Copyright
©The Author(s) 2025.
World J Clin Pediatr. Mar 9, 2025; 14(1): 100938
Published online Mar 9, 2025. doi: 10.5409/wjcp.v14.i1.100938
Published online Mar 9, 2025. doi: 10.5409/wjcp.v14.i1.100938
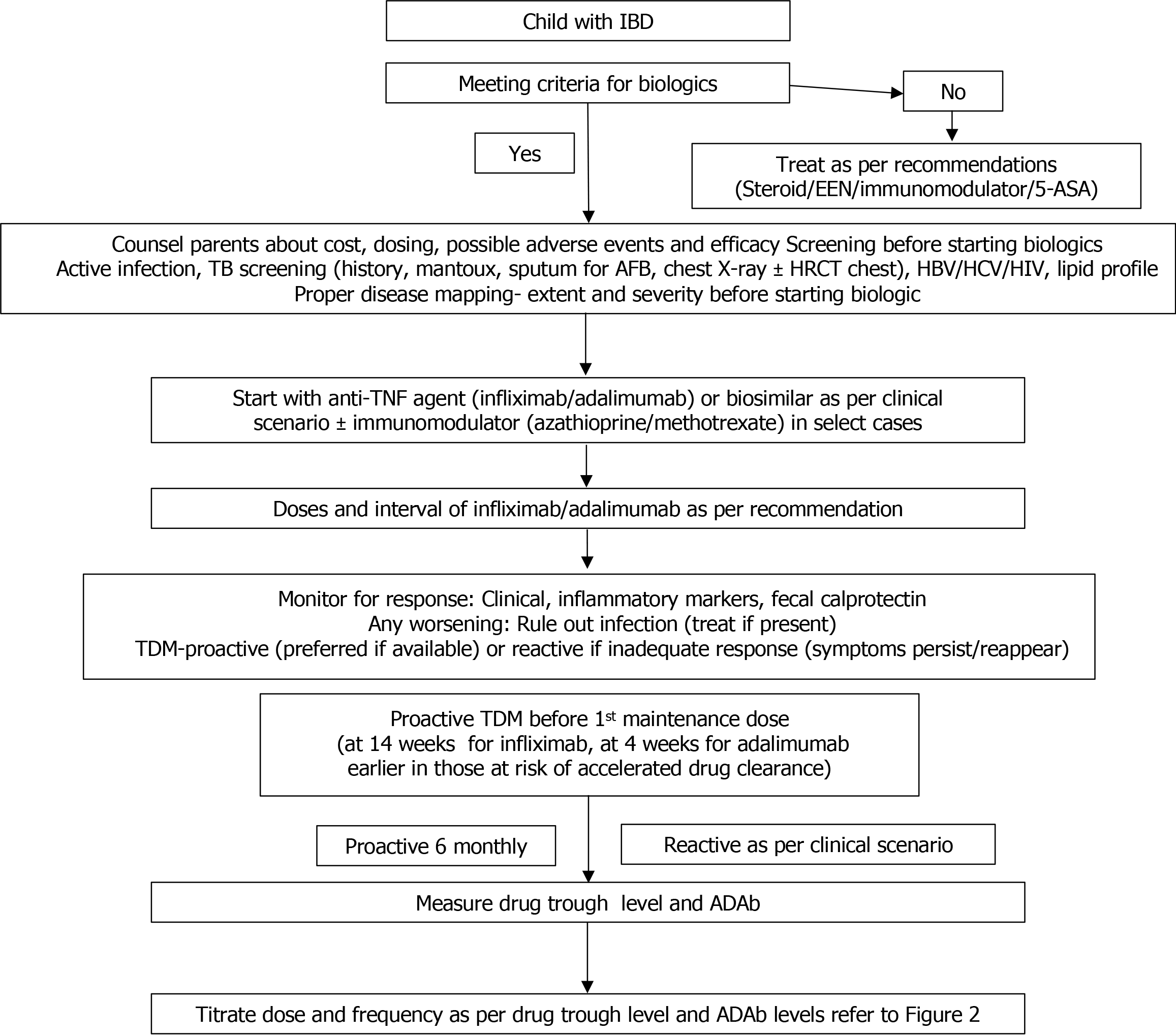
Figure 1 Approach to a child with inflammatory bowel disease requiring biologics.
IBD: Inflammatory bowel disease; EEN: Exclusive enteral nutrition; 5-ASA: 5-aminosalicylic acid; TB: Tuberculosis; AFB: Acid fast bacilli; HRCT: High resolution computed tomography; HBV: Hepatitis B virus; HCV: Hepatitis C virus; HIV: Human immunodeficiency virus; TNF: Tumor necrosis factor; TDM: Therapeutic drug monitoring; ADAb: Anti-drug antibody.
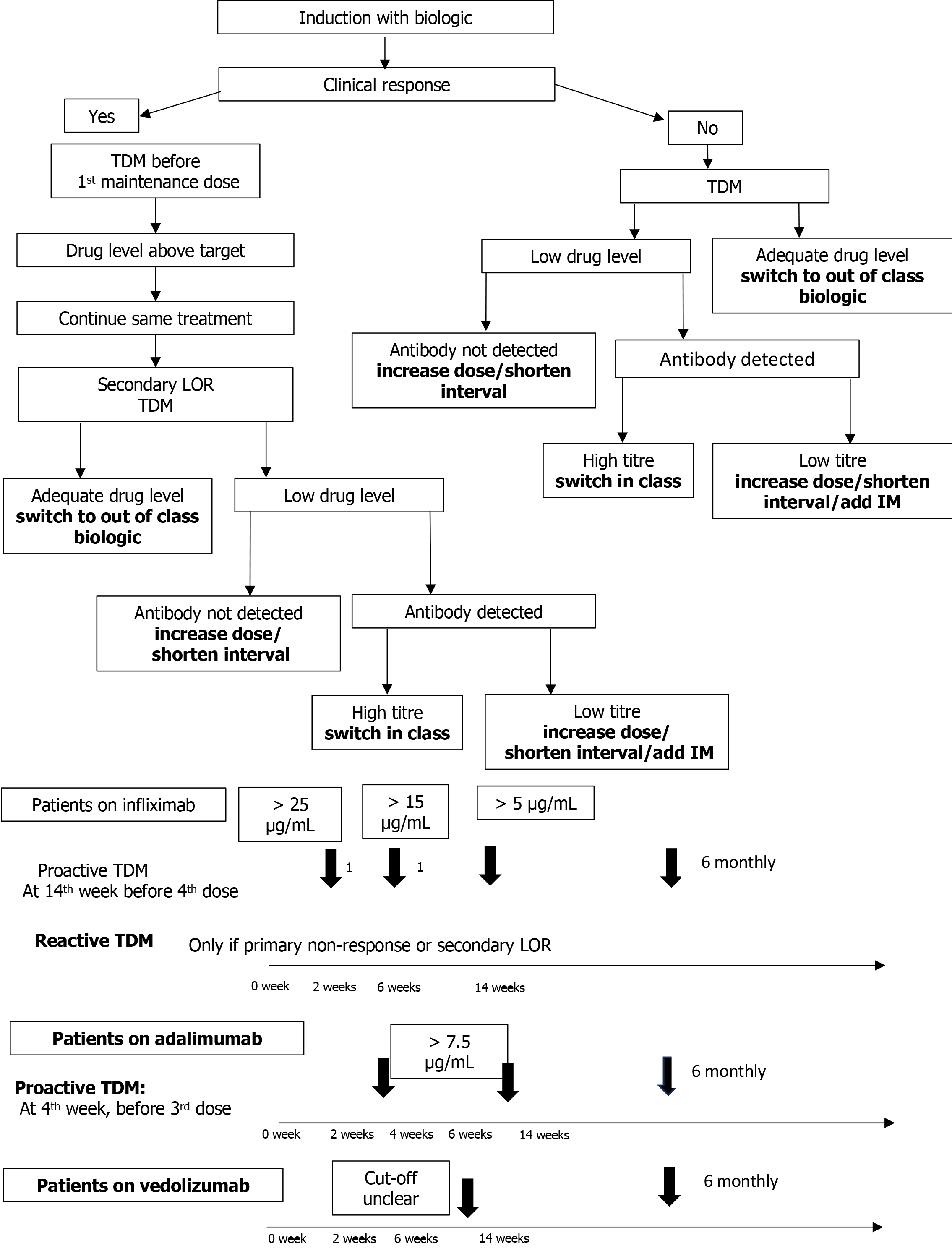
Figure 2 Therapeutic drug monitoring in a child on biologics.
Infliximab and adalimumab are generally not abandoned unless drug concentration is ≥ 10 μg/mL. Target higher drug levels in fistulizing perianal disease. LOR: Loss of response; TDM: Therapeutic drug monitoring; IM: Immunomodulator; 1Arrow: In those at risk of increased drug clearance.
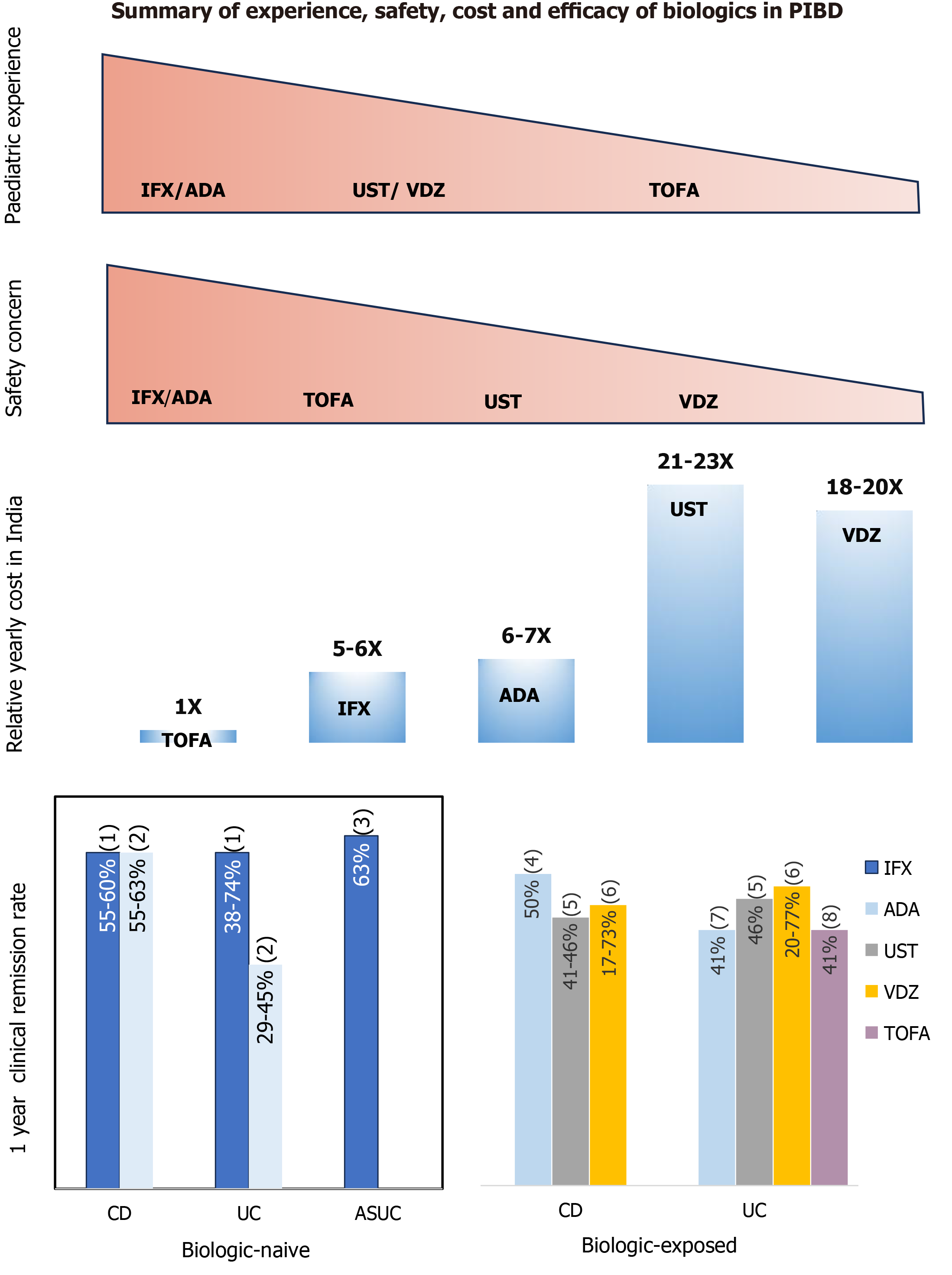
Figure 3 Comparative safety, relative annual cost (in India) and efficacy profile of various biologics in pediatric inflammatory bowel disease.
PIBD: Pediatric inflammatory bowel disease; IFX: Infliximab; ADA: Adalimumab; UST: Ustekinumab; VDZ: Vedolizumab; TOFA: Tofacitinib; CD: Crohn’s disease; UC: Ulcerative colitis; ASUC: Acute severe ulcerative colitis; (1): Response rates to biologics taken from reference[21,25,26,31,34,103]; (2): Response rates to biologics taken from reference[30,31,68]; (3): Response rates to biologics taken from reference[62]; (4): Response rates to biologics taken from reference[36]; (5): Response rates to biologics taken from reference[53,56]; (6): Response rates to biologics taken from reference[51]; (7): Response rates to biologics taken from reference[33]; (8): Response rates to biologics taken from reference[57].
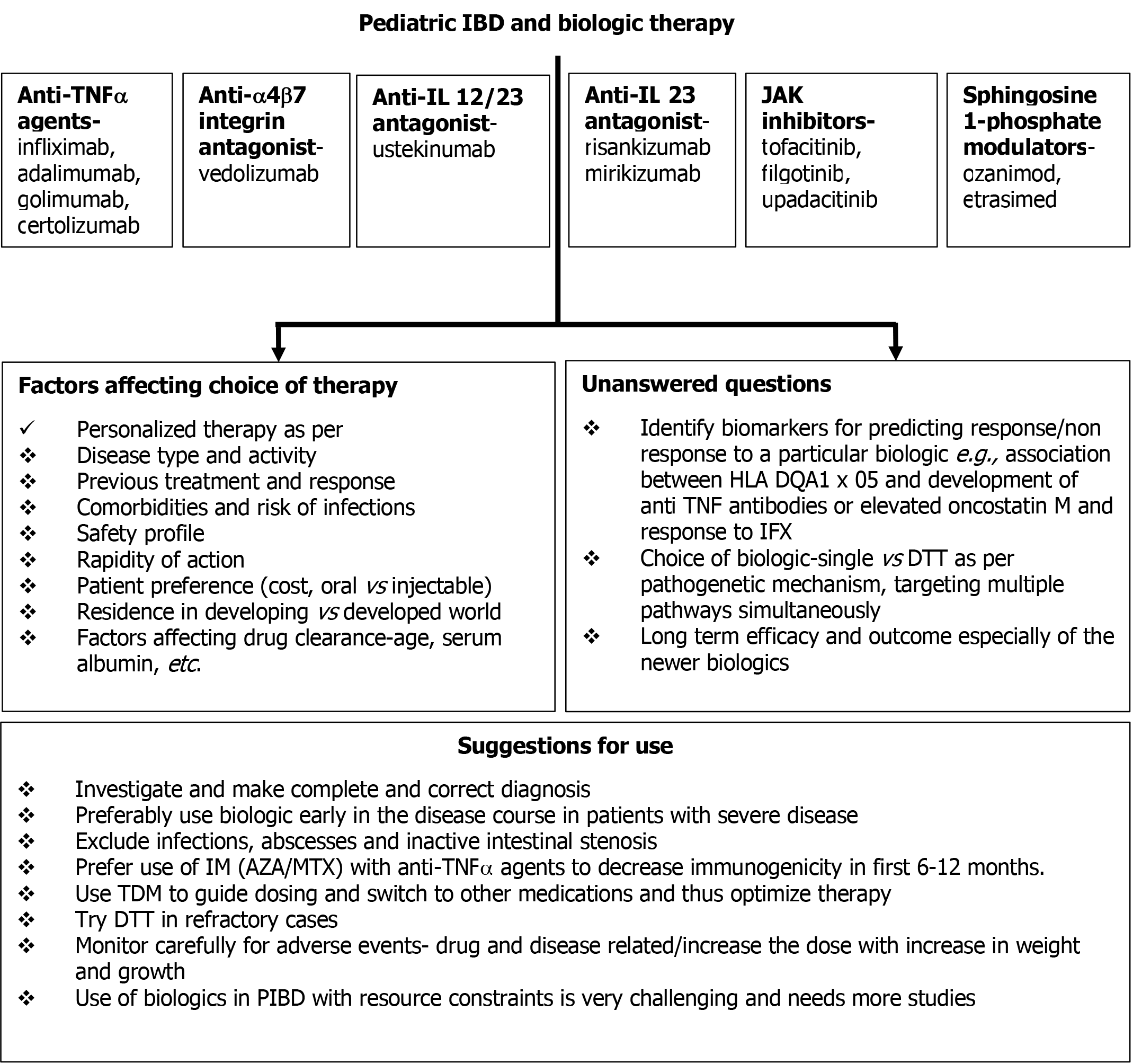
Figure 4 Biologics in children with inflammatory bowel disease: Choice of therapy and future perspectives.
IBD: Inflammatory bowel disease; TNFα:Tumor necrosis factor alpha; Anti-α4β7: Anti-alpha4beta7; IL: Interleukin; JAK: Janus kinase; IFX: Infliximab; HLA: Human leukocyte antigen; DTT: Dual-targeted therapy; IM: Immunomodulators; AZA: azathioprine; MTX: Methotrexate; TDM: Therapeutic drug monitoring; PIBD: Pediatric inflammatory bowel disease.
- Citation: Samanta A, Srivastava A. Biologics in the management of pediatric inflammatory bowel disease: When and what to choose. World J Clin Pediatr 2025; 14(1): 100938
- URL: https://www.wjgnet.com/2219-2808/full/v14/i1/100938.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i1.100938