Published online Nov 20, 2013. doi: 10.5321/wjs.v2.i4.71
Revised: July 31, 2013
Accepted: August 5, 2013
Published online: November 20, 2013
Processing time: 130 Days and 10.3 Hours
Treatment of head and neck cancer with radiotherapy and/or chemotherapy can cause oral damage. Long-term treatment can damage the salivary glands, the oral mucosa, and the maxilla, leading to altered production of saliva and to multiple infections. These lesions can be prevented, limited or avoided by thorough evaluation prior to treatment and by therapeutic follow-up and preventive measures. The dentist must have strong medical knowledge of the possible short-, medium-, and long-term oral complications of the cancer treatment, and must have knowledge of the protocols for oral management of cancer patients. The availability of a multidisciplinary medical team together with a dentist to attend to the patient prior to the cancer treatment, as well as close communication between team members during and after treatment, is crucial. The aim of the present study was review the stomatological management of head and neck cancer patients treated with chemotherapy and radiotherapy and summarizing current treatments, therapeutic innovation and tissue regeneration perspectives.
Core tip: The aim of the present study was to conduct a review of therapeutic advances in the prevention and management of oral disorders in head and neck cancer patients receiving radio- and chemotherapy. The study focuses on possible risk factors and on the prevention of these disorders.
- Citation: Bologna-Molina R, Maglia A, Castañeda-Castaneira RE, Molina-Frechero N. Stomatological management of head and neck cancer patients treated with chemotherapy and radiotherapy. World J Stomatol 2013; 2(4): 71-78
- URL: https://www.wjgnet.com/2218-6263/full/v2/i4/71.htm
- DOI: https://dx.doi.org/10.5321/wjs.v2.i4.71
In recent decades, an increase in the prevalence of oral cancer has been observed in several countries. Surgery, radiotherapy and chemotherapy continue to be the treatments of choice for such cancers, and advances have been made in minimizing their adverse effects[1]. However, treatment of cancer with radiotherapy and/or chemotherapy can cause oral damage. Long-term treatment can damage the salivary glands, the oral mucosa, and the maxilla, leading to altered production of saliva and to multiple infections[2]. The surgical treatment of oral and maxillofacial neoplasms can lead to sequelae such as limited speech, eating disorders, alterations in the patient’s sense of taste and smell, and changes in the patient’s physical appearance[3,4]
Lesions of the oral cavity secondary to head and neck cancer treatment can be prevented, limited or avoided by thorough evaluation prior to treatment and by therapeutic follow-up and preventive measures[5].
The dentist must have strong medical knowledge and be continuously updated on common head and neck malignant neoplasms, their clinical manifestations, therapeutic alternatives, and the complications that may occur as a result of their treatment[6].
The availability of a multidisciplinary medical team consisting of an oncologist, a hematologist, a head and neck surgeon, a radiologist, a physiotherapist, a speech therapist, a social worker, and a psychologist together with a dentist to attend to the patient prior to the cancer treatment, as well as close communication between team members during and after treatment, is crucial[5,7].
Ideally, cancer centers should provide oral health care. However, because patients are frequently referred to the family dentist, dentists must have basic knowledge of the protocols for oral management of cancer patients and of the relevant National Cancer Institute guidelines[8].
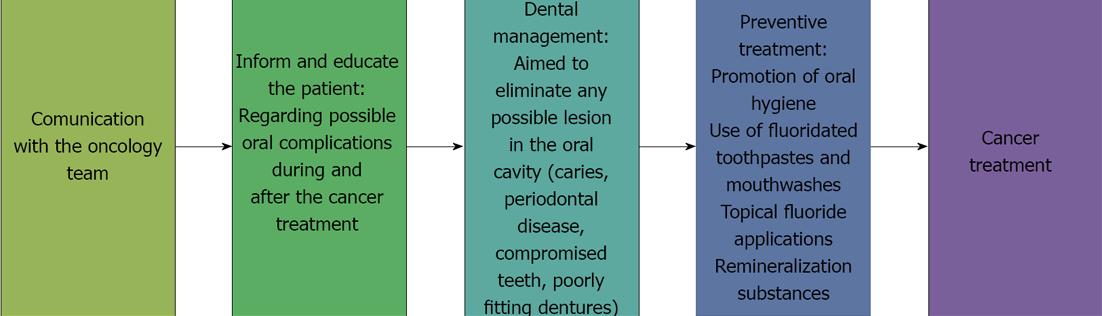
It is important for the dentist to join the oncology team so that he or she is informed of the type of surgery and radio- and/or chemotherapy the patient will receive[5,9].
At the patient’s first visit, the dentist must perform a complete oral health evaluation to establish an integral oral and maxillofacial management plan before the cancer treatment is initiated[5].
The possible short-, medium- and long-term oral complications of the cancer treatment must be explained to the patient and to his or her close relatives[10] in a simple and didactical way, preferably through the use of images, so that they will be able to identify problems such as xerostomia and mucositis. Patients must be instructed in the importance of dental follow-up care before, during, and after chemo- and radiotherapy as a means of preventing radiation-associated dental caries and osteonecrosis. The dentist must provide the patient and the patient’s relatives with an instructional manual on oral hygiene, diet, and measures to be followed before, during, and after cancer treatment[11-14].
The primary objective of providing information to the patient and of diagnosing and treating the patient prior to cancer treatment is to eliminate or stabilize any oral lesions that are present and to minimize the possibility of the occurrence of local or systemic infections during or after cancer treatment[14-16].
Patients often show tumor-related symptoms or dental conditions related to an incisional biopsy or pre-surgical therapy. These symptoms must be evaluated and correctly diagnosed so as to differentiate tumor symptoms from previous oral manifestations of dental caries, periodontal disease, pulpal diseases and soft tissue conditions[13,17].
A detailed exploration of the head and neck region must be performed following a preset order from external to internal, and abnormal growth, asymmetries, and cutaneous lesions must be identified and evaluated. Salivary glands, muscles, and the temporomandibular joint must be inspected. Palpation of submental, submandibular, and cervical lymph nodes is important and must be followed by an intraoral examination starting with the soft tissues, buccal mucosa, tongue, floor of the mouth, and the hard and soft palates. Any lesion, irritation, erosion, ulceration, or hemorrhage must be identified, and the patient’s general periodontal state must be evaluated as well[18].
It is essential to note the presence of caries, damaged restorations, pulpal lesions, necrotic teeth, or apical lesions suggestive of a cyst or granuloma.
The clinical diagnosis is complemented by X-ray imaging with a full set of periapical radiographs and by orthopantomography.
The essential steps of dental pre-treatment prior to anticancer therapy are focused on the elimination or stabilization of oral lesions and are aimed at minimizing the presence of potential sites of infection during or after treatment[19,20].
The important goals of such pre-treatment are as follows: (1) To eliminate any deep carious processes that may compromise pulp vitality during cancer treatment; (2) To control pulp and periapical infections two weeks before therapy to ensure tissue healing; (3) To restore or extract any tooth that shows a periapical lesion because such teeth can become infection sites in patients receiving chemo- and radiotherapy and hese treatments affect the immune response; (4) To extract teeth with poor periodontal or pulpal prognosis, such as teeth with deep caries or deep periodontal pockets and non-vital teeth with an expectancy of less than one year in the mouth. The extraction should be performed as soon as possible and at least three weeks before cancer therapy begins to ensure that the healing process is completed before the onset of therapy; (5) To assess the need for extraction of teeth associated with the tumor or radiation site; (6) To eliminate or restore sharp edges of fractured teeth to reduce mucosal friction or trauma that could aggravate mucositis; (7) To assess the need for extraction of retained teeth and impacted third molars that can cause pericoronitis; (8) The use of pit and fissure plaque sealants on recently erupted teeth is recommended; (9) To perform dental hygiene and scaling to completely eliminate dental and supra- and infra gingival tartar; (10) To inform the patient of the need to change a cariogenic diet[7] and to suspend the consumption of alcohol, tobacco, and any foods or substances that can damage oral structures; (11) To evaluate the need for adjustment or removal of partial or complete dentures or orthodontic appliances that can cause irritation or trauma. During the cancer treatment, dentures must be used by the patient only when eating; and (12) To encourage the patient to maintain proper oral hygiene and to emphasize a preventive treatment aiming on remineralization to minimize caries formation. The patient must be advised to: use fluoride toothpaste; brush his/her teeth four times a day, including after every meal; use topical fluoride gel daily for 5 min at bedtime; use a calcium phosphopeptide remineralization cream; use alcohol-free fluoride mouthwash.
The diagnostic, preventive, and therapeutic steps that should be followed prior to cancer treatment are shown in Figure 1.
Conventional radiotherapy is very useful in the treatment of oral carcinoma; however, it acts on both tumor cells and healthy cells, producing tissue damage. Approximately 50% of malignant head and neck neoplasms are treated with radiotherapy alone or with chemotherapy and surgery. Radiotherapy involves the use of ionizing radiation, which produces morphological and functional changes in tissues and has chemical effects, included the hydrolysis of intracellular constituents and the rupture of DNA strands[20].
The response of tissue to radiation depends on a variety of factors, including the received dose, the fractionation dose, the nature of the radiation, the previous condition of the irradiated tissue, the degree of cell differentiation, cellular kinetics, cell temperature, and the tumor’s sensitivity to radiation, location and oxygenation[21].
Complications are classified depending on their time of appearance (immediate, medium and late side effects), their intensity, and as reversible or irreversible. Immediate complications appear within 1 wk of treatment and may include erythema, mucositis, dysgeusia, glossodynia, infections (candidiasis, herpes), xerostomia, periodontal disease, severe necrosis, and alopecia. Medium-term complications appear after the third month of treatment and may include trismus, caries, dysphagia, and dental hypersensitivity. Late side effects appear months after the treatment and may include osteoradionecrosis, alterations in tooth development (agenesis, coronal hypocalcification such as enamel hypoplasia, and root alterations such as root shortening, early canal closure, and dilaceration), pulpal necrosis, and pain. Table 1 shows the most frequent complications of radiotherapy classified by time of appearance and prognosis[22].
| Complication | Characteristics | Time of appearance | Prognosis |
| Erythema/ radiodermatitis | Redness/decreased skin thickness; skin dryness due to epidermal basal cell damage | Immediate | Reversible |
| Mucositis | Generalized inflammation of the oral mucosa due to basal cell damage; scaling, mucosal ulcerations | Immediate | Reversible |
| Dysgeusia | Altered taste (especially to sour and acid tastes) due to taste bud damage | Immediate | Reversible |
| Glossodynia | Pain and burning sensation in the tongue due to taste bud damage and inflammation | Immediate | Reversible |
| Candidiasis and Herpes simplex | Secondary infections resulting from loss of mucosal protection caused by mucositis and xerostomia | Immediate | Reversible |
| Xerostomia | Decrease in salivary flow and dryness of the mouth caused by alterations in salivary glands | Immediate | Irreversible at high radiation doses (more than 60 Gy) |
| Periodontal disease | Inflammation of the periodontium due to augmented plaque from decreased salivary flow | Immediate | Reversible |
| Alopecia | Hair loss from hair follicle atrophy | Immediate | Reversible |
| Severe necrosis | Loss of tissue, scurvy and malodorous ulcerations | Immediate | Irreversible |
| Trismus | Reduced mouth opening caused by fibrosis of the muscles of mastication or of the temporomandibular joint | Medium-term | Reversible/irreversible |
| Caries | Damage to the cement-enamel junction, incisal edges and cusps caused by decreased salivation | Medium-term | Irreversible |
| Dysphagia | Difficulty swallowing food caused by oropharyngeal alterations; may be evidenced by malnutrition | Medium-term | Reversible |
| Dental hypersensitivity | Dental sensitivity caused by radiation | Medium-term | Reversible |
| Osteoradionecrosis | Aseptic necrosis of the irradiated bone | Late | Irreversible |
| Tooth germ alterations | Alteration in odontogenesis in pediatric patients | Late | Irreversible |
| Pulp necrosis and pain | Pulp necrosis and pain | Late | Irreversible |
The occurrence of oral complications of radiotherapy (Figure 2) can be minimized by taking the following preventive actions: Educating the patient about the importance of oral hygiene and emphasizing the cessation of toxic habits such as alcohol and tobacco consumption. Performing professional dental cleaning including tartar removal, root scaling and planing. Eliminating areas of trauma resulting from ill-fitting dentures and sharp edges. Suspending the use of mucosa-supported dentures for 15 d after radiotherapy begins; if possible, suspending their use indefinitely or using them moderately to avoid trauma. Protecting salivary glands and the mucosa of areas that do not require irradiation. Performing quantitative sialometry to evaluate the production of saliva after radiation doses. Extracting compromised or severely damaged teeth (severe periodontal disease, mobility, fractures, caries). Performing conservative dental treatments that include restorations and root canal therapy. Applying topical fluoride before, during, and after radiation treatment. Recommending the use of 0.12% chlorhexidine mouthwashes. Applying pit and fissure sealants to recently erupted premolars and molars in pediatric patients. Modifying the cariogenic diet. The first side effects of a cariogenic diet may become obvious after radiotherapy.
Chemotherapy in cancer treatment consists of the use of cytotoxic drugs that are intended to destroy or avoid the proliferation of tumor cells. This therapy is not selective; it affects both tumor cells and normal cells, especially cells that undergo rapid cell cycling for continuous replacement. Such cells include bone marrow cells, cells of hair follicles, and gastrointestinal epithelial cells, including oral mucosal cells[23].
Cisplatin, cyclophosphamide, methotrexate, bleomycin, 5-fluorouracil, and vinblastine are used most frequently in the treatment of head and neck neoplasms[24]. The use of these drugs may affect the basal epithelial cells that make up the oral mucosal epithelium. When these cells are damaged, the replacement of the epithelium is compromised and scaling and ulcerations of the mucosa occur. Furthermore, xerostomia caused by salivary gland damage may occur, with resulting alterations in the levels of saliva protectors and ageusia. A high percentage of patients present oral infections, bleeding, or a combination of both, and more than 50% of patients also present complications from surgery and head and neck radiotherapy. Most patients who receive high doses of chemotherapy for head and neck cancer develop severe mucositis[24-26].
Bone marrow suppression is one of the most common side effects of chemotherapy. It is often evident in peripheral blood after 10-14 d of treatment; typical signs are leukopenia, neutropenia, thrombocytopenia and anemia. Hair loss, nausea, vomiting, and palmoplantar erythrodysesthesia syndrome are common. The latter is a palmoplantar erythema with erosions, burning sensation, and local pain[27].
Bisphosphonates are a group of drugs used for the prevention and treatment of bone resorption diseases such as maxillofacial cancer, bone metastasis, malignant hypercalcemia, osteoporosis, Paget’s disease, and multiple myeloma. Their structure is based on that of pyrophosphate, a metabolite that regulates the precipitation and extraction of bone minerals; similar to pyrophosphate, they are sensitive to hydrolysis by phosphatases[28-30].
When bisphosphonates are incorporated into bone, osteoclast-mediated bone resorption is prevented, and osteoclast apoptosis is stimulated. These drugs have affinity for active bone replacement sites and growth plates[30,31].
Bone resorption does not occur in patients undergoing bisphosphonate treatment due to inhibition by bisphosphonates of the osteoclastic activity that normally causes decreased bone replacement and a lack of new bone formation. In these patients, the bone is present for a longer period without replacement, making it prone to chronic infections and necrosis. Bisphosphonates also inhibit angiogenesis, leading to diminished bone vascularization and induction of bone cell apoptosis[27,32-34].
Osteonecrosis of the jaw is one of the complications of treatment with bisphosphonates in cancer patients. It most often occurs in the mandible and is associated with 53% of dental interventions; 48% of affected patients show a response to treatment when the drug has been used for a period of over eight weeks[35-37].
Treatment with bisphosphonates must be considered in the medical history of patients with ulcerated lesions within the jaw with bone exposure for over eight weeks, necrotic bone, or lesions that do not heal spontaneously.
Additional tests such as orthopantomography and computerized tomography scanning are recommended to evaluate the extent of the lesion.
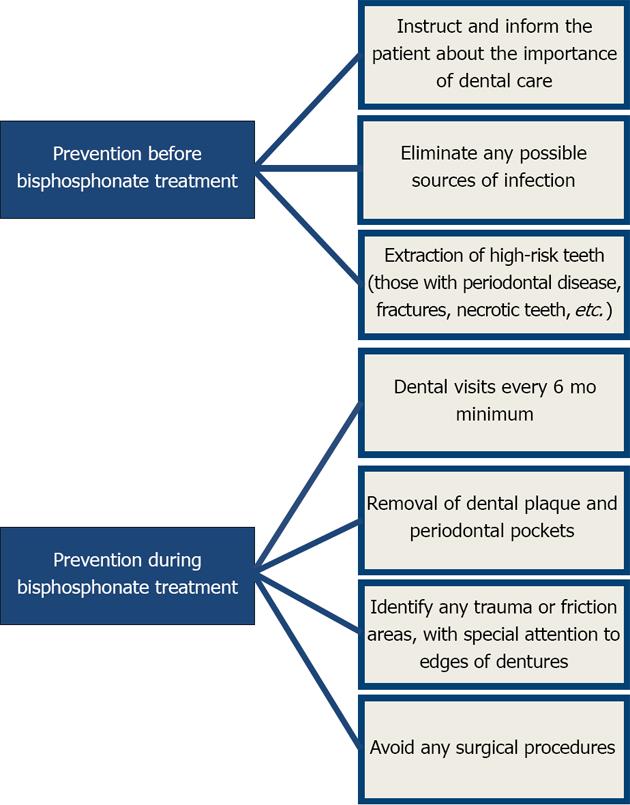
Once oral or intravenous treatment with bisphosphonates has been decided upon, preventive measures must be taken before and after treatment to avoid or minimize osteonecrosis[38,39].
Basic preventive measures prior to treatment focus on eliminating potential sites of infection and extracting teeth with poor prognoses to minimize the risk during therapy[40]. Once treatment has begun, meticulous control of the patient must be maintained so as to detect any sign of osteonecrosis. Good oral hygiene with plaque control and dental cleaning, tartar removal, and periodontal pocket treatment must also be included. Surgical procedures must be minimally invasive, performed only when necessary, and include prophylactic antibiotic treatment and the use of chlorhexidine mouthwashes.
The Figure 3 outlines the preventive measures that must be followed before and after bisphosphonate treatment.
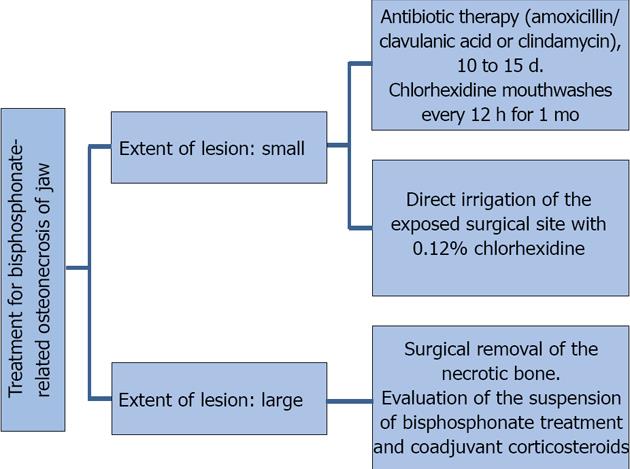
The treatment of osteonecrosis consists of eliminating pain, controlling bone and soft tissue infections, reducing the progression of bone necrosis, decreasing or eliminating possible risk factors, improving oral hygiene, and following a specific antibiotic therapy protocol that is based on a previous culture growth and antibiogram of the exposed bone. If possible, bisphosphonate treatment should be suspended for 6 to 12 mo, which will allow improvement and possible resolution of the condition. Suspension of corticosteroid treatment is recommended when such agents are used as coadjuvants in the maintenance treatment. When conservative management fails, surgical debridement of necrotic bone tissue with a primary tension-free closure is an option, all with coadjuvant antibiotic prophylactic therapy[10,41-43].
Treatment depends on the size and extent of the lesion. Figure 4 outlines the treatment protocol depending on the extent of the lesion.
Tissues injured by chemo- and radiotherapy require preventive measures to minimize damage. Furthermore, severe lesions with no possible repair require additional therapy. In this regard, the stem cell transplant described above is undergoing further development with the goal of achieving the regeneration of tissue damaged by radio- or chemotherapy.
Tissue and organ regeneration requires cells that can regenerate and that are similar to the cells that have been damaged, often irreversibly, by chemo- and radiotherapy. The possibility of restoring these cells allows consideration of tissue regeneration as a therapeutic option.
As the backbone of regenerative medicine, stem cells have acquired a decisive importance; scientific research in the field of stem cell biology has proven to be essential for allowing a transfer from basic to therapeutic research and generating new perspectives for clinical treatment.
Stem cells are defined as “cells that have both the capacity to self-renew (make more stem cells by cell division) and to differentiate into mature, specialized cells”[43]. Therefore, these cells provide a source of cells that can both generate other stem cells and form specific tissues and organs. Their absence limits or prevents regeneration.
Stem cell migration to the damaged site, as well as stem cell transplantation, are being considered as options in therapeutic regeneration, and currently, there are similar initiatives in radiotherapy[44,45]. Although at an experimental stage, the use of stem cells in such regeneration is potentially a viable therapeutic alternative. As indicated above, this approach could potentially be used to repair lesions caused by chemotherapy.
One of the therapeutic advantages of stem cell transplantation is the possibility of autologous transplantation; this would prevent the occurrence of graft versus host disease.
While research is progressing in this field, it must presently be considered an experimental field that, as such, does not permit the formation of any definitive conclusions.
P- Reviewers: Toros SZ, Yokoyama S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Petersen PE. Oral healthcare in people living with cancer. Oral Oncol. 2010;46:399-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Meurman JH. Infectious and dietary risk factors of oral cancer. Oral Oncol. 2010;46:411-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Meurman JH, Grönroos L. Oral and dental health care of oral cancer patients: hyposalivation, caries and infections. Oral Oncol. 2010;46:464-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Scully C, Petti S. Overview of cancer for the healthcare team: aetiopathogenesis and early diagnosis. Oral Oncol. 2010;46:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Joshi VK. Dental treatment planning and management for the mouth cancer patient. Oral Oncol. 2010;46:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Porter SR, Fedele S, Habbab KM. Taste dysfunction in head and neck malignancy. Oral Oncol. 2010;46:457-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Lanza Echeveste DG. Tratamiento odontológico integral del paciente oncológico. Parte I. Comprehensive dental treatment of cancer patients. Part I. Odontoestomatología. 2011;13:14-25. |
| 8. | Lip and Oral Cavity Cancer Treatment (PDQ®). General Information About Lip and Oral Cavity Cancer. Available from: http://www.cancer.gov/cancertopics/pdq/treatment/lip-and-oral-cavity/HealthProfessional. |
| 9. | Madrid C, Bouferrache K, Abarca M, Jaques B, Broome M. Bisphosphonate-related osteonecrosis of the jaws: how to manage cancer patients. Oral Oncol. 2010;46:468-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Bagán J, Blade J, Cozar JM, Constela M, García Sanz R, Gómez Veiga F, Lahuerta JJ, Lluch A, Massuti B, Morote J. Recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw (ONJ) in cancer patients treated with bisphosphonates. Med Oral Patol Oral Cir Bucal. 2007;12:E336-E340. [PubMed] |
| 11. | Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Newton JT. Reactions to cancer: communicating with patients, family and carers. Oral Oncol. 2010;46:442-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Warnakulasuriya S. Living with oral cancer: epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol. 2010;46:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 14. | van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; present concepts of management. Oral Oncol. 2010;46:423-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Rocha Buelvas A. Cancer oral el papel del odontólogo en la detección temprana y control. The role of the dentist in early detection and treatment of oral cancer. Rev Fac Odont Univ Antioq. 2009;21:112-121. |
| 16. | Whitmyer CC, Waskowski JC, Iffland HA. Radiotherapy and oral sequelae: preventive and management protocols. J Dent Hyg. 1997;71:23-29. [PubMed] |
| 17. | Porter SR, Fedele S, Habbab KM. Xerostomia in head and neck malignancy. Oral Oncol. 2010;46:460-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Zheng WK, Inokuchi A, Yamamoto T, Komiyama S. Taste dysfunction in irradiated patients with head and neck cancer. Fukuoka Igaku Zasshi. 2002;93:64-76. [PubMed] |
| 19. | Dios PD, Lestón JS. Oral cancer pain. Oral Oncol. 2010;46:448-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Mikhaĭlova NS, Alekseev NA. Case of nonspherocytic anemia complicated by glucose-6-phosphate dehydrogenase deficiency of the erythrocytes. Probl Gematol Pereliv Krovi. 1976;21:54. [PubMed] |
| 21. | Cano Pérez S, Gutiérrez Villar MD. Complicaciones de la radioterapia en la cavidad oral. Oral complications of radiotherapy. Semergen. 2002;28:363-369. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Rocha Buelvas A, Jojoa Pumalpa A. Manejo odontológico de las complicaciones orales secundarias AL tratamiento oncológico con quimioterapia y radioterapia Dental management of oral complications of cancer treatment with chemotherapy and radiotherapy. CES Odont. 2011;24:71-78. |
| 23. | López-Galindo MP, Bagán JV, Jiménez-Soriano Y, Alpiste F, Camps C. Clinical evaluation of dental and periodontal status in a group of oncological patients before chemotherapy. Med Oral Patol Oral Cir Bucal. 2006;11:E17-E21. [PubMed] |
| 24. | Chaveli Lopez B, Gabaldá Esteve C, Sarrión Pérez M. Dental treatment considerations in the chemoterapy patient. J Clin Exp Dent. 2011;3:e31-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998;34:39-43. [RCA] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 347] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 26. | Logan RM. Advances in understanding of toxicities of treatment for head and neck cancer. Oral Oncol. 2009;45:844-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, Daniele B, De Pouvourville G, Rubenstein EB, Daugaard G. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 315] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 28. | Ata-Ali F, Ata-Ali J, Flichy-Fernández AJ, Bágan JV. Osteonecrosis of the jaws in patients treated with bisphosphonates. J Clin Exp Dent. 2012;4:e60-65. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Carranza Lira S. Mandible osteonechrosis associated to bisfosfonates. Ginecol Obstet Mex. 2007;75:655-660. [PubMed] |
| 30. | Madrid C, Abarca M, Bouferrache K. Osteoradionecrosis: an update. Oral Oncol. 2010;46:471-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Assael LA. Oral bisphosphonates as a cause of bisphosphonate-related osteonecrosis of the jaws: clinical findings, assessment of risks, and preventive strategies. J Oral Maxillofac Surg. 2009;67:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Ruggiero SL, Fantasia J, Carlson E. Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 313] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 33. | Murad OM, Arora S, Farag AF, Guber HA. Bisphosphonates and osteonecrosis of the jaw: a retrospective study. Endocr Pract. 2007;13:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Landesberg R, Wilson T, Grbic JT. Bisphosphonate-associated osteonecrosis of the jaw: conclusions based on an analysis of case series. Dent Today. 2006;25:52, 54-57. [PubMed] |
| 35. | Kerawala CJ. Complications of head and neck cancer surgery - prevention and management. Oral Oncol. 2010;46:433-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Fantasia JE. Bisphosphonates. What the dentist needs to know: Practical considerations. J Oral Maxilofac Surg. 2009;67:53-60. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Silverman SL, Landesberg R. Osteonecrosis of the jaw and the role of bisphosphonates: a critical review. Am J Med. 2009;122:S33-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw: an overview. Ann N Y Acad Sci. 2011;1218:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Pérez SB, Barrero MV, Hernández MS, Knezevic M, Navarro JM, Millares JR. Bisphosphonate-associated osteonecrosis of the jaw. A proposal for conservative treatment. Med Oral Patol Oral Cir Bucal. 2008;13:E770-E773. [PubMed] |
| 40. | Ferlito S, Puzzo S, Liardo C. Preventive protocol for tooth extractions in patients treated with zoledronate: a case series. J Oral Maxillofac Surg. 2011;69:e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Manfredi M, Merigo E, Guidotti R, Meleti M, Vescovi P. Bisphosphonate-related osteonecrosis of the jaws: a case series of 25 patients affected by osteoporosis. Int J Oral Maxillofac Surg. 2011;40:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Bagan J, Scully C, Sabater V, Jimenez Y. Osteonecrosis of the jaws in patients treated with intravenous bisphosphonates (BRONJ): A concise update. Oral Oncol. 2009;45:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Available from: http://www.isscr.org/home/resources/learn-about-stem-cells/stem-cell-glossary#stem. Accessed 01/06/2013.. |
| 44. | Razvi E. Trends in the stem cells marketplace--report from Select Biosciences Stem Cells 2012 Conference. Regen Med. 2012;7:291-294. [PubMed] |
| 45. | Coppes RP, van der Goot A, Lombaert IM. Stem cell therapy to reduce radiation-induced normal tissue damage. Semin Radiat Oncol. 2009;19:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |