Published online Dec 18, 2019. doi: 10.5321/wjs.v7.i3.28
Peer-review started: June 29, 2019
First decision: August 2, 2019
Revised: October 2, 2019
Accepted: November 26, 2019
Article in press: November 26, 2019
Published online: December 18, 2019
Processing time: 173 Days and 21.1 Hours
A key requirement for biomimetic regeneration of tissues is a 3D scaffold. The gold standard scaffold for revascularization is the blood clot, however, an adequate blood clot cannot always be achieved in narrow canals or mature roots. Hereby, we document the effects of platelet-rich fibrin (PRF) for the regenerative endodontic treatment (RET) of two immature permanent teeth with necrotic pulps for up to 48 mo.
The first patient was a 22-year-old female with history of trauma in tooth #9 with a sinus tract and a large periapical lesion. The second was a 9-year-old male presenting with a badly decayed tooth #14. Both cases were treated with RET and PRF prepared from the patients’ blood. PRF and its extract were used as a scaffold for RET. Patients were followed-up to 9 and 48 mo (4 years), respectively. Both patients, were asymptomatic after treatment. At the 9-mo-follow-up of case #1, there was radiographic evidence of periapical bone healing, however, the root apex was still open. In case #2, the roots exhibited apical closure and normal periapical bone architecture at 12-mo follow-up, while no root lengthening was observed. After 48 mo, case #2 showed extensive intracanal calcification in all root canals that complicated conventional root canal treatment.
RET with PRF and its extract could be used in revascularization of immature permanent teeth. However, proper case selection to comply with long-term follow-up is necessary and adverse events such as calcification and canal obliteration should be planned for.
Core tip: This report investigated the effects of platelet-rich fibrin (PRF) for the regenerative endodontic treatment (RET). The first patient had a history of trauma in tooth #9 with a sinus tract and a large periapical lesion. The second patient presented with a badly decayed tooth #14. Both cases were treated with platelet-rich fibrin (PRF) as scaffold for RET. Patients were followed up to 9 and 48 mo, respectively. There was radiographic evidence of periapical bone healing in both cases. The second patient showed root maturation after 12 mo and extensive intracanal calcification at 48 mo.
- Citation: Eltawila AM, El Backly R. Autologous platelet-rich-fibrin-induced revascularization sequelae: Two case reports. World J Stomatol 2019; 7(3): 28-38
- URL: https://www.wjgnet.com/2218-6263/full/v7/i3/28.htm
- DOI: https://dx.doi.org/10.5321/wjs.v7.i3.28
Regenerative endodontic treatments (RETs) require a 3D biological scaffold to promote tissue regeneration[1,2]. The most common scaffold is a blood clot, created from induced periapical bleeding through the root apical foramen[3]. Periapical bleeding delivers mesenchymal stem cells (MSCs)[4] and the blood clot forms a 3-D network for endodontic tissue and root generation. However, an adequate blood clot scaffold cannot always be achieved from periapical bleeding[3]. Most practitioners believe that RETs can be more beneficial than apexification for the treatment of immature teeth with necrotic pulps[5], though they want to know how to make RETs more successful[5], by identifying a more reliable scaffold, such as platelet-rich fibrin (PRF).
PRF is a second-generation platelet-rich concentrate that allows a sustained release in a slowly degrading fibrin matrix for multiple growth factors, such as; platelet derived growth factor; transforming growth factor-β; and vascular endothelial growth factor[6], and numerous cytokines[7]. Platelet-rich concentrates are a naturally occurring cocktail of growth factors and cytokines organized in a 3D framework[7]. These bioactive factors are involved in all phases of wound healing and subsequently tissue regeneration. These properties, in addition to the ease of preparation and manipulation, make PRF an excellent candidate for RETs[8]. Additionally, the fluid obtained when centrifuging PRF (PRF-extract) can further stimulate endogenous regeneration by having synergistic action with mineral trioxide aggregate as it has been shown to enhance odontoblastic differentiation of human dental pulp stem cells[6]. Herein, we describe the successful use of PRF and its extract in conjunction with induction of bleeding, or as an alternative scaffold, for the regenerative endodontic management of two challenging cases.
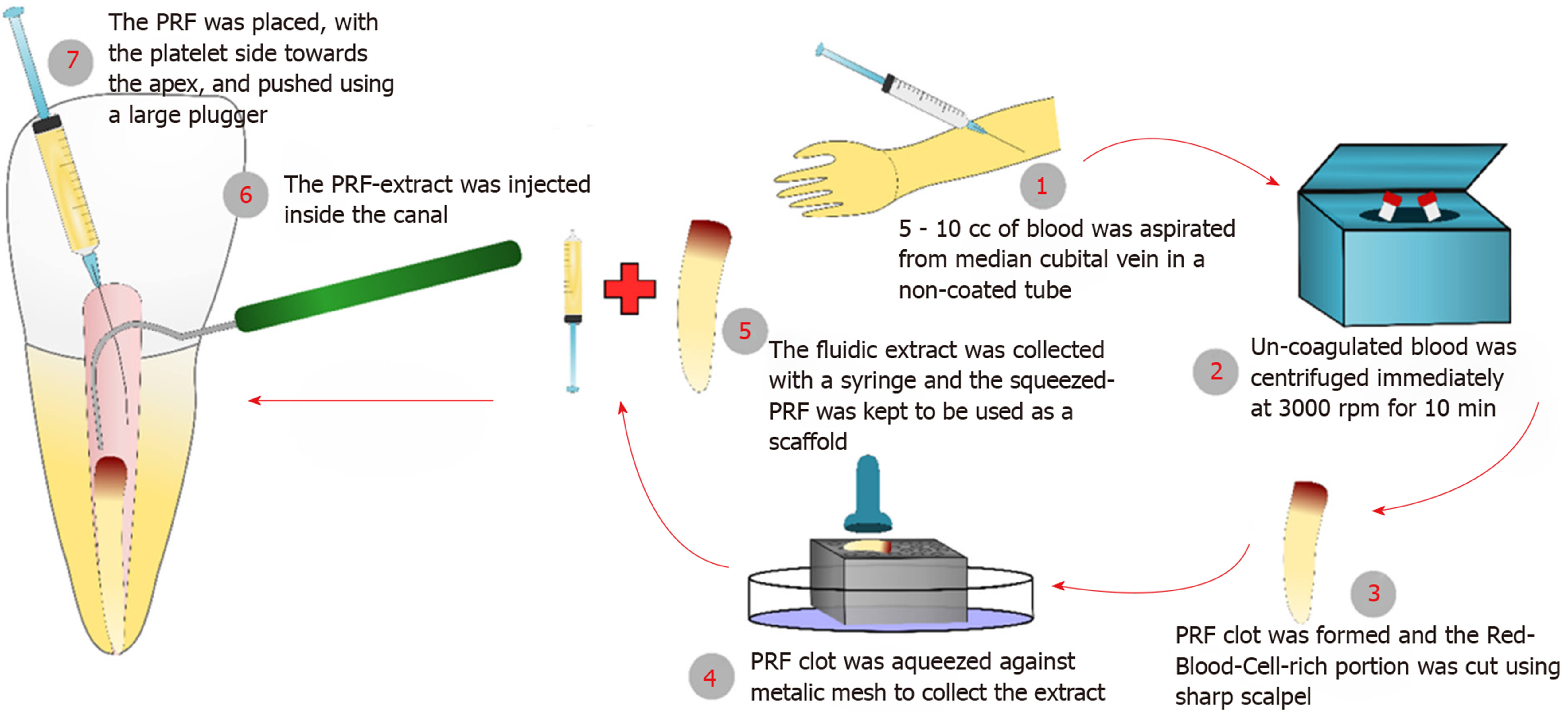
PRF and its extract were prepared from venous blood collected from the patients’ arms immediately prior to centrifugation and use[9], these steps are illustrated in (Figure 1). All procedures were performed per the institutional ethical guidelines instated by the IRB of the Faculty of Dentistry, Alexandria University and following informed patient and parent/guardian consent. 5-10 cc of the patient’s venous blood was collected from their median cubital vein in a non-coated glass tube. The collected blood was immediately centrifuged at 3000 RPM for 10 min. The PRF clot was compressed against a metallic mesh to collect the fluidic extract. The PRF preparation was done chair-side and immediately before use (Figure 2A). The red corpuscle layer was gently wiped off the surface of the PRF clot set aside. At the time of insertion, care was taken to pack the PRF clot with the platelet-plug facing the apical tissues.
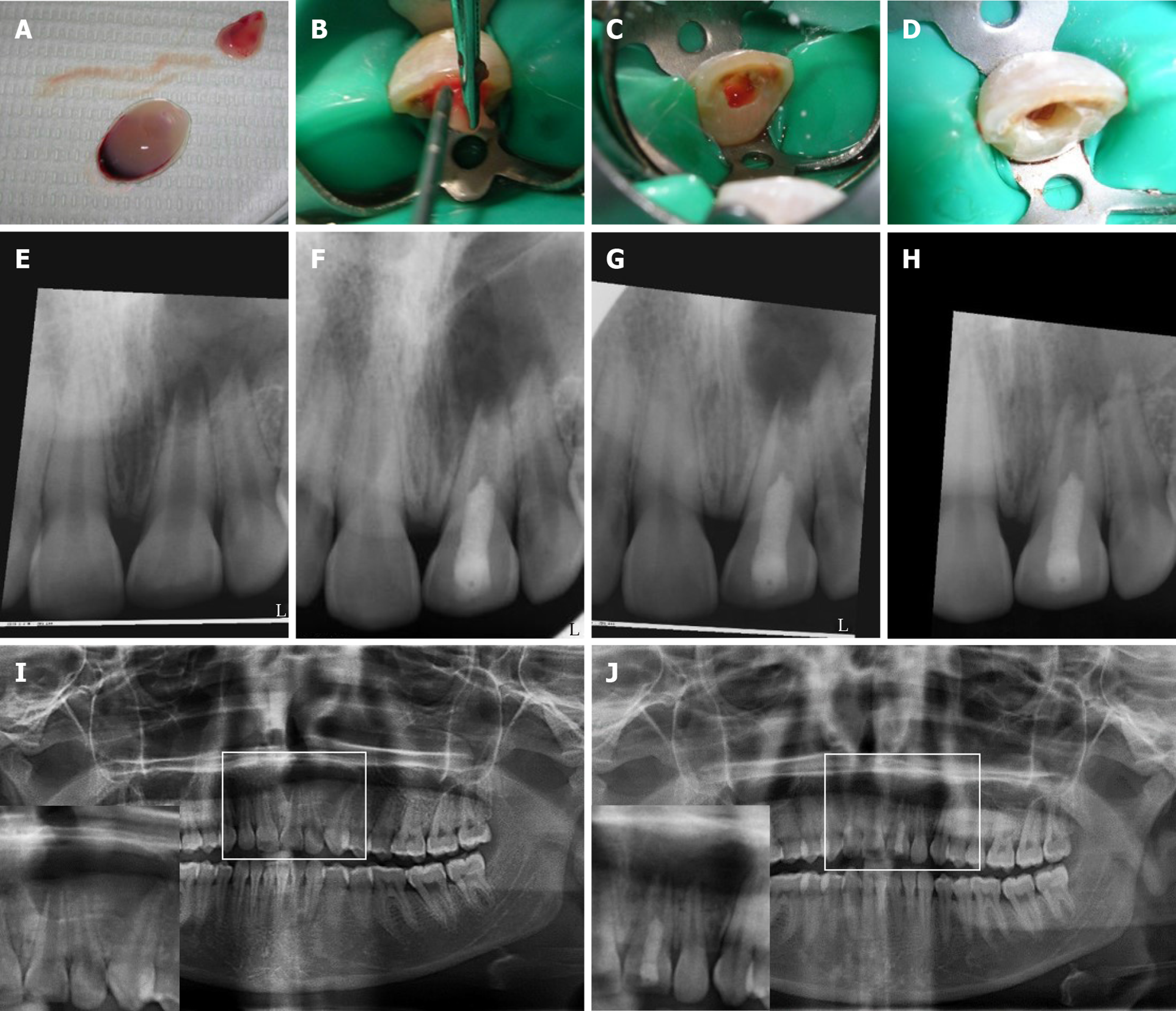
A 22-year-old female patient was referred to the conservative dentistry department clinics at the faculty of dentistry, Alexandria University complaining of a discolored upper anterior tooth. Upon examination, tooth #9 was found to be discolored with a sinus tract opening on the labial surface related to the same tooth when traced with Gutta Percha points. The patient reported a history of trauma when she was 8-years old. The periapical and panoramic radiographs revealed a large periapical lesion related to the teeth #9, #10 and tooth #11. Tooth #9 had an immature root. The tooth was tender to percussion. The cold pulp test was negative for #9 and #10. Tooth #9 responded negatively to hot and tooth #10 showed mild response when compared to the contralateral tooth. Periodontal examination revealed normal probing depths.
A 9-year-old systemically healthy male was referred from the orthodontics department for RET of an immature permanent maxillary first molar. The patient had been complaining of toothache, then the tooth became asymptomatic except the night before treatment. Upon clinical examination the tooth appeared badly decayed, tender to percussion, and negative to both cold and hot tests, however, there was normal periodontal probing depth. Digital periapical radiographs were obtained using Sidexis software system (Sirona Dental Gmbh), and revealed a radiolucent periapical lesion in relation to the palatal root of tooth #14 which appeared to be immature while both buccal roots showed wide apices (Figure 3).
A diagnosis of the immature tooth #9 was concluded as necrotic pulp and chronic periapical abscess. The patient had an irrelevant medical history and a regenerative approach was decided after appropriate consent was obtained.
A diagnosis of necrotic pulp with symptomatic apical periodontitis was made in relation to tooth #14. Since the tooth appeared to have at least one immature root, a decision was made to treat the tooth using RETs. The risks and benefits of the procedure were explained to the parent and informed written consent was obtained.
After local anesthesia and rubber dam isolation of tooth #9, the pulp chamber was accessed. Pus was drained from the canal and flushed with copious saline irrigation. Working length was determined using digital x-rays and the canal was minimally instrumented. Copious irrigation using 20 mL of 1.5% sodium hypochlorite for 5 min was done. Finally, the canal was dried with sterile paper points followed by filling the root canal with Calcium Hydroxide paste (Ultra-Cal XS, Ultradent, United States).
Three weeks later, the tooth was asymptomatic and the labial sinus tract opening had healed. The tooth was anesthetized with 3% Mepivicaine without vasoconstrictor (Alexandria Co. for Pharmaceuticals, Egypt), the canal was found to be dry and then it was copiously irrigated with 1.5% Sodium Hypochlorite followed by 10 mL of sterile saline. After drying the canal with paper points, the dentin was conditioned with 17% EDTA (Glyde) for one minute then it was rinsed with saline irrigation. The canal was dried and intra-canal bleeding was provoked using a K-file #15 extending 2 mm beyond the working length. Meanwhile, PRF was prepared as described before (Figure 2A). PRF-extract was injected inside the canal followed by packing of the PRF clot just apical to the cementoenamel junction (Figure 2B-D). A coronal barrier with MTA was made (MM-MTA™, MicroMega, France) followed by glass ionomer and composite restoration.
The tooth was anesthetized with Mepecaine-L (2% mepivacaine HCl, levonordefrin 1:20000, Alexandria Co. For Pharmaceuticals, Egypt). Caries was removed, the pulp chamber was accessed and the tooth was isolated with rubber dam. Four root canals were identified; two mesiobuccal, distobuccal and palatal canals. Canals were irrigated with 2.5% sodium hypochlorite (NaClO) (Household Cleaning Products Company of Egypt, Cairo, Egypt) and normal saline. The working length was estimated based on the digital periapical x-rays and the canals gently instrumented with #15 K-files, then filled with Calcium hydroxide paste (Ultracal XS, Ultradent, United States), and the tooth was temporary filled (Ora-fil G, Prevest Denpro, India).
After one week, the patient had slight pain on biting and was recalled. The temporary filling was removed after the application of rubber dam. The tooth was etched (Dentsply), bonded (Excite DSC, Ivoclar-Vivadent, Liechtestein) and the missing tooth walls were restored with composite resin (Te econom, Ivoclar-Vivadent, Liechtestein). The canal orifices were enlarged using sizes 3 and 4 Gates Glidden drills and copiously irrigated with 2.5% NaClO, dried and filled with calcium hydroxide. The cavity was filled with a temporary restorative material and Cone Beam Computed Tomography (CBCT) was ordered (Figure 3E-H). After two days, the patient was recalled. The distal and palatal canals were minimally instrumented up to #15 and #20 hand K-files based on the lengths measured on CBCT. The mesial canals were instrumented up to size #30. Then, canals were irrigated, filled with calcium hydroxide paste and the access cavity was sealed with temporary filling.
After 4 wk, the patient was recalled and the tooth was asymptomatic. It was anesthetized with 3% Mepivacaine without vasoconstrictor (Mepivacaine HCl 3%, Alexandria Co. for Pharmaceuticals, Egypt), isolated with rubber dam and the temporary filling was removed. Canals were irrigated with 2.5% NaClO; 10 mL/ canal with a final application of 17% EDTA (Glyde, Dentsply-Maillefer, Switzerland) for one minute then dried with sterile paper points. Bleeding was initiated by passing a pre-curved #25 K-file into the periapical tissues to create an injury milieu. Bleeding appeared minimal from the mesial canals. In parallel, PRF was prepared and used as described above. PRF-extract only was injected inside the mesial canals. A coronal barrier of white mineral trioxide aggregate (MTA) (Pro-root, Dentsply Tulsa, OK, United States) was placed. After two weeks, setting of MTA was checked and the tooth finally restored with composite resin.
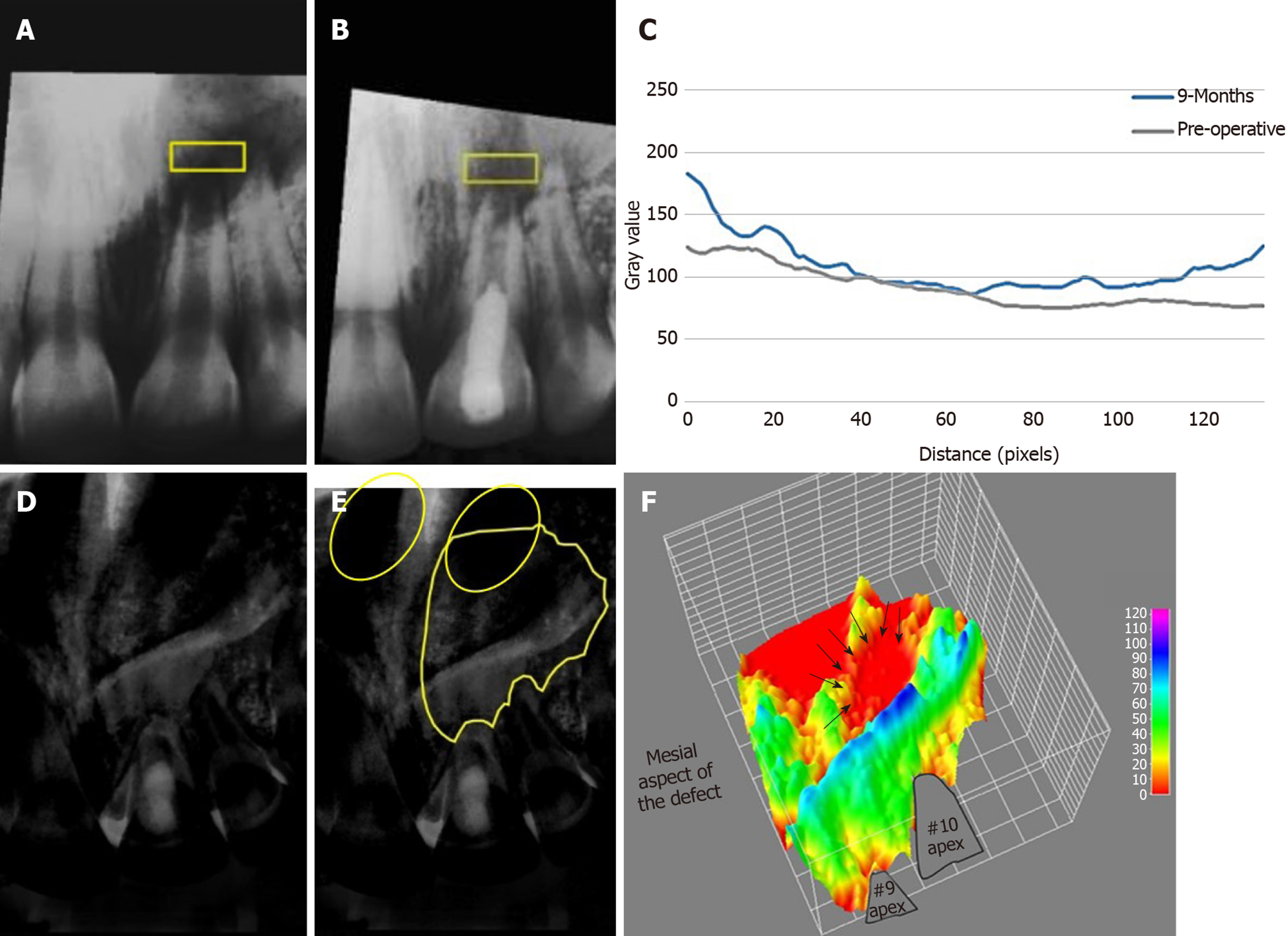
The patient was followed-up at 2-wk, 1, 3 and 9 mo (Figures 2 and 4) and (Table 1). The patient did not return for later follow-up. The sinus tract had completely resolved at the second visit. There was mild pain which subsided after 1 mo, after that, the tooth was asymptomatic and periodontally healthy at all visits (Figure 2E-H). All pulp tests were negative for tooth #9. The periapical radiograph showed root thickening and progressive apical closure of #9. The panoramic radiograph after 9 mo (Figure 2I and J) showed the increase of bone density and diminution of the size of the lesion especially around #9 and at the peripheries of the lesion. However, the lesion had not healed completely. The analysis of bone density of the periapical radiographs (Figure 4) revealed an overall increase in bone density except at the center of the lesion. Details are explained in the supporting information.
| Sinus tract | Pain on percussion | Radiographical | Pulp test | ||||
| Dentin thick | Apical closure | Bone | Hot | Cold | |||
| Day 0 | + | + | NA | NA | NA | - | - |
| 2 wk | - | + | - | - | - | - | - |
| 1 mo | - | - | - | - | - | - | - |
| 3 mo | - | - | - | - | - | - | - |
| 9 mo | - | - | + | - | + | - | - |
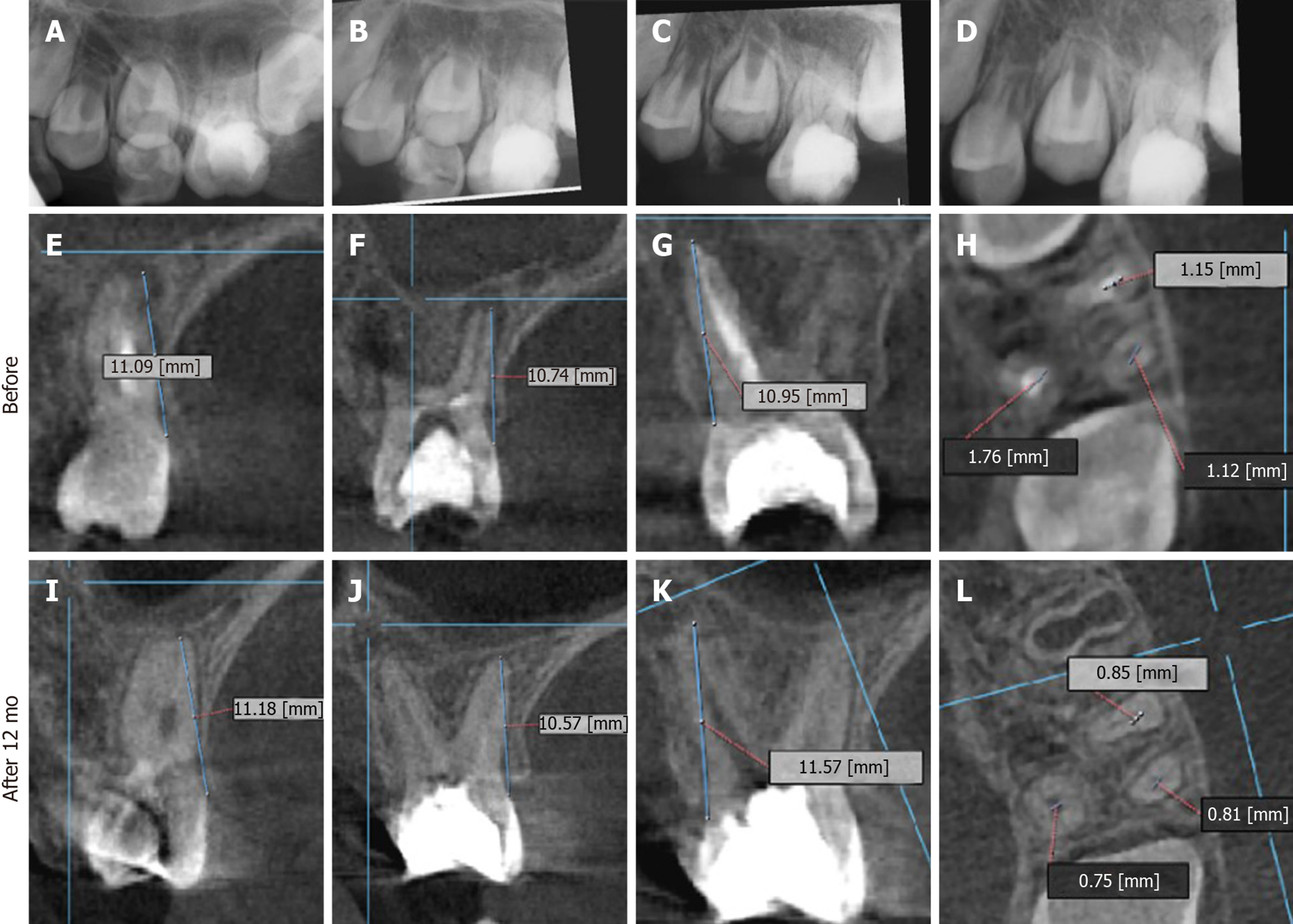
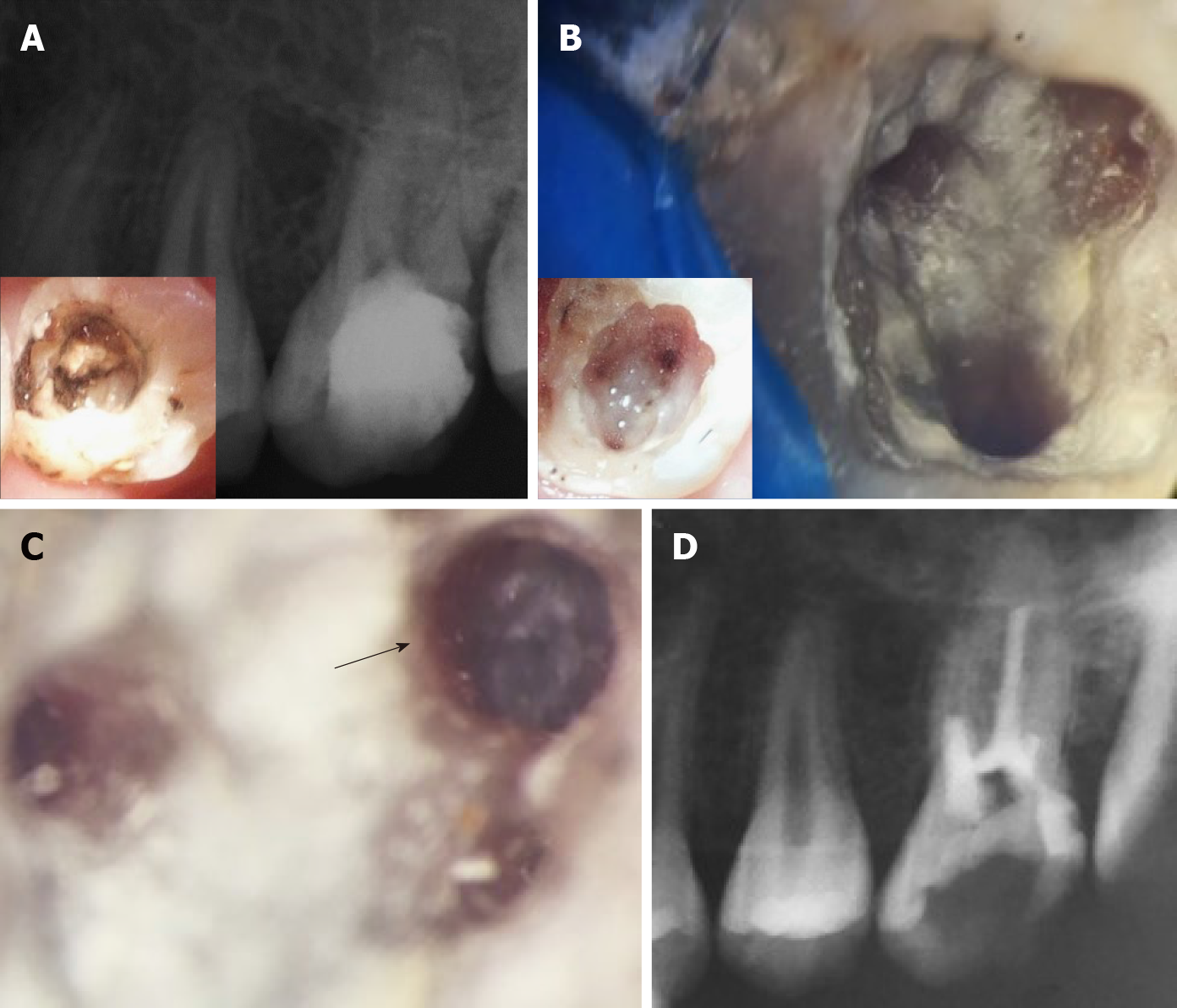
The patient was followed up at 2 wk, then at 1, 3, 6, 12, and 48 mo (Table 2). The patient reported normal function and did not complain about pain at any of the recall visits. There was no pain on palpation or percussion. All pulp tests, cold and hot, were negative throughout the follow-up period. The radiographs showed increased bone density and periapical healing with palatal root maturation (Figure 3). Upon comparing the pre-operative with the CBCT obtained at 12 mo, all roots showed apical closure, narrowing of canal space and complete resolution of the periapical lesion with normal periapical bone architecture. However, no root lengthening was observed in any root. Imaging parameters and image processing details are available in the supporting information. The patient was recalled after 48 mo to initiate orthodontic treatment and the tooth was asymptomatic. However, recurrent decay was noticed at the distal surface of the tooth and it was extending underneath the composite restoration but with normal periapical tissues in the digital periapical radiograph (Figure 5A). The caries, composite restoration and the MTA plug were removed. The canals appeared clean and dry with minimal fragments of tissues retrieved from the pulp chamber and canals (Figure 5B), but appeared with varying degrees of intracanal calcification (Figure 5C). The maximum length reached using a #10 K-file even following negotiation with ultrasonics (E-25 tip, Satelec, Acteon, France) was 16 mm for the palatal canal, 14 mm for the first mesiobuccal canal and 3 mm for the distobuccal canal while only the canal orifice was negotiated for the second mesiobuccal canal. The canals were instrumented to these lengths using manual files to remove any residual biofilm, copiously irrigated with 5.25% NaClO, dried and finally filled with gutta-percha followed by placement of glass ionomer and composite restoration (Figure 5D). The patient was recalled after 6 mo and reported the tooth as being asymptomatic with the coronal restoration intact and he was scheduled to start orthodontic treatment.
| Sinus tract | Pain on percussion | Radiographical | Pulp test | ||||
| Dentin thick | Apical closure | Bone | Hot | Cold | |||
| Day 0 | - | ++ | NA | NA | NA | - | - |
| 2 wk | - | + | - | - | - | - | - |
| 1 mo | - | - | - | - | - | - | - |
| 3 mo | - | - | - | - | - | - | - |
| 6 mo | - | - | + | - | + | - | - |
| 12 mo | - | - | + | + | + | - | - |
| 48 mo | - | - | +++ | ++ | + | - | - |
The present report is the first to document the long-term efficacy of RET for up to 48 mo. Clinical case reports documenting RET normally only extend to one year, with the longer-term outcomes being unpredictable[3], and the reliance on a small number of short-term studies, raises RET failure concerns among dentists[5]. This is also one of the rare clinical studies of using PRF and PRF-extract as scaffolds for RETs. Most RET case reports investigated blood clots[3]. There are a few unorthodox RET cases; which range from the treatment of elderly patient’s teeth to the re-treatment of failed cases[2,10]. Since apical diameter and patient age appear to be two important factors for the success of REPs[11], such novel applications will undoubtedly require modifications to the originally proposed protocol in order to fully encompass the triad of tissue engineering. One interesting observation was that the RET with PRF can initiate root canal wall thickening and lengthening, which is self-limiting over the long-term as the roots did not continue growing abnormally long. This observation resolves a concern among clinicians, about the long-term problems of using RET.
The use of platelet-rich concentrates for RETs including both platelet-rich plasma (PRP) and PRF has been successful in the regenerative applications. While with PRP, there is a release of growth factors within a few hours, PRF scaffolds entrap these factors within the fibrin mesh and slowly release them with a peak at 14 d[12]. In addition, no external chemicals, i.e., thrombin or CaCl2, are needed to prepare PRF.
Additionally, PRF can be divided to a clot and to a liquid (PRF-extract). The PRF-extract itself is a reservoir of bioactive cues that can on its own trigger the migration, proliferation, and differentiation of stem/progenitor cells[6,9,13]. Furthermore, Chai et al[14] recently reported that liquid PRF (prepared by centrifugation at 700 g for 3 min, the upper plasma layer is considered as liquid PRF) promotes dental pulp cellular migration, proliferation, and differentiation. The liquid PRF has also a partial immune defense against the induced inflammation.
The injectable forms of PRF may be used in inaccessible and narrow canals, which is of special importance when there is limited space for placement of a PRF clot and when induced bleeding is insufficient. In retrospective meta-analysis done by Murray[15] on a 222 immature permanent teeth, he found that PRF scaffolds showed higher success rate in terms of periapical lesion healing, root lengthening, and apical closure. While there was no difference in the thickening of dentinal walls. However, the superiority of PRF over blood clot in RETs is still controversial[16,17].
In this report, we presented two challenging regenerative endodontic cases. In case#1, the older age of the patient in addition to the presence of a large long-standing periapical lesion posed significant challenges. For case#2 presented with a permanent maxillary molar with 4 root canals and roots at different developmental stages. Hence, we used PRF and PRF-extract as a natural ameliorate for tissue regeneration. It is also noteworthy to state that induction of bleeding, although expected to be insufficient, was still done to create an injury stimulus to trigger stem/progenitor cell recruitment. The PRF clot was placed so the platelet-plug will face the apical tissues, which may have provided a higher gradient of cytokines and growth factors to enhance the healing process[18]. Moreover, antimicrobial properties of PRF have also been revealed[19]. This coupled with previously mentioned properties of PRF may have contributed to the improved healing outcome in this report.
Incomplete root maturation was observed in case#1 after 9 mo. The patient did not complain about pain, and the bone density had improved. This is analogous to two cases with periapical lesions in middle-aged patients[20,21]. The first reported by Saoud et al[21] involved a large periapical lesion in a 23-year-old patient. The authors found that after one year there was more trabecular bone deposition but incomplete resolution. The second case by Wang et al[20], demonstrated a 39-year-old patient with an extensive bilateral apical radiolucency. The radiolucency resolved 30 mo after treatment. This slow healing could be due to size of the lesion, the long-standing infection and age of the patient. RET outcomes appears to improve if the pulp necrosis occurred less than 6 mo prior to treatment[22].
The effectiveness of disinfection needed for RET is more critical than that needed in traditional endodontics, hence, in these cases, we used lower concentrations of NaClO to avoid killing the stem cells[23,24]. In addition, calcium hydroxide was selected as an intracanal medicament because it could enhance the attachment, viability and proliferation of apical papilla cells in contrast to other medicaments[24].
The use of PRF and its extract could have accelerated the healing in the first case; unfortunately the patient was lost to follow-up prohibiting long-term assessment. Age has also been shown to influence the outcome of regeneration. Dental pulp stem cells from aged individuals have shown impaired proliferation and neuronal differentiation capacities[25]. This may be one factor explaining the delayed healing with age and encourage the use of bioactive materials to enhance stem cell homing and endogenous tissue regeneration.
After 12 mo, in case#2, where PRF-extract was used solely as the bleeding was not successful in the two mesiobuccal canals, demonstrated the greater promise of the PRF approach. Although the roots did not show any root lengthening, they showed maturation and wall thickening. This is with accordance with a meta-analysis by He et al[26], they suggested that apical closure is not necessarily associated with root lengthening. After 4 years, the tooth was asymptomatic and radiographically successful, however, recurrent caries at the distal surface was extensive which necessitated re-intervention. This highlights the importance of proper patient selection and the importance of patients’ oral hygiene for the success of the treatment. Perhaps the most interesting result is the extensive intracanal calcification noted in case #2. This may be attributed to remnants of calcium hydroxide pastes which are difficult to remove and could remain inside root canals. Song et al[27] found that 61.5% of cases (8 of 13 cases) with complete canal obliteration had occurred when Ca(OH)2 was used as an intracanal medication, compared to (30.8%, 4 of 13) when antibiotic pastes were used.
The source of cells appears to plays a key role in root maturation and calcification, where PDL and bone marrow stem cells may contribute to extensive deposition of cementum/bone-like tissues[27] also found that cases with induced bleeding had higher frequency of calcification (69.6%, 16 of 23 cases) than those without bleeding when vital tissues were found in the canals. Documentation of long-term outcomes such as the extensive intracanal calcification seen in case#2 is crucial, particularly for long term treatment planning. RETs assisted by PRF and PRF-extract did help retain tooth#14 until orthodontic treatment commenced. Natera et al[28] reported a similar case that received orthodontic treatment one year after REPs in tooth #20. Although the tooth lacked further root maturation, it remained asymptomatic clinically and radiographically after 4 years and the tooth responded to orthodontic treatment as non-endodontically treated teeth.
A case report has been recently published by Chaniotis et al[29] who started orthodontic treatment 3 years and half after RET in tooth #8. The patient had skeletal Class II division 1, which had subjected tooth #8 to multiple traumas that lead finally to a periapical lesion and fracture. By the end of orthodontic treatment that lasted for 2 years, the periapical radiographs revealed a marked remodeling of the periapical tissues; the fractured apical third showed signs of resorption, along with the resolution of the periapical lesion. The presence of tooth #8 was essential for the success of the orthodontic treatment, however, the orthodontic forces may have disturbed the reattachment of the fractured root part. Similarly, retention of the tooth#14 in case#2 in this report was essential in spite of the need to later retreat the tooth. The decision to retreat calcified/obliterated root canals is based on multiple factors yet calcification itself may not be a failure per se[30] unless coronal seal is lost and bacterial re-infection is a risk as was the case in this report.
Both the RETs in this present report exhibited negative responses to pulp testing. However, this does not negate the possibility of re-vitalization[31]. A longer-term follow-up may be required to accurately assess the pulp re-vitalization parameters.
Some previous histological studies have shown that the use of PRP did not change the nature of the regenerated tissues, when compared to blood clots within root canals[32]. It was not possible to conduct a histologic analysis of the teeth following RET, due to the lack of patient consent to extract healthy functioning teeth.
In conclusion and within the limitations of this report, it was observed that: RET with PRF can generate the root structure of immature permanent teeth with a necrotic pulp following caries and trauma. RET with PRF can initiate root canal wall thickening and lengthening, which is self-limiting over the long-term as the roots did not continue to grow abnormally long. RET with PRF could benefit patients by saving immature teeth with a necrotic pulp from subsequent fracture and requiring extraction.
The authors would like to thank Dr. Mohamed Hesham for his assistance in patient follow-up, Dr. Ayat Hamdy and Dr. Noha El Shazley for their assistance in radiographic assessment, and Dr. Semha El Naggar for assistance with clinical procedures.
Manuscript source: Unsolicited manuscript
Specialty type: Dentistry, oral surgery and medicine
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang AHC, Paredes-Vieyra JP, Teragawa H S-Editor: Ma YJ L-Editor: A E-Editor: Liu JH
| 1. | Nosrat A, Seifi A, Asgary S. Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: a review and report of two cases with a new biomaterial. J Endod. 2011;37:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Saoud TM, Martin G, Chen YH, Chen KL, Chen CA, Songtrakul K, Malek M, Sigurdsson A, Lin LM. Treatment of Mature Permanent Teeth with Necrotic Pulps and Apical Periodontitis Using Regenerative Endodontic Procedures: A Case Series. J Endod. 2016;42:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Garcia-Godoy F, Murray PE. Recommendations for using regenerative endodontic procedures in permanent immature traumatized teeth. Dent Traumatol. 2012;28:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Lovelace TW, Henry MA, Hargreaves KM, Diogenes A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod. 2011;37:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 5. | Epelman I, Murray PE, Garcia-Godoy F, Kuttler S, Namerow KN. A practitioner survey of opinions toward regenerative endodontics. J Endod. 2009;35:1204-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Woo SM, Kim WJ, Lim HS, Choi NK, Kim SH, Kim SM, Jung JY. Combination of Mineral Trioxide Aggregate and Platelet-rich Fibrin Promotes the Odontoblastic Differentiation and Mineralization of Human Dental Pulp Cells via BMP/Smad Signaling Pathway. J Endod. 2016;42:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 692] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 8. | Hotwani K, Sharma K. Platelet rich fibrin - a novel acumen into regenerative endodontic therapy. Restor Dent Endod. 2014;39:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Huang FM, Yang SF, Zhao JH, Chang YC. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod. 2010;36:1628-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Saoud TM, Huang GT, Gibbs JL, Sigurdsson A, Lin LM. Management of Teeth with Persistent Apical Periodontitis after Root Canal Treatment Using Regenerative Endodontic Therapy. J Endod. 2015;41:1743-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Estefan BS, El Batouty KM, Nagy MM, Diogenes A. Influence of Age and Apical Diameter on the Success of Endodontic Regeneration Procedures. J Endod. 2016;42:1620-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 345] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 13. | Ji B, Sheng L, Chen G, Guo S, Xie L, Yang B, Guo W, Tian W. The combination use of platelet-rich fibrin and treated dentin matrix for tooth root regeneration by cell homing. Tissue Eng Part A. 2015;21:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Chai J, Jin R, Yuan G, Kanter V, Miron RJ, Zhang Y. Effect of Liquid Platelet-rich Fibrin and Platelet-rich Plasma on the Regenerative Potential of Dental Pulp Cells Cultured under Inflammatory Conditions: A Comparative Analysis. J Endod. 2019;45:1000-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Murray PE. Platelet-Rich Plasma and Platelet-Rich Fibrin Can Induce Apical Closure More Frequently Than Blood-Clot Revascularization for the Regeneration of Immature Permanent Teeth: A Meta-Analysis of Clinical Efficacy. Front Bioeng Biotechnol. 2018;6:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Lv H, Chen Y, Cai Z, Lei L, Zhang M, Zhou R, Huang X. The efficacy of platelet-rich fibrin as a scaffold in regenerative endodontic treatment: a retrospective controlled cohort study. BMC Oral Health. 2018;18:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Ulusoy AT, Turedi I, Cimen M, Cehreli ZC. Evaluation of Blood Clot, Platelet-rich Plasma, Platelet-rich Fibrin, and Platelet Pellet as Scaffolds in Regenerative Endodontic Treatment: A Prospective Randomized Trial. J Endod. 2019;45:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 18. | Dohan Ehrenfest DM, Bielecki T, Jimbo R, Barbé G, Del Corso M, Inchingolo F, Sammartino G. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF). Curr Pharm Biotechnol. 2012;13:1145-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 19. | Bayer A, Lammel J, Rademacher F, Groß J, Siggelkow M, Lippross S, Klüter T, Varoga D, Tohidnezhad M, Pufe T, Cremer J, Gläser R, Harder J. Platelet-released growth factors induce the antimicrobial peptide human beta-defensin-2 in primary keratinocytes. Exp Dermatol. 2016;25:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Zhu X, Zhang C. Pulp Revascularization on Permanent Teeth with Open Apices in a Middle-aged Patient. J Endod. 2015;41:1571-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Saoud TM, Sigurdsson A, Rosenberg PA, Lin LM, Ricucci D. Treatment of a large cystlike inflammatory periapical lesion associated with mature necrotic teeth using regenerative endodontic therapy. J Endod. 2014;40:2081-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Nosrat A, Homayounfar N, Oloomi K. Drawbacks and unfavorable outcomes of regenerative endodontic treatments of necrotic immature teeth: a literature review and report of a case. J Endod. 2012;38:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 23. | Fouad AF, Verma P. Healing after regenerative procedures with and without pulpal infection. J Endod. 2014;40:S58-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Kitikuson P, Srisuwan T. Attachment Ability of Human Apical Papilla Cells to Root Dentin Surfaces Treated with Either 3Mix or Calcium Hydroxide. J Endod. 2016;42:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Wu W, Zhou J, Xu CT, Zhang J, Jin YJ, Sun GL. Derivation and growth characteristics of dental pulp stem cells from patients of different ages. Mol Med Rep. 2015;12:5127-5134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | He L, Zhong J, Gong Q, Kim SG, Zeichner SJ, Xiang L, Ye L, Zhou X, Zheng J, Liu Y, Guan C, Cheng B, Ling J, Mao JJ. Treatment of Necrotic Teeth by Apical Revascularization: Meta-analysis. Sci Rep. 2017;7:13941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Song M, Cao Y, Shin SJ, Shon WJ, Chugal N, Kim RH, Kim E, Kang MK. Revascularization-associated Intracanal Calcification: Assessment of Prevalence and Contributing Factors. J Endod. 2017;43:2025-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Natera M, Mukherjee PM. Regenerative Endodontic Treatment with Orthodontic Treatment in a Tooth with Dens Evaginatus: A Case Report with a 4-year Follow-up. J Endod. 2018;44:952-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Chaniotis A. Orthodontic Movement after Regenerative Endodontic Procedure: Case Report and Long-term Observations. J Endod. 2018;44:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Oginni AO, Adekoya-Sofowora CA, Kolawole KA. Evaluation of radiographs, clinical signs and symptoms associated with pulp canal obliteration: an aid to treatment decision. Dent Traumatol. 2009;25:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Topçuoğlu G, Topçuoğlu HS. Regenerative Endodontic Therapy in a Single Visit Using Platelet-rich Plasma and Biodentine in Necrotic and Asymptomatic Immature Molar Teeth: A Report of 3 Cases. J Endod. 2016;42:1344-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Torabinejad M, Faras H, Corr R, Wright KR, Shabahang S. Histologic examinations of teeth treated with 2 scaffolds: a pilot animal investigation. J Endod. 2014;40:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |