Published online Feb 20, 2013. doi: 10.5321/wjs.v2.i1.12
Revised: January 4, 2013
Accepted: January 17, 2013
Published online: February 20, 2013
Processing time: 192 Days and 12.9 Hours
AIM: To investigate low intensity laser irradiation phototherapy (LILIP) on the proliferation, mineralization and degradation of dental pulp constructs.
METHODS: Stem cells from human exfoliated deciduous teeth (SHED) were grown to confluence and seeded on collagen scaffolds to create dental pulp constructs. LILIP was delivered to the dental pulp constructs using an 830 nm GaAIAs laser at an output power of 20 mW. The LILIP energy density was 0.4, 0.8, 1.2, and 2.4 J/cm2. After 8 d, the cell proliferation and degradation within the dental pulp constructs were measured using histologic criteria. After 28 d, the effect of LILIP on SHED mineralization was assessed by von Kossa staining.
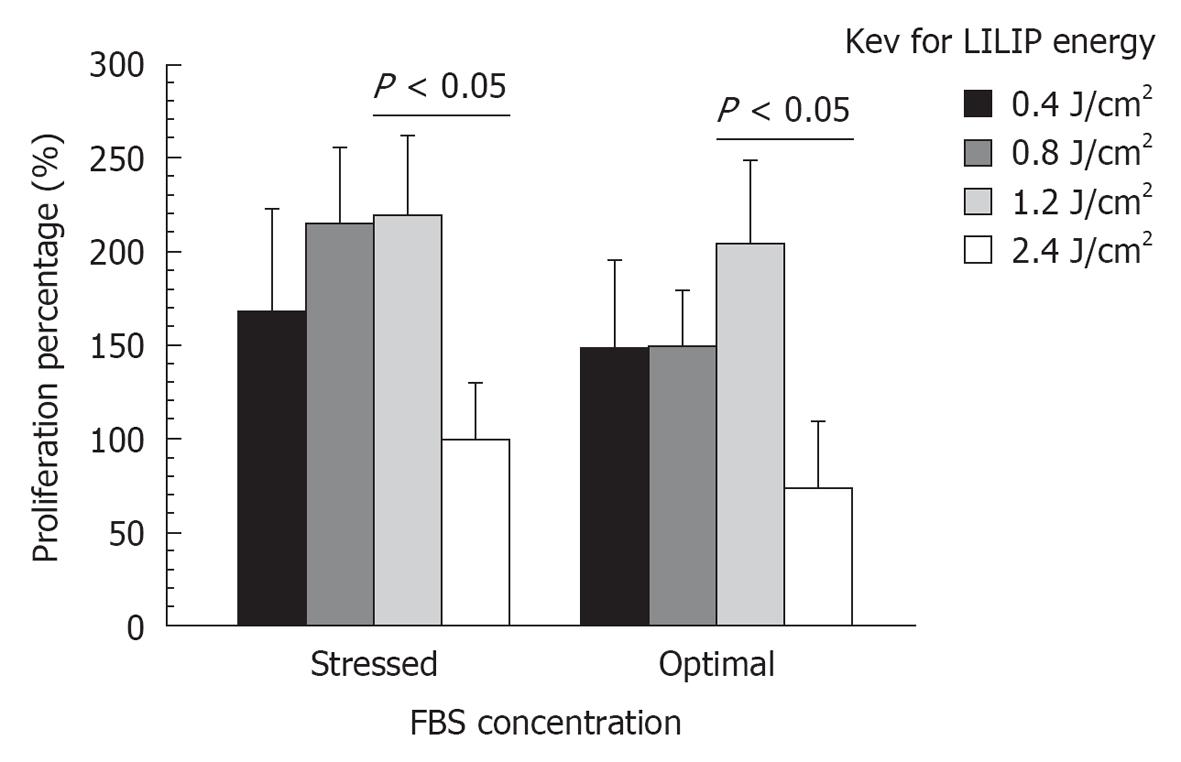
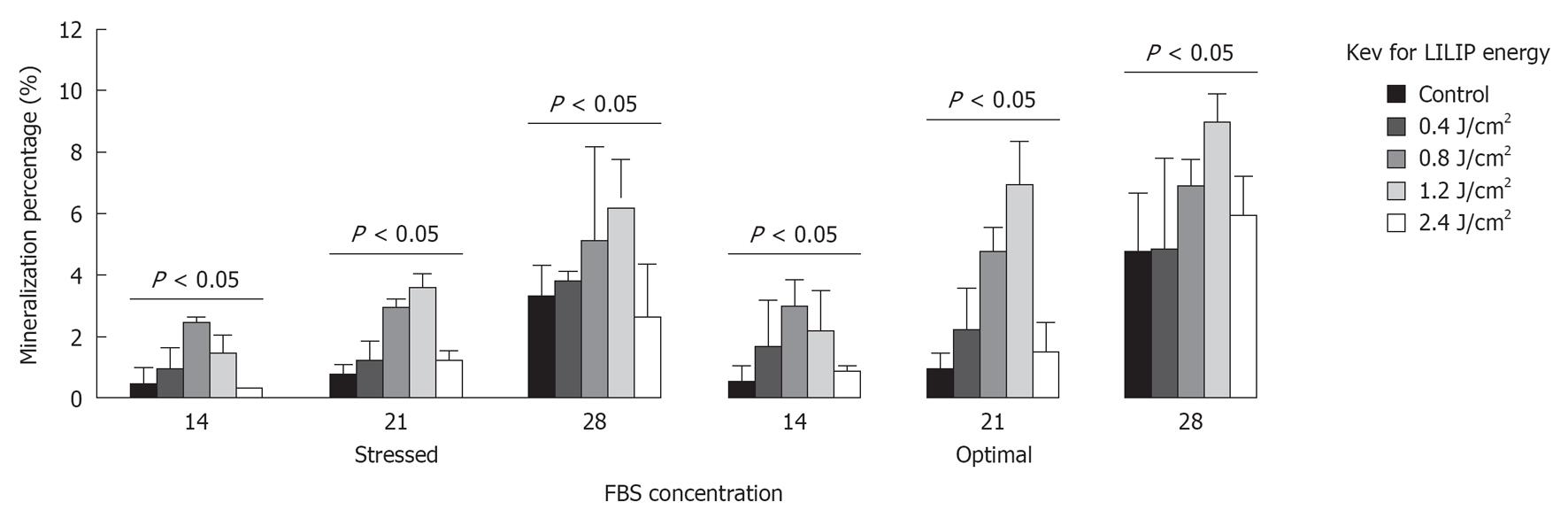
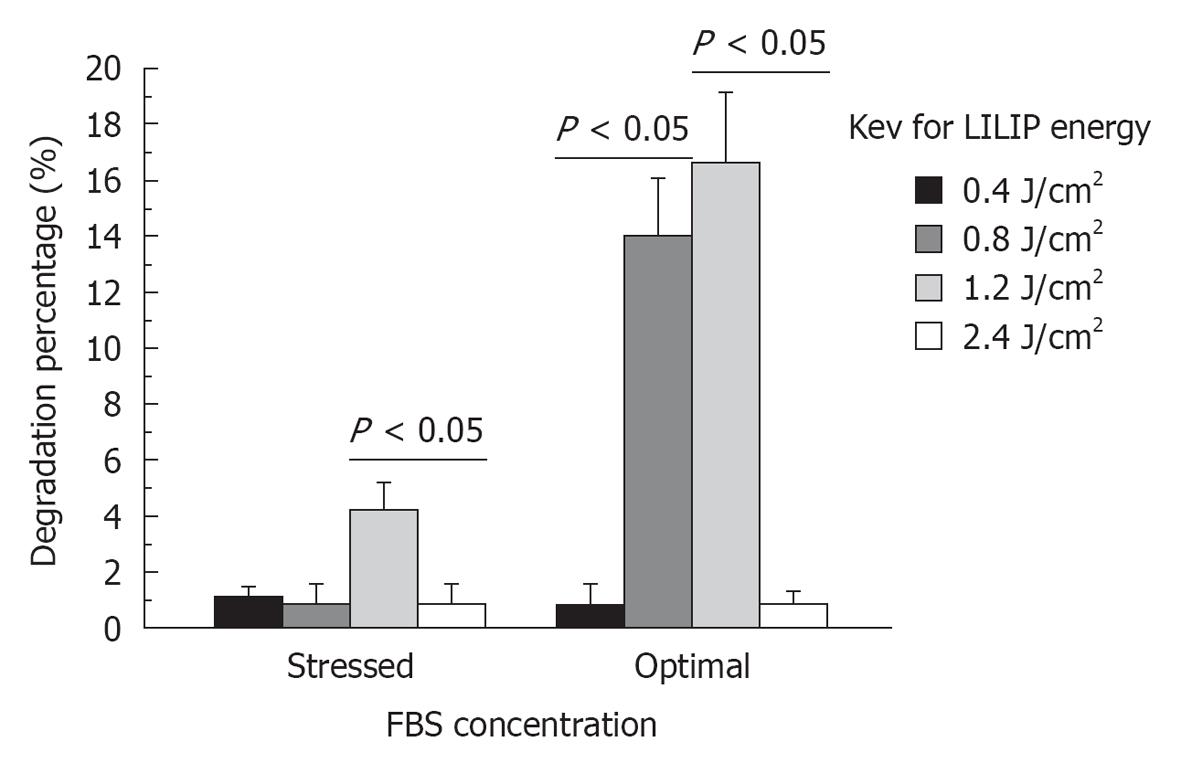
RESULTS: SHED proliferation within the dental pulp constructs varied after exposure to the 0.4, 0.8, 1.2, and 2.4 J/cm2 LILIP energy densities (P < 0.05). The maximum proliferation of SHED in nutrient deficient media was 218% after exposure to a 1.2 J/cm2 LILIP energy density. SHED grown in nutrient deficient media after exposure to a 0.4, 0.8, and 1.2 J/cm2 LILIP energy density, proliferated by 167-218% compared to the untreated (non-LILIP) control group (P < 0.05). SHED exposed to a 0.4, 0.8, and 1.2 J/cm2 LILIP energy density, and grown in optimal nutritional conditions and proliferated by 147%-164% compared to the untreated (non-LILIP) control group (P < 0.05). The exposure of SHED to the highest LILIP energy density (2.4 J/cm2) caused a reduction of the cell proliferation of up to 73% of the untreated (non-LILIP) control (P < 0.05). The amount of mineral produced by SHED increased over time up to 28 d (P < 0.05). The 0.8 and 1.2 J/cm2 LILIP energy densities were the most effective at stimulating the increased the mineralization of the SHED from 150%-700% compared to untreated (non-LILIP) control over 28 d (P < 0.05). The degradation of dental pulp constructs was affected by LILIP (P < 0.05). The dental pulp constructs grown in optimal nutritional conditions exposed to a 0.8 J/cm2 or 1.2 J/cm2 LILIP energy density had 13% to 16% more degradation than the untreated (non-LILIP) control groups (P < 0.05). The other LILIP energy densities caused a 1% degradation of dental pulp constructs in optimal nutritional conditions (P > 0.05).
CONCLUSION: LILIP can enhance or reduce SHED proliferation, degradation and mineralization within dental pulp constructs. LILIP could promote the healing and regeneration of dental tissues.
- Citation: Elnaghy AM, Murray PE, Bradley P, Marchesan M, Namerow KN, Badr AE, El-Hawary YM, Badria FA. Effects of low intensity laser irradiation phototherapy on dental pulp constructs. World J Stomatol 2013; 2(1): 12-17
- URL: https://www.wjgnet.com/2218-6263/full/v2/i1/12.htm
- DOI: https://dx.doi.org/10.5321/wjs.v2.i1.12
Regenerative endodontics procedures are biologically based procedures that are used to replace the damaged dentin and root structures of teeth as well as cells of the pulp-dentin complex[1]. Regenerative endodontic procedures are: root canal revascularization, apexogenesis, apexification, partial pulpotomy, direct pulp capping, stem cell therapy and dental pulp constructs[1]. Endodontic regenerative procedures are widely expected to become more common in coming decades[2]. The increased usage of regenerative therapies is likely because of the discovery of dental stem cells, the use of improved treatment protocols, and the availability of new technologies[1]. The success of regenerative endodontic procedures is dependent on stimulating the proliferation and mineralization activity of stem cells from human exfoliated deciduous teeth (SHED) and other dental stem cells[3]. Previous research has demonstrated pulp healing and regeneration by adding growth factors to increase dental pulp stem cells (DPSCs) activity[4]. No previous research has investigated the possibility of using lasers to increase SHED proliferation, mineralization or degradation of scaffolds.
Lasers are beneficial for some dental treatments, such as oral surgery[5], endodontics[6], periodontology[7], and restorative dentistry[8]. Low intensity laser irradiation phototherapy (LILIP) can change cell activity[9]. LILIP has been used in the treatment of dentin hypersensitivity, gingivitis, periodontitis, and to heal oral ulcers[10,11]. In response to LILIP, fibroblast cells can increase their rate of proliferation by 300% to 600%[12]. In response to LILIP, epithelial cells cultured in a nutritionally deficient state can dramatically increase their rate of proliferation[13]. LILIP can be effective in stimulating the proliferation and mineralization activity of osteoblasts and fibroblasts[12-14]. LILIP can increase the proliferation of DPSCs, as indicated by measuring their cell mitochondrial activity using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay[15]. Regenerative endodontic procedures require SHED proliferation, mineralization and degradation of scaffolds if they are delivered into teeth as a dental construct[16,17] to attempt to regenerate teeth. However, the SHED responses to LILIP have not been evaluated. Consequently, there is a need to investigate the effects of LILIP on SHED proliferation, mineralization, and scaffold degradation, to identify its optimal and injurious effects, prior to its potential use as part of future regenerative endodontic procedures. The aim of this research was to investigate the effects of LILIP on the proliferation and mineralization SHED, and the degradation of dental pulp constructs.
The SHED was donated under a material transfer agreement with the National Institutes of Dental and Craniofacial Research (Bethesda, MD). Rat fibroblast L929 cells (ATTC, Manassas, VA) were used as a control treatment group cell line. The SHED were cultured in Dulbecco’s modified Eagles medium (DMEM; BD Biosciences, Franklin Lakes, NJ) supplemented with 10% or 2.5% fetal bovine serum (FBS) (HyClone, Logan, UT) and 1% gentamycin and amphotericin antibiotic supplement. Cell cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2 with the culture media being replenished every second day. Confluent cultures of SHED were collected by trypsinization (0.25% trypsin/EDTA; Mediatech, Inc., Herndon, VA).
Three-dimensional collagen scaffolds (Collacote; Zimmer Dental, Carlsbad, CA) were cut into 2 mm × 2 mm sheets. Each scaffold was soaked in Hanks’ balanced salt solution (HBSS; Cellgro, Herndon, VA) and stored at 4 °C. Before use, the HBSS was replaced by culture medium. The scaffolds were incubated in DMEM at 37 °C for 30 min before application of the cells to equalize culture conditions and temperature between the scaffolds and cells.
The SHED were added to the scaffolds in fresh aliquots; each scaffold was seeded with a million (× 106) cells to create dental pulp constructs[16,17]. The L929 (× 106) cells were also applied to the scaffolds as a control treatment. The scaffolds were maintained in 6-well culture plates (BD Biosciences, Franklin Lakes, NY) containing 5 mL of culture media. The DMEM culture media was removed and replenished every 2 d. After 8 d of cell culture, the dental pulp constructs were transferred to 96-well plates.
Laser irradiation was delivered with Gallium-Aluminum-Arsenide (GaAlAs) laser (Asah Medico Uni-Laser, Hvidovre, Denmark). The irradiations were performed in contact with the plate base using the punctual irradiation mode in a 0.252 cm2 area[15]. The 830 nm laser was applied with output power setting of 20 mW. The laser device was calibrated using laser power meter (Model OPM-572; Sanwa, Tokyo, Japan). The clear base of a well from 96-well plate was separated and the laser power output was measured after the laser passed through the base to determine the exact energy density on the cells. This measurement was repeated three times and the average was calculated. The energy density was applied using 6, 12, 18, and 36 s of irradiation time. The energy density was calculated using the following formula: energy density (J/cm2) = [power (W) × time (s)]/area (cm2).
During the cell culture of the dental pulp constructs, 0.0016% neutral red dye (JT Baker, Phillipsburg, NJ) cell viability marker was added to the DMEM in order to stain the vital cells dark red[1]. The SHED and L929s were cultured for 8 d on the collagen scaffolds, with 10 culture replicates for each of the constructs treatments. For each cell type, the experimental groups were: lased 6 s with 0.4 J/cm2, lased 12 s with 0.8 J/cm2, lased 18 s with 1.2 J/cm2, and lased 36 s with 2.4 J/cm2. The control for this investigation was including constructs without irradiation.
The dental pulp constructs were removed from cell culture and fixed by submerging them in a 10% neutral-buffered formalin (BDH Chemicals, Poole, United Kingdom) solution for 24 h. All the tissue constructs were then dehydrated in a graded series of alcohols from 70% to 100%. The constructs were then embedded in paraffin wax blocks and cut into serial histologic sections of 5-μm thickness using a microtome. The histology sections were then mounted onto glass slides and covered with a cover slip using adhesive.
The numbers of stained neutral red SHED were counted as the number of vital metabolically active cells within each of the dental pulp constructs[16]. The cells were counted using pathohistometric analysis, the numbers of cells per microscope field with 5 random microscope fields being counted per specimen using a light microscope (Vista vision, VWR Scientific, West Chester, PA) at × 200 magnifications with a reticule[18]. Construct degradation was measured as an area of scaffold that no longer existed using a light microscope at × 200 magnifications with a reticule.
SHED and L929s were cultured and treated with LILIP using the same energy density and 6, 12, 18, and 36 s of irradiation time which was described previously. The SHED and L929s cells were incubated with DMEM mineralization induction media, supplemented with 10 mmol/L sodium β-glycerophosphate (Sigma, St. Louis, MO, United States), for 28 d. The mineralization was assessed by a von Kossa staining (Diagnostic BioSystems, Pleasanton, CA)[19]. The mineralized cultures were fixed with 10% buffered formalin for 30 min. Subsequently, they were washed and stained with von Kossa silver and exposed to ultraviolet light for 30 min. Then cells were treated with 5% sodium thiosulfate for 2 min and washed again. The mineralization capacity of each cell line was determined and compared by measuring the density of mineral nodules formed in each cell type using the tagged image format (tif) image for manipulation in Adobe Photoshop (Adobe Systems, San Jose, CA). Mineralization was measured in three random areas of each specimen.
The data were analyzed using an analysis of variance statistical test, followed by Scheffe’s multiple comparison tests between treatment groups (Statview, SAS Institute Inc., Cary, NC). A P value of P < 0.05 was considered statistically significant.
The output power setting of 20 mW of the GaAlAs laser was measured by the laser energy meter as 16.83 mW reaching the SHED through the plastic base of the 6 well plates. After applying the LILIP energy density for 6, 12, 18, and 36 s, the energy density was calculated using a formula to be 0.4, 0.8, 1.2, and 2.4 J/cm2.
Prior to experimentation a pilot study of the effects FBS concentrations (10%-1%) within the DMEM culture media found that a 2.5% FBS concentration, was the minimal concentration of FBS necessary to avoid SHED death and reduced cell proliferation. The 2.5% FBS concentration met the criteria[12,13,15] to be the conditions of SHED nutritional deficit, and the 10% FBS concentration was the optimal nutritional condition.
A pilot study revealed that the maximum SHED responses were seen 8 d or more following exposure to LILIP, consequently the SHED responses in this present study were measured 8 d after exposure to LILIP. SHED proliferation within the dental pulp constructs varied after exposure to the 0.4, 0.8, 1.2, and 2.4 J/cm2 LILIP energy densities (P < 0.05). The maximum proliferation of SHED in nutrient deficient FBS media was 218% after exposure to a 1.2 J/cm2 LILIP energy density. SHED grown in nutritional deficient media after exposure to a 0.4, 0.8, and 1.2 J/cm2 LILIP energy density, proliferated by 167%-218% compared to the untreated (non-LILIP) control group (P < 0.05). SHED exposed to a 0.4, 0.8, and 1.2 J/cm2 LILIP energy density, and grown in optimal nutritional conditions and proliferated by 147%-164% compared to the untreated (non-LILIP) control group (P < 0.05). The exposure of SHED to the highest LILIP energy density (2.4 J/cm2) caused a reduction of the cell proliferation of up to 73% of the untreated (non-LILIP) control (P < 0.05). The nutrient deficient (2.5%) FBS culture media and optimal (10%) FBS culture media had little effect on the loss of SHED proliferation following exposure to the highest (2.4 J/cm2) LILIP energy density (P > 0.05). The loss of proliferation (62%) of L929 was less than the loss of proliferation (73%) of SHED after exposure to the highest (2.4 J/cm2) LILIP energy density (Figure 1).
The amount of mineral produced by SHED varied 28 d after exposure to 0.4, 0.8, 1.2, and 2.4 J/cm2 LILIP energy densities (P < 0.05). SHED produced (106%-255%) more mineral than L929 cells (P < 0.05). The amount of mineral produced by SHED increased over time up to 28 d (P < 0.05). The 0.8 and 1.2 J/cm2 LILIP energy densities were the most effective at stimulating the SHED to produce minerals over 28 d (P < 0.05) (Figure 2). The 0.8 and 1.2 J/cm2 LILIP energy densities increased the mineralization of the SHED from 150%-700% compared to untreated (non-LILIP) control SHED.
The degradation of dental pulp constructs was affected by LILIP (P < 0.05). The dental pulp constructs grown in optimal nutritional conditions exposed to a 0.8 J/cm2 or 1.2 J/cm2 LILIP energy density had 13% to 16% more degradation than the untreated (non-LILIP) control groups (P < 0.05). The other LILIP energy densities were less effective at causing the degradation of dental pulp constructs (Figure 3).
Regenerative endodontic procedures are successful because SHED and other dental stem cells can regenerate dentin and dental-pulp tissues[20]. The stimulation of SHED to increase proliferation, mineralization and scaffold degradation can be important to ensure that endodontic healing is quick and effective. This is the first investigation of using LILIP to control SHED proliferation, mineralization and construct degradation. This is also the first investigation to determine the optimal and injurious ranges of LILIP energy densities to enhance and inhibit the activity of a dental stem cell line.
The GaAlAs laser was used with an output power setting of 20 mW, but the laser energy meter measured only 16.83 mW reaching the SHED through the plastic base of the 96 well plates. The loss of 15.9% of the laser energy was factored into the energy densities (0.4, 0.8, 1.2, and 2.4 J/cm2) of this present study. A limiting factor in some previous laser studies[15,21-23] is that the energy densities reaching the cells were not measured by a meter, and so it is not clear what actual energy density was used. In this study, the LILIP parameters (wavelength, power output, irradiated area), were kept constant, except the irradiation times (6, 12, 18, and 36 s, and their corresponding energy densities (0.4, 0.8, 1.2, and 2.4 J/cm2). The GaAlAs laser wavelength was 830 nm, which is ideal for LILIP[22,23], but low energy compared to other laser types.
The effect of LILIP has been studied on several cell types[12-14]. A previous study found that LILIP can increase the proliferation of DPSCs, by measuring their cell mitochondrial activity using the MTT assay[15]. However, it is not clear what the precise change in the rate of DPSCs proliferation was, or if the 20 mW and 6 s of LILIP energy density[15] was the most optimal, since no other energy densities were investigated, or if DPSCs and SHED used in this present study, share similar responses to LILIP.
The present study discovered that the LILIP energy density could enhance or reduce SHED proliferation and degradation within dental pulp constructs. SHED proliferation increased following exposure to 0.4, 0.8, and 1.2 J/cm2 LILIP energy densities following culture in both the optimum and nutrient deficient FBS conditions. After prolonged exposure to 2.4 J/cm2 LILIP energy density, the proliferation of SHED was inhibited. The results indicate that a 1.2 J/cm2 LILIP energy density is optimal to enhance SHED proliferation. This is consistent with previous research demonstrating that LILIP can stimulate cell proliferation in a narrow energy range, and with low energy density[24]. Excessive LILIP energy densities can inhibit cell proliferation[12,23]. The range of energy densities in this current study which could enhance or reduce SHED activity is in accordance with the Arndt-Shultz Law[25]. The Arndt-Shultz Law predicts that a small amount of laser or other source of energy will increase physiological activity, and that a larger amount of the energy will kill cells[12,23]. In this study, the energy stimulation range was between 0.4-1.2 J/cm2 and the energy inhibition occurred at a 2.4 J/cm2 energy density.
SHED are beneficial for regenerative endodontics because they can differentiate into mineralizing cells which can regenerate teeth[26]. In the von Kossa staining part of the present study, the SHED treated with mineralization induction media, we observed to deposit substantial amounts of mineral nodules (black color). The formation of mineral nodules suggests that the SHED differentiated into an odontoblast-like type of cell[27]. The SHED had a greater capacity for mineralization than the control L929 cultures. All the cell cultures showed ascending mineralization percentages from 14-28 d regardless the cell line or the FBS concentrations. The cultures supplemented with optimal FBS concentration showed higher percentages of mineralization than the stressed cultures. This indicates that nutrient deficient SHED has a lower capacity for mineralization, suggesting that a nutrient deficit can reduce cell mineralization activity.
The molecular mechanism whereby LILIP can increase cell activity is reported to be its ability to increase the concentration of calcium in the cytoplasm from the mitochondria[9,28,29]. Consequently, the calcium transported into the cytoplasm can increase the rate of cell mitosis and improve cell proliferation. Further research is needed to identify how the molecular mechanisms of cells can be targeted to cause them to proliferate, differentiate and mineralize, as an alternative to the traditional use of growth factors for this purpose[4].
In conclusion, a 1.2 J/cm2 energy density of LILIP enhances SHED proliferation, dental pulp construct degradation, and mineralization. These results are significant because SHED and other dental cell proliferation, dental pulp construct degradation, and mineralization are needed to make regenerative endodontics quick and effective. Future clinical research is needed to more completely identify the regeneration benefits of using LILIP, such as following the accidental exposure of the dental pulp, Cvek pulpotomy, tooth revascularization and regeneration.
This is the first article to describe using low intensity laser irradiation phototherapy (LILIP) to control stem cells from human exfoliated deciduous teeth (SHED) proliferation, mineralization and construct degradation. This is also the first article to determine the optimal and injurious ranges of LILIP energy densities to enhance and inhibit the activity of a dental stem cell line.
A hotspot of dental research is to develop new therapies which can promote dental tissue healing and regeneration. LILIP could be used to activate SHED and potentially other stem cells to regenerate missing dental tissues.
At the current time, lasers are most often used to cut soft dental tissues. LILIP is a new type of laser therapy. LILIP could be used more frequently in future dental practice to regenerate missing tissues for patients.
Future clinical research is needed to more completely identify the applications of using LILIP, such as to promote the healing of the exposed dental pulp and in conjunction with Cvek pulpotomy, tooth revascularization and regeneration procedures.
The reviewers found the article to be innovative and interesting. The article is significant because it is the first article to evaluate the effects of LILIP on SHED within dental pulp constructs.
P-Reviewer Spagnuolo G S- Editor Wen LL L- Editor A E- Editor Zheng XM
| 1. | Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 552] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 2. | Epelman I, Murray PE, Garcia-Godoy F, Kuttler S, Namerow KN. A practitioner survey of opinions toward regenerative endodontics. J Endod. 2009;35:1204-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807-5812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 1985] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 4. | Kikuchi N, Kitamura C, Morotomi T, Inuyama Y, Ishimatsu H, Tabata Y, Nishihara T, Terashita M. Formation of dentin-like particles in dentin defects above exposed pulp by controlled release of fibroblast growth factor 2 from gelatin hydrogels. J Endod. 2007;33:1198-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Kucerová H, Dostálová T, Himmlova L, Bártová J, Mazánek J. Low-level laser therapy after molar extraction. J Clin Laser Med Surg. 2000;18:309-315. [PubMed] |
| 6. | Kimura Y, Yamazaki R, Goya C, Tomita Y, Yokoyama K, Matsumoto K. A comparative study on the effects of three types of laser irradiation at the apical stop and apical leakage after obturation. J Clin Laser Med Surg. 1999;17:261-266. [PubMed] |
| 7. | Yilmaz S, Kuru B, Kuru L, Noyan U, Argun D, Kadir T. Effect of gallium arsenide diode laser on human periodontal disease: a microbiological and clinical study. Lasers Surg Med. 2002;30:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Ceballos L, Toledano M, Osorio R, García-Godoy F, Flaitz C, Hicks J. ER-YAG laser pretreatment effect on in vitro secondary caries formation around composite restorations. Am J Dent. 2001;14:46-49. [PubMed] |
| 9. | Friedmann H, Lubart R, Laulicht I, Rochkind S. A possible explanation of laser-induced stimulation and damage of cell cultures. J Photochem Photobiol B. 1991;11:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 116] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Mercer C. Lasers in dentistry: a review. Part 1. Dent Update. 1996;23:74-80. [PubMed] |
| 11. | Walsh LJ. The current status of low level laser therapy in dentistry. Part 2. Hard tissue applications. Aust Dent J. 1997;42:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Pereira AN, Eduardo Cde P, Matson E, Marques MM. Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med. 2002;31:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Eduardo FP, Mehnert DU, Monezi TA, Zezell DM, Schubert MM, Eduardo CP, Marques MM. Cultured epithelial cells response to phototherapy with low intensity laser. Lasers Surg Med. 2007;39:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Karu T. Laser biostimulation: a photobiological phenomenon. J Photochem Photobiol B. 1989;3:638-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Eduardo Fde P, Bueno DF, de Freitas PM, Marques MM, Passos-Bueno MR, Eduardo Cde P, Zatz M. Stem cell proliferation under low intensity laser irradiation: a preliminary study. Lasers Surg Med. 2008;40:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Gebhardt M, Murray PE, Namerow KN, Kuttler S, Garcia-Godoy F. Cell survival within pulp and periodontal constructs. J Endod. 2009;35:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Gotlieb EL, Murray PE, Namerow KN, Kuttler S, Garcia-Godoy F. An ultrastructural investigation of tissue-engineered pulp constructs implanted within endodontically treated teeth. J Am Dent Assoc. 2008;139:457-465. [PubMed] |
| 18. | Murray PE, Smith AJ, Garcia-Godoy F, Lumley PJ. Comparison of operative procedure variables on pulpal viability in an ex vivo model. Int Endod J. 2008;41:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Liu L, Ling J, Wei X, Wu L, Xiao Y. Stem cell regulatory gene expression in human adult dental pulp and periodontal ligament cells undergoing odontogenic/osteogenic differentiation. J Endod. 2009;35:1368-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 21. | Tuby H, Maltz L, Oron U. Low-level laser irradiation (LLLI) promotes proliferation of mesenchymal and cardiac stem cells in culture. Lasers Surg Med. 2007;39:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Renno AC, McDonnell PA, Parizotto NA, Laakso EL. The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed Laser Surg. 2007;25:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Renno AC, McDonnell PA, Crovace MC, Zanotto ED, Laakso L. Effect of 830 nm laser phototherapy on osteoblasts grown in vitro on Biosilicate scaffolds. Photomed Laser Surg. 2010;28:131-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Loevschall H, Arenholt-Bindslev D. Effect of low level diode laser irradiation of human oral mucosa fibroblasts in vitro. Lasers Surg Med. 1994;14:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 110] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Tuner J, Hode L. Laser therapy. Grangesberg: Prima Books 2002; . |
| 26. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 27. | Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, Jin Y. Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell. 2007;99:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Manteifel V, Bakeeva L, Karu T. Ultrastructural changes in chondriome of human lymphocytes after irradiation with He-Ne laser: appearance of giant mitochondria. J Photochem Photobiol B. 1997;38:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Marques MM, Pereira AN, Fujihara NA, Nogueira FN, Eduardo CP. Effect of low-power laser irradiation on protein synthesis and ultrastructure of human gingival fibroblasts. Lasers Surg Med. 2004;34:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |