally deaf, and respond to sound only through the sense of touch[24].
Profound congenital SNHL is managed by a multidisciplinary team (MDT), which includes the pediatrician, the ENT surgeon, the genetic scientist, the clinical audiogist, the speech and language therapist, the psychologist, the teacher for the deaf, and the social worker. Ideally, infants with profound congenital SNHL should be bilaterally fitted with hearing aids, no later than 3 mo after birth. The child’s progress should be monitored by the speech and language therapist and the teacher for the deaf, which should report their findings to the MDT.
If the child is profoundly deaf and is not progressing linguistically, despite the consistent use of bilateral hearing aids, and the intensive speech and language therapy, a cochlear implantation can be considered after thorough preoperative assessment. Neuroplasticity and neurolinguistic issues have led cochlear implant centers to the decision of implanting children younger than 12 mo of age. Despite the relative lack of robust and reliable outcome measures of monitoring implanted infants[25], it has been reported that implanted infants demonstrate improved auditory, speech language and cognitive performances compared to children implanted later[26].
Cochlear implants represent one of the most important achievements of modern medicine, as for the first time in history an electronic device is able to restore a lost sense-hearing[27].
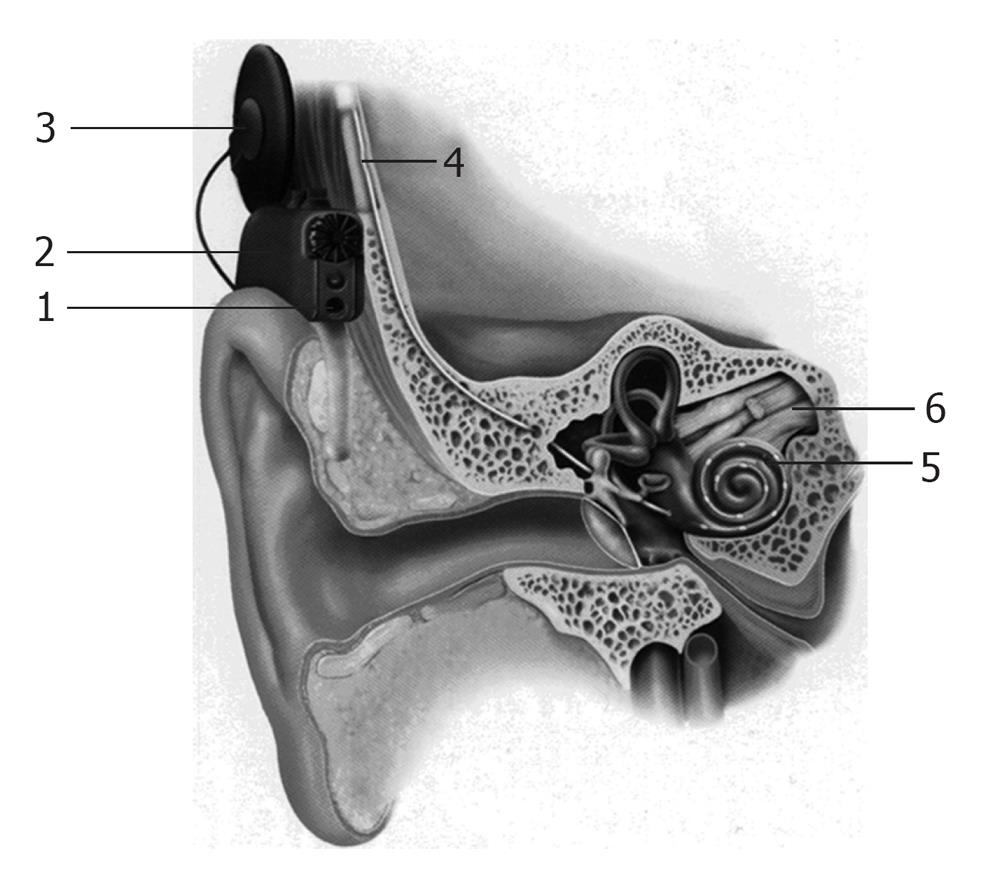
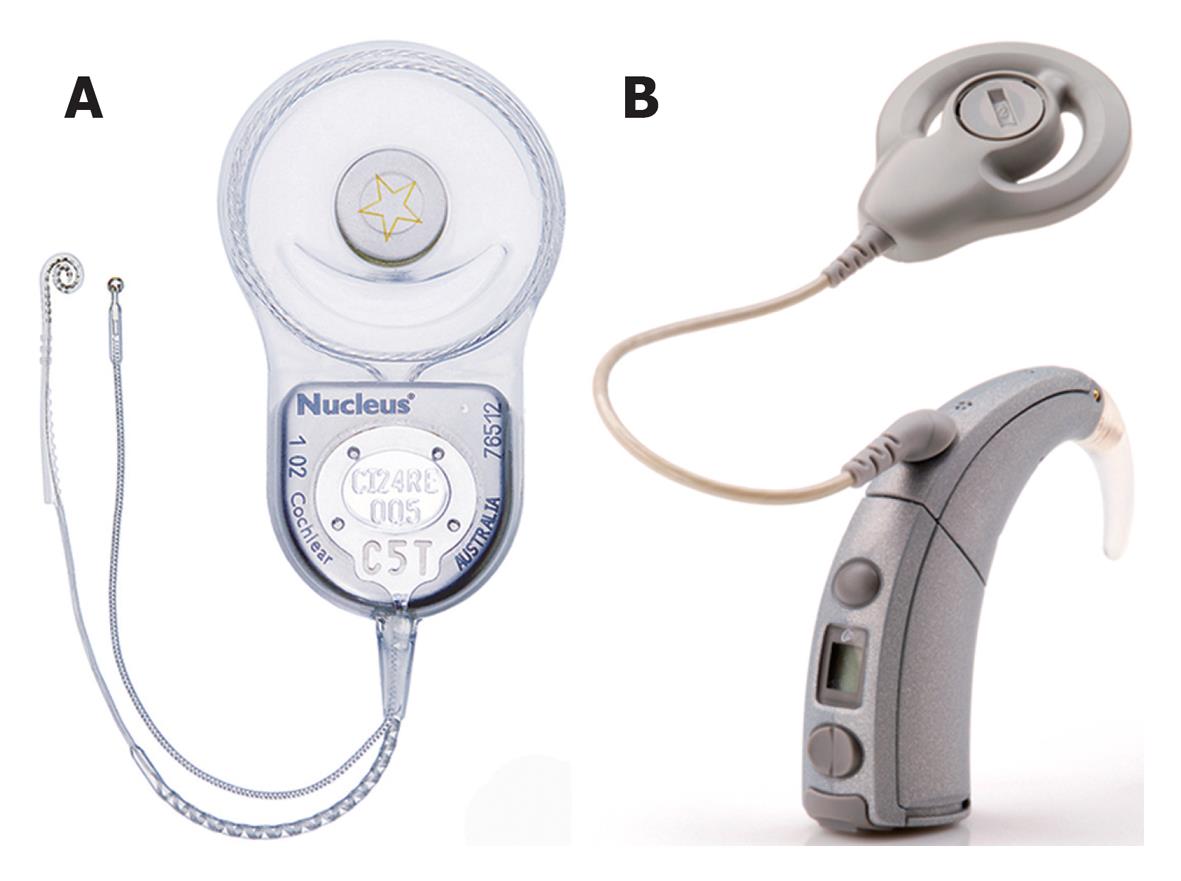
A cochlear implant system comprises of the following components (Figures 1 and 2): (1) A multi-channel receiver - stimulator, which has several electrodes, and is placed under the skin behind the ear at the time of surgery (cortical mastoidectomy and posterior tympanotomy). The other end of the receiver (the electrodes) is delicately placed in the scala tympani of the cochlea; (2) A transmitter coil - a small external device (usually about 30 mm in diameter), which is held securely in place over the internal receiver/stimulator by magnetic attraction; (3) A microphone which is fitted behind the ear; and (4) A speech processor-a device that looks like a post-auricular hearing aid.
The microphone picks up sounds from the environment and sends them to the speech processor, through a thin cord that connects them. The speech processor converts the sounds into electronic signals, which are sent to the transmitter coil, through a cable. The transmitter sends these signals to the receiver across the intact skin, via an FM carrier wave. The signals are then converted back into electronic signals, and stimulate the implanted electrodes, and the cochlear nerve fibers. The nerve fibers send the signals to the brain, and a sensation of hearing is experienced. Hence, unlike a hearing aid, a cochlear implant can by-pass the damaged inner ear, and directly stimulate the auditory nerve fibers, in order to restore hearing[28].
It is essential that parents have realistic expectations prior to embarking on cochlear implantation. In addition, parents and carers should be informed in detail about the need for long-term commitment to the child’s rehabilitation.
However, the results regarding the acquisition of spoken language in implanted children with profound deafness are astonishing. Prelingually deaf children develop significant speech perception and production abilities over time. These achievements may appear limited in the first two years, but show significant improvement after the second year of implantation, and do not reach a plateau, even 5 years following implantation[29].
Prelingually deaf children also develop significant speech intelligibility, but a long period of cochlear implant use (sometimes more than 5 years) is needed prior to the emergence of intelligible speech[30].
The age at intervention, and the mode of communication are the most important determinants of outcomes following cochlear implantation in young prelingually deaf children[31]. Implanted children ought to be operated early in life, and placed in an environment that has a strong oral component, in order to maximize the respective outcomes. Children implanted prior to educational placement are significantly more likely to go to mainstream schools following implantation, than those implanted when they are already in school[32].
It needs to be mentioned as a concluding remark that the selection of the appropriate pediatric population, the existence of a dedicated cochlear implant MDT, with long-term commitment to the rehabilitation of the young patients, the adequacy of resources, and the strong support of the implant program by parents, community members, professional bodies, and political authorities, are the necessary parameters for the acquisition of spoken language by the prelingually deaf children following cochlear implantation, thus maximizing the future earnings of pediatric cochlear implantation for human societies.
To my Mentor, Professor Nikolopoulos TP, for everything he’s taught me. To Charis, the little angel that changed my life for ever.
Peer reviewer: Mohammad MSAl-Haggar, MD, Professor, Genetics Unit, Mansoura University Children’s Hospital, Mansoura 35516, Egypt
S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Nikolopoulos TP. Outcomes and predictors in cochlear implantation [doctoral thesis]. Nottingham: University of Nottingham 2000; 138, 166. |
| 2. | Beeghly M, Bretherton I, Mervis CB. Mothers internal state language to toddlers. Br J Develop Psychol. 1986;4:247-261 [doi: 10.1111/j.2044-835X.1986.tb01016.x]. |
| 3. | Ruben RJ. Language and the plastic brain. Otolaryngology: Basic science and clinical review. New York: Thieme 2005; 1-10. |
| 4. | Tait M, De Raeve L, Nikolopoulos TP. Deaf children with cochlear implants before the age of 1 year: comparison of preverbal communication with normally hearing children. Int J Pediatr Otorhinolaryngol. 2007;71:1605-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Gallaway C. Early interaction. Issues in Deaf Education. London: David Fulton Publishers Ltd 1998; 49-57. |
| 6. | Pinker S. The language instinct: How the mind creates language. London: Penguin Press 1994; . |
| 7. | Mártony J. On the correction of the voice pitch level for severely hard of hearing subjects. Am Ann Deaf. 1968;113:195-202. [PubMed] |
| 8. | Angelocci AA, Kopp GA, Holbrook A. The vowel formants of deaf and normal-hearing eleven- to fourteen-year-old boys. J Speech Hear Disord. 1964;29:156-160. [PubMed] |
| 9. | Monsen RB. Acoustic qualities of phonation in young hearing-impaired children. J Speech Hear Res. 1979;22:270-288. [PubMed] |
| 10. | Osberger MJ, McGarr NS. Speech production characteristics of the hearing impaired. Speech and language: Advances in basic research and practice. New York: Academic Press 1982; 227-288. |
| 11. | Tobey EA. Speech Production. Cochlear Implants: Audiological Foundations. San Diego: Singular Publishing Group 1993; 257-316. |
| 12. | Gold T. Speech production in hearing-impaired children. J Commun Disord. 1980;13:397-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Smith CR. Residual hearing and speech production in deaf children. J Speech Hear Res. 1975;18:795-811. [PubMed] |
| 14. | Di Carlo LM. The deaf: Englewood Cliffs. New Jersey: Prentice-Hall 1964; . |
| 15. | Rawlings BW. Summary of selected characteristics of hearing impaired students–United States: 1969-70. Washington: Gallaudet College 1971; . |
| 16. | Goffman E. Stigma: Notes on the management of spoiled identity. New Jersey: Prentice-Hall 1963; . |
| 17. | Rance G, Roper R, Symons L, Moody LJ, Poulis C, Dourlay M, Kelly T. Hearing threshold estimation in infants using auditory steady-state responses. J Am Acad Audiol. 2005;16:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Roland NJ, McRae RDR, McCombe AW. Key topics in Otolaryngology. 2nd ed. Oxford: BIOS Scientific Publishers Ltd 2000; . |
| 19. | Clifton RK, Morrongiello BA, Kulig JW. Developmental changes in auditory localization in infancy. Development of perception. New York: Academic Press 1981; 141-160. |
| 20. | Norton SJ, Gorga MP, Widen JE, Folsom RC, Sininger Y, Cone-Wesson B, Vohr BR, Mascher K, Fletcher K. Identification of neonatal hearing impairment: evaluation of transient evoked otoacoustic emission, distortion product otoacoustic emission, and auditory brain stem response test performance. Ear Hear. 2000;21:508-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Valencia DM, Rimell FL, Friedman BJ, Oblander MR, Helmbrecht J. Cochlear implantation in infants less than 12 months of age. Int J Pediatr Otorhinolaryngol. 2008;72:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Graungaard AH, Skov L. Why do we need a diagnosis A qualitative study of parents' experiences, coping and needs, when the newborn child is severely disabled. Child Care Health Dev. 2007;33:296-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Vlastarakos PV, Candiloros D, Papacharalampous G, Tavoulari E, Kampessis G, Mochloulis G, Nikolopoulos TP. Diagnostic challenges and safety considerations in cochlear implantation under the age of 12 months. Int J Pediatr Otorhinolaryngol. 2010;74:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Boothroyd A, Cawkwell S. Vibrotactile thresholds in pure tone audiometry. Acta Otolaryngol. 1970;69:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Vlastarakos PV, Proikas K, Papacharalampous G, Exadaktylou I, Mochloulis G, Nikolopoulos TP. Cochlear implantation under the first year of age--the outcomes. A critical systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2010;74:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Colletti L, Mandalà M, Zoccante L, Shannon RV, Colletti V. Infants versus older children fitted with cochlear implants: performance over 10 years. Int J Pediatr Otorhinolaryngol. 2011;75:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Vlastarakos PV, Nikolopoulos TP, Pappas S, Buchanan MA, Bewick J, Kandiloros D. Cochlear implantation update: contemporary preoperative imaging and future prospects - the dual modality approach as a standard of care. Expert Rev Med Devices. 2010;7:555-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Vlastarakos PV, Nikolopoulos TP, Tavoulari E, Papacharalambous G, Tzagaroulakis A, Dazert S. Sensory cell regeneration and stem cells: what we have already achieved in the management of deafness. Otol Neurotol. 2008;29:758-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | O'Donoghue GM, Nikolopoulos TP, Archbold SM, Tait M. Speech perception in children after cochlear implantation. Am J Otol. 1998;19:762-767. [PubMed] |
| 30. | Allen MC, Nikolopoulos TP, O'Donoghue GM. Speech intelligibility in children after cochlear implantation. Am J Otol. 1998;19:742-746. [PubMed] |
| 31. | Nikolopoulos TP, O'Donoghue GM, Archbold S. Age at implantation: its importance in pediatric cochlear implantation. Laryngoscope. 1999;109:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 199] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 32. | Archbold S, Nikolopoulos TP, O'Donoghue GM, Lutman ME. Educational placement of deaf children following cochlear implantation. Br J Audiol. 1998;32:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |