Published online Nov 20, 2013. doi: 10.5321/wjs.v2.i4.86
Revised: August 5, 2013
Accepted: August 20, 2013
Published online: November 20, 2013
Processing time: 158 Days and 23.1 Hours
AIM: To evaluate the direct and indirect biocompatibility of Filtek Silorane on human gingival fibroblastic cells.
METHODS: Sixty-three standardized cylindrical specimens (8 mm diameter and 2 mm thickness) of restorative material were prepared using a light emitting diode-curing unit. The sample were built up in one increment and divided in 2 groups. In the first group, 21 samples (unpolished samples) were left without a specific polishing procedure; in the second one, 42 samples (polished samples) were polished with 4 different grains of discs. Fibroblast cultures, obtained from gingiva of 2 subjects without systemic and oral disease, were used to assess the direct and indirect biocompatibility. Cells cultured for 48 h in normal culture medium were used as a control.
RESULTS: The scanning electron microscope observations of fibroblasts cultured on the silorane samples, either polished or unpolished, confirmed the good biocompatibility of the material, favouring the cellular spreading. 3-dimethylthiazol-2, 5-diphenyltetrazolium bromide tests showed a significant reduction (P < 0.01) of gingival fibroblasts viability cultured both in polished samples (90.05% ± 19.00%) and unpolished samples (78.15% ± 11.00%) compared with the control. Cells growth in medium conditioned with the samples for 1 wk showed a significant viability reduction (P < 0.01) compared to the control. A reduction of cell viability was observed even in the groups containing the material for 3 wk (polished: 89.45% ± 10.00%; unpolished: 65.97% ± 10.00%), even if the cytotoxicity was reduced after this long time exposure.
CONCLUSION: Although the poor chromatic availability of this material remains a big limit that restricts its use to posterior sectors, the silorane-based material can be considered an option to perform restorations when aesthetic demands are not the priority, such as the class II restorations
Core tip: The behaviour of silorane-based materials seems to be comparable to the one observed for conventional composite material, thus decreasing the cytotoxicity after long time exposure. Further studies are still needed to characterize the biological response of these methacrylate-free composite formulations, in order to definitely demonstrate their safe use in restorative dentistry.
- Citation: Orsini G, Catellani A, Ferretti C, Gesi M, Mattioli-Belmonte M, Putignano A. Cytotoxicity of a silorane-based dental composite on human gingival fibroblasts. World J Stomatol 2013; 2(4): 86-90
- URL: https://www.wjgnet.com/2218-6263/full/v2/i4/86.htm
- DOI: https://dx.doi.org/10.5321/wjs.v2.i4.86
Recently, the use of composite materials for restoring dental elements has significantly increased due to the growing aesthetic demand of patients[1].
Despite extensive improvements in mechanical and aesthetic properties of dental composites, volumetric shrinkage and contraction stress during polymerization are still a problem[1]. Contraction stress transferred to the tooth may lead to cusp deflection or enamel micro cracks; additionally, contraction stress of tooth-composite interface can determinate post-operative sensitivity, microleakage, marginal discoloration and recurrent caries[2].
In several studies different techniques have been investigated in order to minimize polymerization shrinkage and contraction stress[3-7]. At the same purpose low-shrinkage materials have been proposed, but none of them offered significant improvement to Bis-GMA-based composites[8].
In 2007, a low shrinkage dental composite based on silorane monomers has been introduced. This material contains traditional filler particles (quartz) and monomers based on a silane or a siloxane core bonded with several oxirane functional groups. The silorane monomers polymerize by a ring-opening polymerization process of the oxirane groups. According to its composition, this resin has two advantages: low polymerization shrinkage, due to the ring-opening oxirane monomer, and increased hydrophobicity, due to the presence of the siloxanes[9].
The release of substances from dental composite materials after polymerization and their possible toxicity have been widely examined during previous years[10-12]. Several in vitro studies have shown cytotoxic, genotoxic, mutagenic, or estrogenic effects of some monomers released by composite materials[13-17].
Limited information is available about the substance eluted from silorane composite and its cell or tissue compatibility. Kopperud et al[18] found no substance eluted from Filtek silorane in water, while silorane were found in ethanol solution. Krifka et al[19] revealed no significant signs of cytotoxicity on human pulp-derived cells caused by silorane-based materials, while a slight increase in reactive oxygen species was detected.
The aim of present study was to evaluate the biocompatibility of Filtek silorane. The maintaining of surface architecture after finishing was also investigated. These properties were investigated in polished and unpolished silorane polymerized samples.
As regards biocompatibility, we studied the viability of human fibroblastic cells both after direct contact with silorane composite and after cells conditioning using a medium exposed to silorane.
Sixty-three standardized cylindrical specimens (8 mm in diameter and 2 mm in thickness) were prepared using a transparent plastic molds. The molds were positioned on a glass plate and filled with Filtek silorane (3 mol/L ESPE, Seefeld, Germany). The samples were built up in one increment. The specimens were polymerized using a diode unit with a power of 1100 Mw/cm2 for 60 s (LE Demetron I; Kerr, Bioggio, Switzerland). Forty two of these samples were polished using a slow speed hand-piece using 4 polishing discs of different grains (Sof-Lex discs, 3 mol/L ESPE; Seefeld, Germany), from the most (2382 C) to the least (2382 SF) abrasive. The remaining samples were left unpolished. All the samples were processed for observation under a scanning electron microscope (SEM: Philips XL20; FEI, Milano, Italy).
Cultured fibroblasts were obtained from subjects without systemic and oral disease, after signing informed consent. Biopsies (2 cm × 2 cm were taken from the gingiva of 2 subjects (40 years old), rinsed twice with phosphate buffered saline (PBS) at pH 7.4, containing penicillin (100 U/mL), streptomycin (100 μg/mL) and amphotericin B (2.5 μg/mL; all from Sigma Aldrich, Milan, Italy) and cut in small pieces with a sterile blazer. The tissue fragments were placed in culture flasks of 25 cm2 with Dulbecco Modified Essential Medium (DMEM), containing 1 mg/mL of collagenase (all from Sigma Aldrich), and incubated for 3 h at 37 °C. Afterwards, fragments were incubated at 37 °C (5% CO2) in Petri plates of 35 mm containing DMEM supplemented with 10% of fetal bovine serum (FBS, Life Technologies, Monza, Italy), 4.5 g/L of glucose, penicillin (100 U/mL) and streptomycin (100 μg/mL) all from Sigma Aldrich. The first fibroblast cells were visible after 3-4 d. Culture medium was changed twice a week until cells confluence (2 wk). Using a trypsin/EDTA treatment (0.25% trypsin, 0.02% EDTA; Sigma Aldrich), the cells were detached and cultured in flasks of 75 cm2 until a new confluence was achieved. Cells between the 2nd and the 4th passage of subculture have been used.
For direct toxicity test, silorane samples have been disinfected with alcohol at 70% for 3 h and washed with PBS for 24 h after the alcohol removing. After a conditioning treatment in DMEM containing 10% FBS and penicillin (100 U/mL) and streptomycin (100 μg/mL) for 24 h, the medium was discarded and samples considered suitable for cell seeding. Specimens were placed in ultra-low attachment 24/well plates (Corning, Tewksbury, MA, United States) and seeded with 1 × 104 cells/cm2.
To assess indirect toxicity assay, samples disinfected as previously described were placed in agitation in DMEM containing 10% FBS and penicillin (100 U/mL) and streptomycin (100 μg/mL) for 1 and 3 wk. The conditioned medium was placed in contact with fibroblasts (1 × 104 cells/cm²) seeded in 24/well polystyrene plates for 48 h. Cells cultured for 48 h in normal culture medium were used as a control.
The obtained monolayer cells were fixed in 2% glutaraldehyde in cacodylate buffer for one hour at 4 °C. After fixation, cells were rinsed in cacodylate buffer 0.1 mol/L, pH 7.4 and 7% sucrose; cells were then post-fixed using 0.1% OsO4 in cacodylate buffer 0.1 mol/L, at 7.4 pH (1 h in dark at 4 °C). After a second rinse in cacodylate buffer for 10 min, samples were dehydrated using a growing grade of ethanol (from 25% to 100%) at 4 °C with Critical Point Drying at 31.3 °C and 72.9 Atm. The samples were placed on aluminium stubs with a graphite-based glue, covered with gold, using an Edwards sputtering device, and observed with a SEM operating at 20 kV.
After 48 h of culture, medium was removed and 200 μL of a solution (5 mg/mL in medium without phenol red) containing 3-dimethylthiazol-2, 5-diphenyltetrazolium bromide (MTT; Aldrich, Sigma) and 1.8 mL of medium was added to the monolayer cells. The plates were incubated at 37 °C for 4 h. The supernatant was removed, the blue-violet formazan crystals were dissolved adding 2 mL of solvent (HCL 4% in isopropanol) and quantified with the spectrophotometer (Secoman; Anthelie light, 3.8 version, Contardi, Italia) at 570 and 690 nm. The results have been reported as viability percentage compared with the control culture.
Statistical analysis of the data was performed using two-ways analysis of variance. In detail, cell viability was evaluated on fibroblasts: (1) directly cultured on polished samples (P), unpolished samples (UnP) and control (CTRL); and (2) in contact with the eluates of P, UnP and CTRL samples at 1 and 3 wk.
Levels of P < 0.05 were considered to be statistically significant. The results were also evaluated in accordance with ISO standard 10993-5[20] which describes less than 25% inhibition as non-cytotoxic, 25% to 50% inhibition as slightly cytotoxic, 50% to 75% inhibition as moderately cytotoxic and more than 75% inhibition as highly cytotoxic[21].
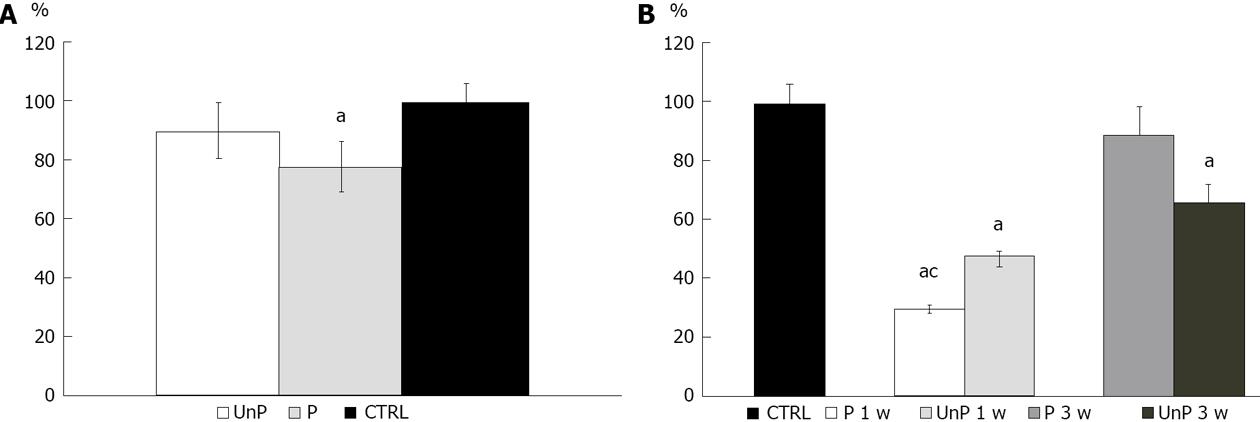
MTT tests showed a significant reduction (P < 0.01) of gingival fibroblasts viability cultured both in P (90.05% ± 19.03%) and in UnP (78.15% ± 11.01%) compared with the CTRL (100.00% ± 6.00%), as shown in Figure 1A.
As regards to indirect toxicity, the viability of fibroblastic cells incubated in a medium conditioned with both P and UnP, for 1 or 3 wk, respectively, was studied using MTT test.
Cells growth in medium conditioned for 1 wk showed a significant viability reduction (P < 0.01) compared to the CTRL: the group conditioned with P showed a viability of 29.83% ± 1.92%, the one with UnP: 47.06% ± 1.87% (Figure 1B).
A reduction of cell viability was also observed in both groups conditioned for 3 wk (P: 89.45% ± 10.11%; UnP: 65.97% ± 9.89%), but only in the second group this reduction was statistically significant (Figure 1B).
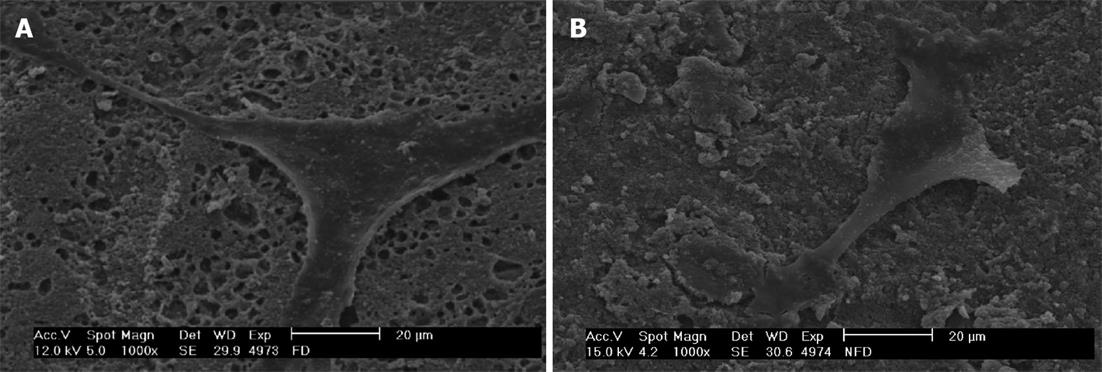
As shown in Figure 2, SEM observations of fibroblasts cultured on the silorane samples, either P or UnP, confirmed the good biocompatibility of this material, which favoured cell spreading. These observations showed that the surface of the silorane-based material is able to absorb a big quantity of the serum component from the culture medium.
Silorane-based composite is a candidate for use in conservative dentistry due to its low polymerization shrinkage. However, it cannot be excluded that the potential release of remaining monomer substances may exert harmful effects on cells of periodontal tissues[22]. The current limited literature indicates that silorane-based composite has a low toxicity presumably due to the low rate of free monomers released after polymerization[18]. In order to ensure a safe use of silorane-based materials, studies on the biocompatibility of this material are still needed.
Biocompatibility of a dental material can be studied exposing tissue directly to the material (direct toxicity) or placing it in a medium (conditioning), which will be used for additional tests (indirect toxicity)[23].
The results obtained in our study show a low direct cytotoxicity of both samples: P and UnP. The percentage of survival is lower in UnP than in P probably due to the larger surface contact area between composite and fibroblasts. Furthermore, the presence of oxygen inhibits the polymerization, resulting in a higher percentage of unreacted composite on the composite surface. Incomplete polymerization not only causes a decrease in the mechanical properties, but it can cause tissue reaction, as shown by Spangberg et al[24]. Composite finishing and polishing may indeed decrease the toxicity, as hypothesized in the study of Mohsen and Vankerchoven[25,26]. A moderate (with a few peaks of high toxicity) indirect cytotoxicity was observed in the samples placed in culture medium conditioned for 1 wk with silorane eluates (being the UnP slightly less cytotoxic than the P ones). Slight indirect cytotoxicity values were obtained for the samples placed in culture with medium conditioned for 3 wk. Under this condition, the fibroblast cultures show a different behaviour, since cell viability was slightly greater in case of contact with P than with UnP ones. These findings are in agreement with Sheridan et al[27], reporting that the cytotoxic effect of acrylic resin was greater after polymerization and decreased with time for many resins. The authors concluded that the longer a prosthesis is soaked, the less cytotoxic effects it is likely to have regardless of the denture base resin it is manufactured from[27]. Due to the not univocal data among P and UnP, the surface roughness does not seem to be a determining factor in the study of indirect toxicity. Indirect toxicity can be determined by release of substances from silorane as widely described in scientific literature[22].
Scanning electron micrographs allow observing the characteristic fibroblastic spreading. This is consistent with a study of Balcells et al[28], which states that the adsorption of serum proteins present in the culture medium is the first event that occurs when cells are seeded on a material and the adsorbed protein layer influences cell adhesion, spreading and proliferation.
In conclusion, although the poor chromatic availability of this material remains a big limit that restricts its use to posterior sectors, the silorane-based material can be considered an option to perform restorations when aesthetic demands are not the priority, such as the class II restorations[29]. The behaviour of silorane-based materials seems to be comparable to the one observed for conventional composite material[30], thus decreasing the cytotoxicity after long time exposure. Further studies are still needed to characterize the biological response of these methacrylate-free composite formulations, in order to definitely demonstrate their safe use in restorative dentistry.
Dr. Marcantoni, Dr. Morici and Dr. Kyriakidou are kindly acknowledged for technical assistance.
Despite extensive improvements in mechanical and aesthetic properties of dental composites, volumetric shrinkage and contraction stress during polymerization are still a problem.
In several studies different techniques have been investigated in order to minimize polymerization shrinkage and contraction stress at the same purpose low-shrinkage materials have been proposed but none of them offered significant improvement to Bis-GMA-based composites.
The behaviour of silorane-based materials seems to be comparable to the one observed for conventional composite material, thus decreasing the cytotoxicity after long time exposure.
Further studies are still needed to characterize the biological response of these methacrylate-free composite formulations, in order to definitely demonstrate their safe use in restorative dentistry.
The authors considered and concluded that the materials are biocompatible.
P- Reviewers: Brasileiro B, Eugenia KK, Jeng JH S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Condon JR, Ferracane JL. Assessing the effect of composite formulation on polymerization stress. J Am Dent Assoc. 2000;131:497-503. [PubMed] |
| 2. | Hilton TJ. Can modern restorative procedures and materials reliably seal cavities? In vitro investigations. Part 1. Am J Dent. 2002;15:198-210. [PubMed] |
| 3. | Gonçalves F, Pfeifer CS, Meira JB, Ballester RY, Lima RG, Braga RR. Polymerization stress of resin composites as a function of system compliance. Dent Mater. 2008;24:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Braga RR, Ballester RY, Ferracane JL. Factors involved in the development of polymerization shrinkage stress in resin-composites: a systematic review. Dent Mater. 2005;21:962-970. [PubMed] |
| 5. | Witzel MF, Ballester RY, Meira JB, Lima RG, Braga RR. Composite shrinkage stress as a function of specimen dimensions and compliance of the testing system. Dent Mater. 2007;23:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Chen HY, Manhart J, Hickel R, Kunzelmann KH. Polymerization contraction stress in light-cured packable composite resins. Dent Mater. 2001;17:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Bouschlicher MR, Vargas MA, Boyer DB. Effect of composite type, light intensity, configuration factor and laser polymerization on polymerization contraction forces. Am J Dent. 1997;10:88-96. [PubMed] |
| 8. | Braga RR, Ferracane JL. Alternatives in polymerization contraction stress management. Crit Rev Oral Biol Med. 2004;15:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Ilie N, Hickel R. Resin composite restorative materials. Aust Dent J. 2011;56 Suppl 1:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Yap AU, Han VT, Soh MS, Siow KS. Elution of leachable components from composites after LED and halogen light irradiation. Oper Dent. 2004;29:448-453. [PubMed] |
| 11. | Lee SY, Greener EH, Menis DL. Detection of leached moieties from dental composites in fluids simulating food and saliva. Dent Mater. 1995;11:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Geurtsen W. Substances released from dental resin composites and glass ionomer cements. Eur J Oral Sci. 1998;106:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 237] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Geurtsen W, Lehmann F, Spahl W, Leyhausen G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res. 1998;41:474-480. [PubMed] |
| 14. | Volk J, Leyhausen G, Dogan S, Geurtsen W. Additive effects of TEGDMA and hydrogenperoxide on the cellular glutathione content of human gingival fibroblasts. Dent Mater. 2007;23:921-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Reichl FX, Simon S, Esters M, Seiss M, Kehe K, Kleinsasser N, Hickel R. Cytotoxicity of dental composite (co)monomers and the amalgam component Hg(2+) in human gingival fibroblasts. Arch Toxicol. 2006;80:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Yoshii E. Cytotoxic effects of acrylates and methacrylates: relationships of monomer structures and cytotoxicity. J Biomed Mater Res. 1997;37:517-524. [PubMed] |
| 17. | Poplawski T, Pawlowska E, Wisniewska-Jarosinska M, Ksiazek D, Wozniak K, Szczepanska J, Blasiak J. Cytotoxicity and genotoxicity of glycidyl methacrylate. Chem Biol Interact. 2009;180:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Kopperud HM, Schmidt M, Kleven IS. Elution of substances from a silorane-based dental composite. Eur J Oral Sci. 2010;118:100-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Krifka S, Seidenader C, Hiller KA, Schmalz G, Schweikl H. Oxidative stress and cytotoxicity generated by dental composites in human pulp cells. Clin Oral Investig. 2012;16:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | International Standards Organization, ISO 10993-5: Biological evaluation of Medical Devices-Part 5. Tests for Cytotoxicity: In Vitro Methods. Geneva: ISO 1992; . |
| 21. | Jorge JH, Giampaolo ET, Vergani CE, Machado AL, Pavarina AC, Carlos IZ. Cytotoxicity of denture base resins: effect of water bath and microwave postpolymerization heat treatments. Int J Prosthodont. 2004;17:340-344. [PubMed] |
| 22. | Schulz SD, König A, Steinberg T, Tomakidi P, Hellwig E, Polydorou O. Human gingival keratinocyte response to substances eluted from silorane composite material reveal impact on cell behavior reflected by RNA levels and induction of apoptosis. Dent Mater. 2012;28:e135-e142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Schweikl H, Hiller KA, Bolay C, Kreissl M, Kreismann W, Nusser A, Steinhauser S, Wieczorek J, Vasold R, Schmalz G. Cytotoxic and mutagenic effects of dental composite materials. Biomaterials. 2005;26:1713-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Spangberg L, Rodrigues H, Langeland L, Langeland K. Biologic effects of dental materials. 2. Toxicity of anterior tooth restorative materials on HeLa cells in vitro. Oral Surg Oral Med Oral Pathol. 1973;36:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Mohsen NM, Craig RG, Hanks CT. Cytotoxicity of urethane dimethacrylate composites before and after aging and leaching. J Biomed Mater Res. 1998;39:252-260. [PubMed] |
| 26. | Vankerckhoven H, Lambrechts P, van Beylen M, Davidson CL, Vanherle G. Unreacted methacrylate groups on the surfaces of composite resins. J Dent Res. 1982;61:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 105] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Sheridan PJ, Koka S, Ewoldsen NO, Lefebvre CA, Lavin MT. Cytotoxicity of denture base resins. Int J Prosthodont. 1997;10:73-77. [PubMed] |
| 28. | Balcells M, Klee D, Fabry M, Höcker H. Quantitative Assessment of Protein Adsorption by Combination of the Enzyme-Linked Immunosorbent Assay with Radioisotope-Based Studies. J Colloid Interface Sci. 1999;220:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Goncalves FS, Castro CD, Bueno AC, Freitas AB, Moreira AN, Magalhaes CS. The short-term clinical performance of a silorane-based resin composite in the proximal contacts of class II restorations. J Contemp Dent Pract. 2012;13:251-256. [PubMed] |
| 30. | Hahnel S, Henrich A, Bürgers R, Handel G, Rosentritt M. Investigation of mechanical properties of modern dental composites after artificial aging for one year. Oper Dent. 2010;35:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |