Published online Aug 8, 2012. doi: 10.5409/wjcp.v1.i2.3
Revised: July 31, 2012
Accepted: August 5, 2012
Published online: August 8, 2012
Just like children are not small adults, pediatric studies are not just subgroup-adult studies. Clinical pharmacology aims to predict these effects based on drug, population and/or patient-specific pharmacokinetics (concentration-time profiles) and -dynamics (concentration-effect profile). The most essential characteristics of childhood are growth and maturation. Both phenomena are most prominent during infancy making the claim that “an infant is not just a small child” as relevant compared to the paradigm that “a child is not just a small adult”. From a clinical pharmacology perspective, the consequence of such a dynamic setting is extensive variability throughout childhood in both the pharmacokinetics and pharmacodynamics. Trial design probably has impact on recruitment to an even greater extent compared to adult studies. In general, if a study is designed well, with a clear clinical question with which parents and children can identify, they are likely to consider participation. Open communication with all stakeholders involved will most likely result in ethically correct, practically feasible, scientifically sound, and economical reasonable studies to provide children with the appropriate treatment. From an academic perspective, feasibility, relevance, applicability and costs of clinical pharmacological studies in children can be significantly improved by new sampling concepts (e.g., saliva, urine, dried spot blood) and the systematic introduction of already known information into the trial design through model based pediatric drug development, that mainly affect feasibility of pharmacokinetic studies. In contrast, for the pharmacodynamic part of pediatric studies, development and validation of population specific biomarkers or robust outcome variables is urgently needed.
- Citation: Allegaert K. Clinical pharmacological studies in children: From exploratory towards confirmation driven methodology. World J Clin Pediatr 2012; 1(2): 3-7
- URL: https://www.wjgnet.com/2219-2808/full/v1/i2/3.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v1.i2.3
By virtue of their developmental and cognitive abilities, children are a vulnerable population, depending on adults for protection. This is also true when participation in a clinical trial is considered. In addition to adaptations of the clinical protocol design (e.g., drug formulation, dosing, sampling strategy, and clinical indication), recruitment and retention strategies, incentives and the process to obtain informed consent must be modified. The current limited experience with pediatric recruitment is the result of a historical swing. In the 18th and 19th century, children were often viewed as “property” of their parents or legal representatives, and frequently recruited for research, with subsequent protection since the mid 20th century, formalized in e.g., the Helsinki declaration. Unfortunately, protecting children, got translated to excluding these populations from clinical trials, resulting in “therapeutic orphans”[1,2]. The danger of extrapolation of findings collected in adults to children has been recognized and some of the anecdotal errors (chloramphenicol in neonates, formulation errors, human immune deficiency virus related drugs in children, inappropriate questionnaires) are well known to the pediatric community. Differences in physiological responses to disease, in disease characteristics, in treatment modalities and in drug disposition necessitate collection of age-specific observations at various stages of pediatric maturation[1-3]. At present, unlicensed or off-label administration of drugs is frequent and in part depends on disease severity. Ironically, unlicensed or off-label administration is most frequent (60%-80%) the most critically ill patients (neonatal and pediatric intensive care), still 40%-60% in a pediatric hospital setting and 20%-40% in ambulatory care[1,2]. This is not without risk: unknown adverse events, poorly established efficacy, hazardous dose calculation and inappropriate formulation.
Since the late 90ies in the United States (Food and Drug Administration) and more recently (2006) in Europe (European Pediatric Regulation, 1901/2006) and in line with World Health Organization initiatives (Better medicines for children initiative/list of essential medicines), (legal) frameworks have been developed to re-launch clinical research on drugs in children. In the European Union (EU), this pediatric regulation governing the development and authorization of medicines of pediatric use (children and adolescents aged 0 to 17 years) has been introduced in December 2006 and entered into force in 2007. This regulation makes pediatric development an obligation in the EU for all new medicinal products unless a waiver has been granted. By making pediatric development mandatory, this regulation will lead to profound changes in pediatric (drug) development and will increase the number of studies performed in the pediatric population[3-5].
Effective implementation of such a launch necessitates collaboration of all stakeholders involved in search for a “good clinical practice” approach: i.e., ethically correct, practically feasible, scientifically sound, and economical reasonable. Open communication between the stakeholders (patients and their parents, academia, industry and government) will be crucial to achieve the common goal, i.e., to reduce off-label use of medicines in children so that their medical treatment is based on evidence and on understanding developmental physiology. From an industrial or clinical research organization point of view, this will necessitate networking and collaboration with health care professionals, initially and mainly trained for clinical care and parents/patients. For health care professionals, this necessitates readiness to develop clinical research networks and specific research skills in addition to the clinical care.
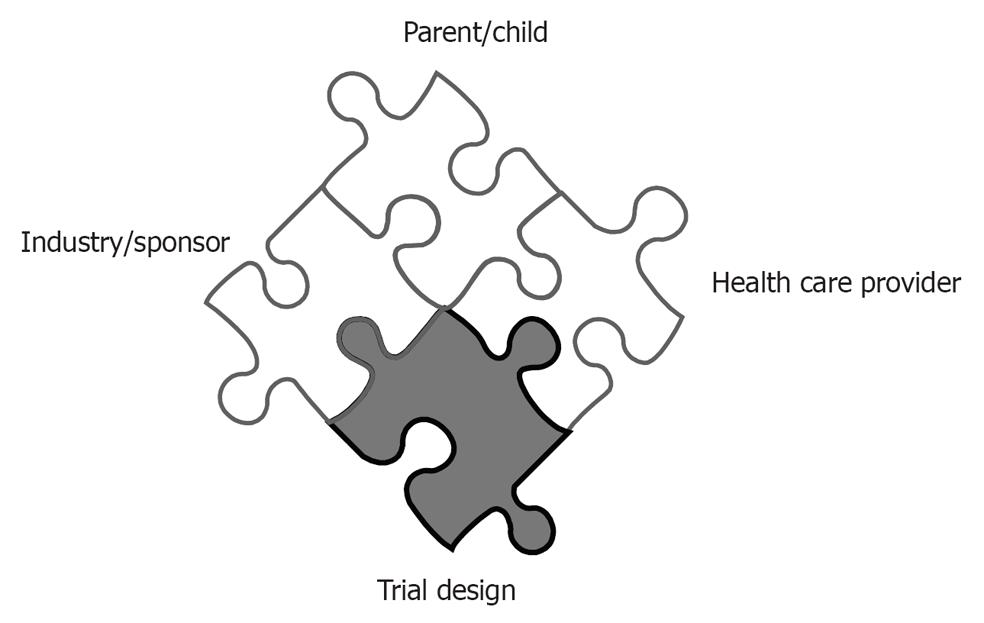
Besides regulatory requirements for receiving approval and final marketing authorization, the clinical trial design of pediatric studies probably effects recruitment to a greater extent than in adult studies (Figure 1). In general, the more complex and the more invasive, the harder to recruit. To enhance recruitment, the study should aim to use patients as close to the clinical situation as possible. Potential risks and inconveniences perceived to be of relevance in children include discomfort, inconvenience, pain, fear, separation from parents and family, effects on growing and developing organs, and size or volume of biological samples[1-3]. A feasible trial design, which do not impose a burden markedly beyond routine clinical care should be aimed for. Use of placebo in children is more restricted, because children cannot consent. Placebo should not be used when it means withholding effective treatment[2,5]. As the level of evidence in favour of an effective treatment increases, the ethical justification for placebo decreased. Placebo use in not equivalent to absence of treatment, but could be used on top of standard care. As many medicines used in children have not been fully assessed and authorized, medicinal products without marketing authorisation may be considered suitable as controls if they represent standard of care. The inclusion of an active control product not authorised in a clinical trial to be entered in a marketing authorisation file, should always be discussed with a scientific body of a (national) competent authority. In this editorial, we would like to focus on the contributions that can be made by academic research centers to further improve the study design, both for aspects of pharmacokinetics as well as -dynamics[6-13].
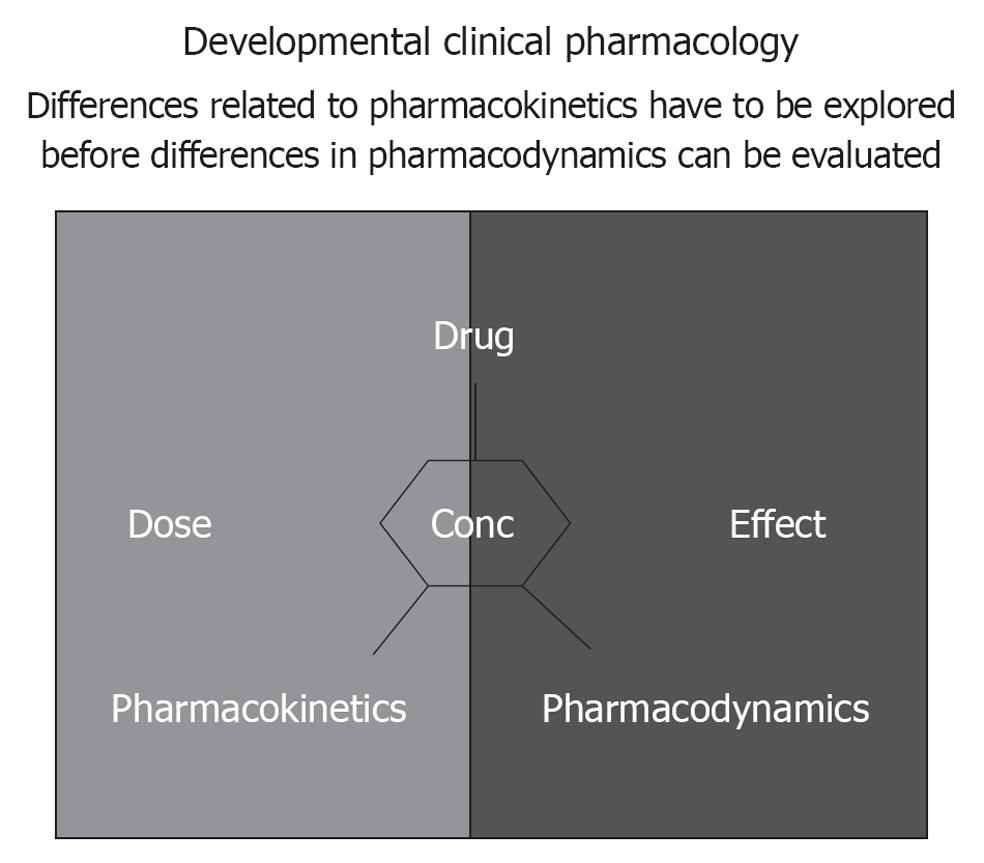
When a drug is prescribed, it is with the intention to attain a proportional therapeutic effect, preferably without side-effects. Clinical pharmacology aims to predict these effects based on drug, population and/or patient-specific pharmacokinetics (concentration-time profiles) and -dynamics (concentration-effect profile) (Figure 2). The most essential characteristics of childhood are growth and maturation. Both phenomena are most prominent during infancy making the claim that “an infant is not just a small child” as relevant compared to the more commonly used paradigm that “a child is not just a small adult”. From a clinical pharmacology perspective, the consequence of such a dynamic setting is extensive variability throughout childhood in both the pharmacokinetics and pharmacodynamics[1].
Differences related to pharmacokinetics have to be explored before differences in pharmacodynamics can be evaluated (Figure 2). The feasibility to perform studies in children relates in part to the introduction of adapted sampling techniques (e.g., saliva samples, dried spots blood) and more accurate quantification of metabolites in low volume samples. In addition, the burden for each individual infant can be further minimized by modeling and simulation approaches using non-linear mixed effect modeling to develop effective and safe dosing regimens since these population pharmacokinetic approaches result in the capability to analyze sparse, unbalanced datasets[7-11]. The variability can - to a certain extent - be anticipated. We would like to raise awareness in the pediatric clinical research community for the relevance of “rich data sets” that contain clinical characteristics and concentration-time (pharmacokinetics) or concentration-effect (pharmacodynamics) profiles: the description of a compound specific pattern is beyond compound specific relevance since it reflects maturational changes[7-11].
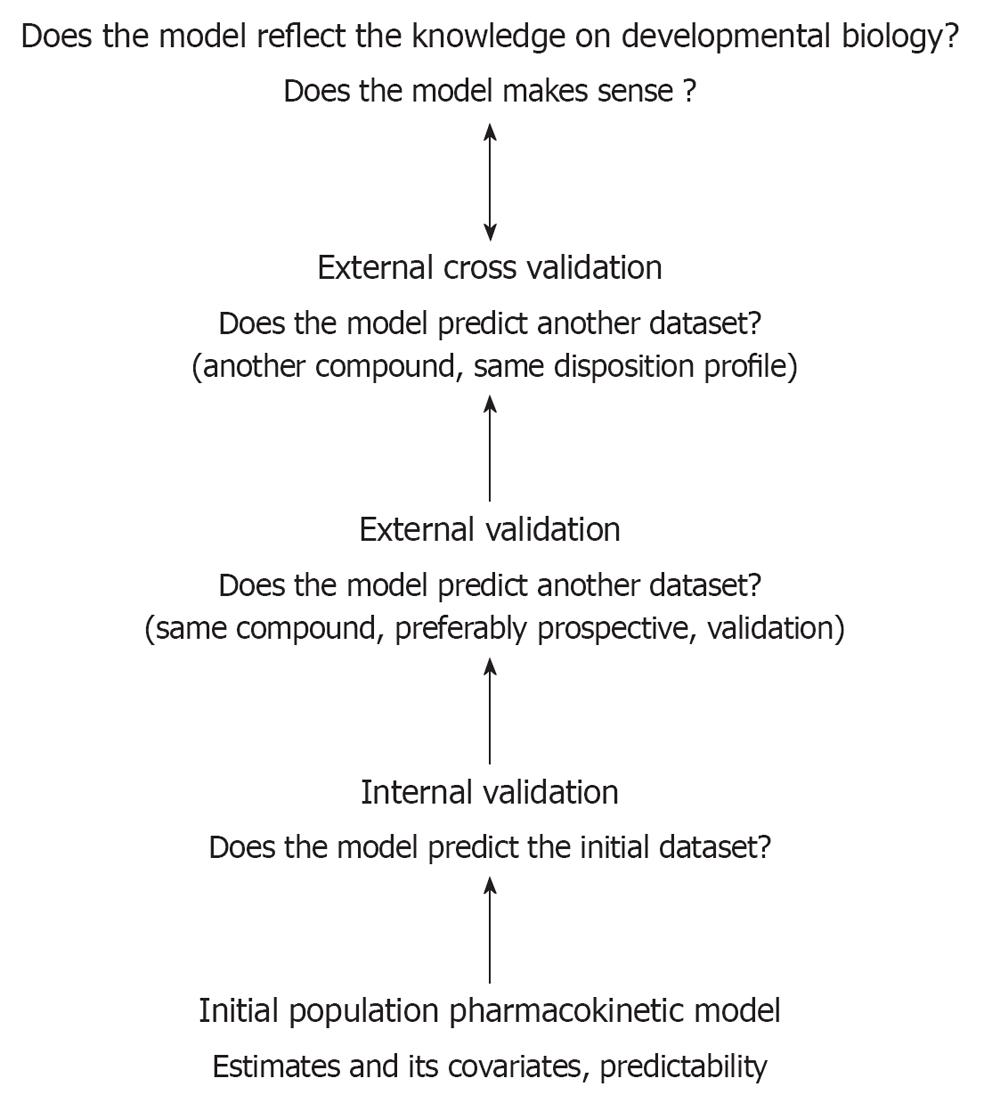
The maturational patterns described and the extent of the impact of covariates can be used to predict in vivo time-concentration profiles for compounds that undergo similar routes of elimination. However, following initial development, the clinical research community still fails to a certain extent to validate such models in the clinical setting. Besides internal and external validation, prospective clinical trials, which allow for the evaluation of the model-based dosing regimens are needed, not only to adjust the proposed dosing regimen, but also to convince pediatricians to use the information that has been generated using these modeling exercises [7-11]. This general concept has been illustrated in Figure 3. The development and validation of analytic methods adapted for pediatric applications and the modeling and simulation concept should be further developed within the academic research groups active in the field of pediatrics.
Improved knowledge on developmental pharmacokinetics is in the majority of drugs only a first, but essential step to describe the impact of maturation on the concentration/effect relation (Figure 2). Similar to the expansion of biomarker research in adult medicine, there is an active search for valid biomarkers to evaluate effects and side effects of interventions in children[12,13]. In essence, a biomarker is a characteristic or quantitative indicator that reflects either normal biologic processes, or pathological processes or pharmacological responses[13-16].
Since the research field of clinical pediatrics is broad, we would like to use an illustrative, anecdotal approach to make this point obvious for the readers.
Pulmonary hypertension: How to adapt the 6 min walking test routinely used in adults to quantify the impact of therapeutic interventions (e.g., endarterectomy, pharmacological, physiotherapy) for children, infants or even neonates[17-19]
Depression in children: To quantify the severity of a depression, questionnaires are used. However, these questionnaires (e.g., sleeping disorders, vital signs, sexual dysfunction) need validation in adolescence or childhood[20-22].
Gastro-oesophageal reflux disease in infants: While outcome in adults is based on subjective comfort and on findings during gastroscopy, the symptoms (crying, apnoea, milk intake) are different in infants[23-25].
Long term neurodevelopmental outcome: Although assessment based on Bayley scales or similar does result in quantitative results, early (first 12-18 mo) developmental assessment is only a weak (sensitivity, specificity) predictor for late neurodevelopmental outcome. This outcome is further biased by social factors and seems not to remain stable over time[26-29].
Renal failure: Creatinine reference values in neonates depend on birth weight, postnatal age but also on the measurement technique (either Jaffe or enzymatic quantification) applied[30-36].
Progressive neuromuscular diseases, type Duchenne: How to quantify the impact of a given intervention on muscular strength and to what extent is such a biomarker also of clinical relevance in children[37,38]
Biomarkers for patent ductus arteriosus: How sensitive and specific are clinical symptoms, specific flow measurements by echocardiography or should we consider the N-terminal pro-brain-type natriuretic peptide measurement[15,16,39]
Since the implementation of the pediatric regulation in the United States and the subsequent initiatives throughout the world including Europe, there is an increase in ongoing research in the field of pediatric product development[40]. The results obtained during such a pediatric product development however are beyond the compound specific observations[40-45]. Research groups are made to collaborate and to agree on assessment tools, definitions and treatment strategies, and results obtained can be extrapolated to other compounds or other subpopulation within the pediatric age range[46-50]. The advantage of such an extrapolation is that the clinical research can shift from exploratory approach to confirmation, hypothesis-driven methods[7-13]. Collaborative efforts to improve research tools on pharmacokinetics (e.g., model-based pediatric medicinal development) and -dynamics (e.g., biomarkers) likely will result not only in improved pediatric drug therapy in near future, but are also a potent drivers to learn more of the intriguing world of developmental life[7-13].
Peer reviewer: Sinem Akgül, MD, Assistant Professor, Institute of Population Studies, Hacettepe University, Ankara 06100, Turkey
S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157-1167. [PubMed] |
| 2. | Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet. 2004;364:803-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 318] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 3. | Caldwell PH; EMA. Committee for medicinal products for human use. Guideline on clinical trials in small populations. Available from: http://www.ema.europa.eu/pdfs/human.ewp/8356105en.pdf. |
| 4. | Hoppu K, Anabwani G, Garcia-Bournissen F, Gazarian M, Kearns GL, Nakamura H, Peterson RG, Sri Ranganathan S, de Wildt SN. The status of paediatric medicines initiatives around the world--What has happened and what has not. Eur J Clin Pharmacol. 2012;68:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Pinxten W, Dierickx K, Nys H. Ethical principles and legal requirements for pediatric research in the EU: an analysis of the European normative and legal framework surrounding pediatric clinical trials. Eur J Pediatr. 2009;168:1225-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Laughon MM, Benjamin DK, Capparelli EV, Kearns GL, Berezny K, Paul IM, Wade K, Barrett J, Smith PB, Cohen-Wolkowiez M. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | De Cock RF, Piana C, Krekels EH, Danhof M, Allegaert K, Knibbe CA. The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol. 2011;67 Suppl 1:5-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 8. | Khalil F, Läer S. Physiologically based pharmacokinetic modeling: methodology, applications, and limitations with a focus on its role in pediatric drug development. J Biomed Biotechnol. 2011;2011:907461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Allegaert K, Smits A, van den Anker JN. Physiologically based pharmacokinetic modeling in pediatric drug development: a clinician's request for a more integrated approach. J Biomed Biotechnol. 2012;2012:103763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Leeder JS, Kearns GL, Spielberg SP, van den Anker J. Understanding the relative roles of pharmacogenetics and ontogeny in pediatric drug development and regulatory science. J Clin Pharmacol. 2010;50:1377-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | van den Anker J, Allegaert K. Clinical pharmacology in neonates and young infants: the benefit of a population-tailored approach. Expert Rev Clin Pharmacol. 2012;5:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Manolis E, Pons G. Proposals for model-based paediatric medicinal development within the current European Union regulatory framework. Br J Clin Pharmacol. 2009;68:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Kearns GL. Beyond biomarkers: an opportunity to address the 'pharmacodynamic gap' in pediatric drug development. Biomark Med. 2010;4:783-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Rakhmanina NY, van den Anker JN. Pharmacological research in pediatrics: From neonates to adolescents. Adv Drug Deliv Rev. 2006;58:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Buijs EA, Zwiers AJ, Ista E, Tibboel D, de Wildt SN. Biomarkers and clinical tools in critically ill children: are we heading toward tailored drug therapy. Biomark Med. 2012;6:239-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Sasi A, Deorari A. Patent ductus arteriosus in preterm infants. Indian Pediatr. 2011;48:301-308. [PubMed] |
| 17. | Abman SH, Ivy DD. Recent progress in understanding pediatric pulmonary hypertension. Curr Opin Pediatr. 2011;23:298-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Roofthooft MT, van Loon RL, Berger RM. Management of pulmonary arterial hypertension in children. Paediatr Respir Rev. 2010;11:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Oishi P, Datar SA, Fineman JR. Advances in the management of pediatric pulmonary hypertension. Respir Care. 2011;56:1314-1339; discussion 1339-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Rutherford BR, Sneed JR, Tandler JM, Rindskopf D, Peterson BS, Roose SP. Deconstructing pediatric depression trials: an analysis of the effects of expectancy and therapeutic contact. J Am Acad Child Adolesc Psychiatry. 2011;50:782-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Liu HY, Potter MP, Woodworth KY, Yorks DM, Petty CR, Wozniak JR, Faraone SV, Biederman J. Pharmacologic treatments for pediatric bipolar disorder: a review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2011;50:749-62.e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Gentile S. Clinical usefulness of second-generation antipsychotics in treating children and adolescents diagnosed with bipolar or schizophrenic disorders. Paediatr Drugs. 2011;13:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Apps JR, Flint JD, Wacogne I. Towards evidence based medicine for paediatricians. Question 1. Does anti-reflux therapy improve symptoms in infants with laryngomalacia. Arch Dis Child. 2012;97:385-387; discussion 387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Malcolm WF, Cotten CM. Metoclopramide, H2 blockers, and proton pump inhibitors: pharmacotherapy for gastroesophageal reflux in neonates. Clin Perinatol. 2012;39:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Fike FB, Mortellaro VE, Pettiford JN, Ostlie DJ, St Peter SD. Diagnosis of gastroesophageal reflux disease in infants. Pediatr Surg Int. 2011;27:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Moore T, Johnson S, Hennessy E, Marlow N. Screening for autism in extremely preterm infants: problems in interpretation. Dev Med Child Neurol. 2012;54:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Mulder H, Pitchford NJ, Marlow N. Inattentive behaviour is associated with poor working memory and slow processing speed in very pre-term children in middle childhood. Br J Educ Psychol. 2011;81:147-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Mulder H, Pitchford NJ, Hagger MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol. 2009;34:393-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 272] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 29. | Johnson S, Marlow N, Wolke D. Assessing educational outcomes in middle childhood: validation of the Teacher Academic Attainment Scale. Dev Med Child Neurol. 2012;54:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Cataldi L, Leone R, Moretti U, De Mitri B, Fanos V, Ruggeri L, Sabatino G, Torcasio F, Zanardo V, Attardo G. Potential risk factors for the development of acute renal failure in preterm newborn infants: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2005;90:F514-F519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | George I, Mekahli D, Rayyan M, Levtchenko E, Allegaert K. Postnatal trends in creatinemia and its covariates in extremely low birth weight (ELBW) neonates. Pediatr Nephrol. 2011;26:1843-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Vieux R, Hascoet JM, Merdariu D, Fresson J, Guillemin F. Glomerular filtration rate reference values in very preterm infants. Pediatrics. 2010;125:e1186-e1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Puddu M, Fanos V, Podda F, Zaffanello M. The kidney from prenatal to adult life: perinatal programming and reduction of number of nephrons during development. Am J Nephrol. 2009;30:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Cobbaert CM, Baadenhuijsen H, Weykamp CW. Prime time for enzymatic creatinine methods in pediatrics. Clin Chem. 2009;55:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Kuppens M, George I, Lewi L, Levtchenko E, Allegaert K. Creatinaemia at birth is equal to maternal creatinaemia at delivery: does this paradigm still hold. J Matern Fetal Neonatal Med. 2012;25:978-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Cuzzolin L, Fanos V, Pinna B, di Marzio M, Perin M, Tramontozzi P, Tonetto P, Cataldi L. Postnatal renal function in preterm newborns: a role of diseases, drugs and therapeutic interventions. Pediatr Nephrol. 2006;21:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Bendixen RM, Senesac C, Lott DJ, Vandenborne K. Participation and quality of life in children with Duchenne muscular dystrophy using the International Classification of Functioning, Disability, and Health. Health Qual Life Outcomes. 2012;10:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Ergul Y, Ekici B, Nisli K, Tatli B, Binboga F, Acar G, Ozmen M, Omeroglu RE. Evaluation of the North Star Ambulatory Assessment scale and cardiac abnormalities in ambulant boys with Duchenne muscular dystrophy. J Paediatr Child Health. 2012;48:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Allegaert K, Langhendries JP, van den Anker JN. Educational paper : Do we need neonatal clinical pharmacologists. Eur J Pediatr. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Kern SE. Challenges in conducting clinical trials in children: approaches for improving performance. Expert Rev Clin Pharmacol. 2009;2:609-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Dessì A, Salemi C, Fanos V, Cuzzolin L. Drug treatments in a neonatal setting: focus on the off-label use in the first month of life. Pharm World Sci. 2010;32:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Jacqz-Aigrain E. Drug policy in Europe Research and funding in neonates: current challenges, future perspectives, new opportunities. Early Hum Dev. 2011;87 Suppl 1:S27-S30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Fanos V, Yurdakök M. Personalized neonatal medicine. J Matern Fetal Neonatal Med. 2010;23 Suppl 3:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | van den Anker JN. Managing drugs safely. Semin Fetal Neonatal Med. 2005;10:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Dabliz R, Levine S. Medication safety in neonates. Am J Perinatol. 2012;29:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Tuleu C. 'Formulating better medicines for children' - still paving the road. Int J Pharm. 2012;435:99-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Turner MA. Neonatal drug development. Early Hum Dev. 2011;87:763-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Choonara I. WHO wants safer medicines for children. Arch Dis Child. 2008;93:456-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Rieder M. If children ruled the pharmaceutical industry: the need for pediatric formulations. Drug News Perspect. 2010;23:458-464. [PubMed] |
| 50. | Whitney SN. The python's embrace: clinical research regulation by institutional review boards. Pediatrics. 2012;129:576-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |