INTRODUCTION
According to the 2015 reports from Global initiative for Asthma and Global initiative for chronic obstructive lung disease, the prevalence of asthma ranges from 1% to 18% worldwide, while prevalence of chronic obstructive pulmonary disease (COPD) is about 6%[1,2]. Obstructive airway diseases, both asthma and COPD, are characterized by abnormal inflammation and bronchoconstriction. Bronchospasm is contributed by both airway smooth muscle contraction and mucus production by the epithelial cells. Pathogenesis of obstructive airway disease is therefore a complex interaction among inflammatory cells, epithelial cells of the bronchial airway, smooth muscle cells and fibroblasts. While the role of inflammation is emphasized in the pathogenesis and treatment of airway diseases, especially asthma, the role of airway smooth muscle cells beyond inflammation has been gaining increased recognition. This has led to the development of new β2-agonists, especially the long-acting β2-agonists since the 1990s. Their introduction into clinical practice however has generated some controversy. Recently, there was a paradigm shift in the understanding of obstructive airway disease and increasing evidence points to the role of β-blockers, especially those with inverse agonist action (or negative intrinsic efficacy), in the management of obstructive airway diseases.
Adrenoceptors in the airways
Adrenoceptors (AR) belong to the G protein-coupled receptor family and are activated by endogenous hormone adrenaline and neurotransmitter noradrenaline. Receptor activation stimulates the heterotrimeric G proteins (Gα and Gβγ subunits) and, in turn, the Gα subunit activates effector molecule (e.g., adenylyl cyclase, phospholipase Cβ, and transducin) for signal transduction. Various subtypes of Gα protein have been described, including Gαq, Gαt, Gαs and Gαi proteins.
There are two main groups of AR which have been classified as α- and β-subtypes, and are encoded by at least nine unique genes (α1A, α1B, α1D, α2A/D,α2B,α2C,β1,β2 and β3)[3]. α1-AR typically induce vascular smooth muscle contraction via a Gαq protein. α2-AR are mainly expressed in presynaptic terminals and regulate release of neurotransmitters. Despite evidence for α-AR distribution in the lung, neither receptor subtype has a clear role in regulating human airway smooth muscle tone or plays a significant role in the pathogenesis of asthma or COPD[4]. In contrast, β-AR activate adenylyl cyclase via the Gαs protein to produce cyclic adenosine monophosphate (cAMP), which promotes airway smooth muscle relaxation (Figure 1).
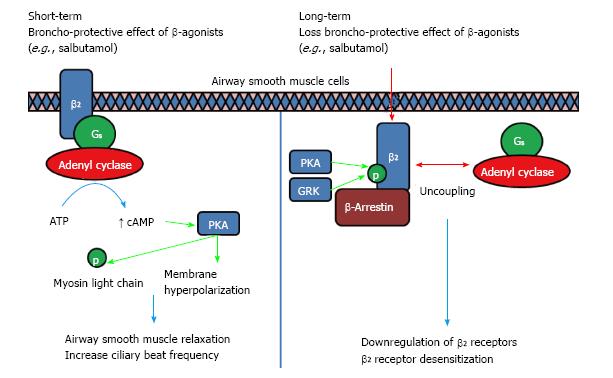
Figure 1 Long-term usage of β-agonists will result in a loss of Broncho-Protective Effect where β-adrenoceptors desensitization occurs.
Broncho-Protective Effect is conferred when β-agonist binds to β2-AR, activating adenyl cyclase through Gαs, leading to an increase in cAMP levels. The surge in cAMP in turn activates PKA which phosphorylates myosin light chain to inhibit contraction. PKA also activates K+ channels, inducing membrane hyperpolarization which counteracts electrical excitation leading to contraction. Chronic use of β-agonist will lead to a loss of this Broncho-Protective Effect due to the uncoupling of Gαs from β2-AR, phosphorylation by PKA/GRK and the binding of β-arrestin which leads to internalization, downregulation and desensitization towards β-agonist[15,27,28]. PKA: Protein kinase A; GRK: G-protein receptor kinases; AR: Adrenoceptors.
β-AR are subdivided into at least three distinct groups: β1, β2, and β3. In mouse or guinea pig trachea, airway bronchial tissues have twice the density of β2-AR compared to β1, and the density of β3 is much less[5]. In humans, however, quantitative autoradiographic analyses of human isolated bronchus have shown that β-AR of airway smooth muscle are entirely of the β2-receptor subtype. Similarly, β-AR of airway epithelium are also entirely of the β2-receptor subtype. Only in bronchial sub-mucosal glands was β1-AR found[6]. As such, β2-AR play a more important role than β1-AR in the pathogenesis of obstructive airway diseases.
Role of β2-AR in obstructive airway disease
Studies using non-selective β-blockers with inverse agonism or β2-AR-/- knockout mice demonstrated that β2-AR signaling is required for the full asthma phenotypic development in mice[7].
Smooth muscle relaxation in the airways is one of the most critical targets of drug therapy during acute exacerbation of bronchial asthma. It is believed that β2 agonist action is primarily mediated by cAMP-dependent protein kinase A (PKA). Activated PKA will phosphorylate myosin light chain kinase, reducing its ability to activate myosin light chain which is essential for airway smooth muscle contraction, hence, leading to the bronchodilatory effect[3]. Another biologically important action of β2-AR agonist is to induce membrane hyperpolarization via activation of the K+ channels in the plasma membrane by PKA, which counteracts the electrical excitation and subsequent Ca2+ influx contributing to contraction[8]. Cyclic AMP has also been shown to cross-talk with the mitogen-activated protein kinase (MAPK) pathway through the inhibition of Ras-dependent activation of Raf, resulting in inhibition of this proliferative pathway. β2-agonist usage may prevent smooth muscle remodeling as well as contraction[9].
β2-AR are also found on the surface of bronchial epithelial cells. A study in transgenic mice shows that an over-expression of β2-AR on the epithelial cells of bronchial airway could prevent bronchoconstriction and hyperresponsiveness to methacholine. β-AR activation could lead to increase ciliary beat frequency and increase alveolar fluid clearance in animal and human lung tissues. β2-AR appear to be responsible for most of the β-receptor-sensitive alveolar active Na+ transport which facilitates alveolar fluid removal[10]. Experimental data also suggest that β2-agonist inhibits endothelial cell contraction and reduces intercellular gap, improving the endothelial barrier function. Human β2-AR have been shown to regulate mucin production and increase mucous viscosity. In animals, usage of β2-agonist is associated with increasing goblet cell hyperplasia[11], while the treatment with β-blockers in mouse epithelial cells significantly reduces the density of mucus-producing goblet cells[12].
The role of β2-AR in inflammatory cells is more controversial. In vitro studies of long-acting β2-agonists (LABA) formoterol and salmeterol show that activation of β2 receptors inhibited neutrophil and eosinophil adhesion to tracheal venules, and interlukin (IL-1) and leukotriene B4 secretion from human alveolar macrophages[13]. β2-receptor activation inhibits the production of IL-6, IL-8, RANTES, eotaxin, granulocyte-macrophage colony stimulating factor, and monocyte chemotactic protein 1. However, some recent evidence has pointed towards the detrimental effects of LABA in promoting further inflammation in asthma. Loza et al[14] showed that β2-agonist promoted IL-13+ T-helper 2 cell survival by activation of the PKA pathway. An in vitro study by Oehme et al[15] demonstrated that prolonged treatment with β2-agonists reduced β2-receptor expression and stimulated IL-6 and IL-8 production in human bronchial epithelial cell line.
β2-agonist and its role in obstructive airway disease
The Chinese have been inhaling herbs containing ephedrine for asthma from centuries ago. In 1698, John Foyer[16] understood that asthma treatment is “both in fit and out of it”, suggesting early recognition of both acute treatment and maintenance therapy. Since the early 1900s, direct adrenergic bronchodilators were introduced in Western medicine for the treatment of asthmatic attacks[17], way before the usage of corticosteroids in the 1940s. During the 1960s and 1970s, relatively specific β2-agonists were developed for inhalational use[18]. The introduction of LABA such as salmeterol and formoterol in the 1990s was considered a major advancement in asthma therapy with evidence of improved lung function and quality of life. In 2011, the once daily β2-agonist indacaterol is being used in COPD patients[19].
Drugs that act on β2-AR are classified by their speed of onset, duration of action, affinity, intrinsic efficacy and potency. The duration of action and onset of action is influenced by lipophilicity and kinetics of binding. Among the agents currently used, salmeterol and formoterol sustain longer duration of action than salbutamol as their lipophilicity produces a depot effect at the cell membrane, allowing slow and sustained release of the drugs[20]. Formoterol has a shortened lipophilic side chain compared to salmeterol and hence while it’s moderate lipophilicity allows it to enter and be retained in the plasmalemma, sufficient drugs are still available in the aqueous biophase to allow immediate interaction with the active site of the receptor, accounting for its rapid onset of action.
The affinity of a drug depends on its specific binding to the β2-AR and is usually described in terms of dissociation constant between the agonist and the receptors. The intrinsic efficacy of a β2-AR agonist will depend on the ability of the drug to activate its receptor. Drugs that have high intrinsic efficacy are termed full agonist while drugs with lower intrinsic efficacy are termed partial agonist. The potency of a drug depends on both its affinity and intrinsic efficacy. Drugs that inhibit the β-AR (β-blockers) are either antagonists or inverse agonists. Antagonists are drugs that prevent the agonist from binding to the receptors, while inverse agonists are drugs that bind the receptor and inactivate constitutive downstream signaling. Many β-blockers in the market possess inverse agonist action on the β-AR, such as propranolol and nadolol, where they are able to inhibit constitutively active receptors[7].
Although the role of the β2-adrenergic agonists had long been recognized, their long term usage has been controversial. Occasional epidemics of asthma-related deaths have been linked to the use of β2-agonists such as fenoterol[21]. The Serevent National Survey (SNS)[22] study in the United Kingdom and the Salmeterol Multicenter Asthma Research Trial (SMART)[23] study in the United States raised the concern that regular usage of LABAs such as salmeterol may increase asthma-related mortality. This mortality is not seen when a LABA is used concomitantly with an inhaled corticosteroid[24]. The increased mortality is attributed to increased bronchial hyperresponsiveness, loss of protection against bronchoconstrictor stimuli and the development of tolerance[25].
It has long been appreciated that the ability of β2-agonist to induce bronchodilatation weans over time[26]. This is termed as loss of Broncho-Protective Effect of β2-agonist, which was initially attributed to desensitization and down-regulation of the β2-AR (Figure 1). The mechanism for desensitization and down-regulation of β2-AR is linked to receptor phosphorylation by PKA and by β-adrenergic receptor kinase (βARK), a member of the G-protein receptor kinases, leading to conformational change in the receptor and its consequent reduced coupling to G proteins, leading to desensitization[27,28]. βARK also promotes the binding of β-arrestin proteins to the receptor[29]. Arrestins act as scaffolding proteins that allow desensitized receptors to undergo endocytosis into the cells, lysis, and termination of further signaling process.
β2-blocker or inverse agonist and their role in obstructive airway disease
Traditionally β-blockers have been contraindicated in various diseases including obstructive airways disease and congestive cardiac failure. A recently published study by Bellocchia et al[30], which recruited 229 patients, showed that 51% COPD and 30% asthmatic patients had cardiovascular disease. Congestive heart failure (CHF) in COPD patients range from 8% to 27% while coronary artery disease (CAD) in COPD patients range from 15% to 25%[31]. In a recent RHYTHMOS study, in a population of 280 CAD with COPD patients, only 52.8% were treated with β-blockers, where most were treated with sub-optimal dosages[32]. In another study by Puente-Maestu et al[31], only 58% of COPD patients with indication for CHF/CAD were prescribed with β-blockers, while 97% of non-COPD patients with indications were treated with β-blockers. Studies of using β-blockers in asthma and COPD have demonstrated decreased airway reversibility[33] and reduction in FEV1[34,35]. A large retrospective electronic medical record database review of 11592 adult patients with asthma and COPD by Brooks et al[36] in 2007 revealed that patients with asthma with or without COPD who were taking selective or non-selective β-blockers had an increased risk of hospitalization and emergency department visits. All these added to the reluctance to use β-blockers in obstructive airway disease.
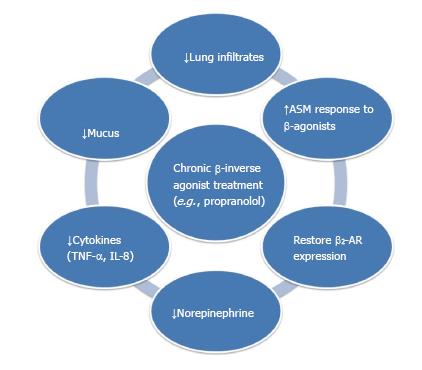
However, a recent single center randomized double-blind placebo-controlled trial with a sample size of 16 in the United Kingdom showed that 80 mg/d of propranolol given to patients with persistent asthma did not cause adverse effects[37,38]. Using an OVA-induced murine asthma model, nadolol, a non-selective β-blocker with inverse agonist action, was shown to reduce mucous metaplasia, BALF cellular infiltrates and airway hyperresponsiveness[7]. In a 4-mo rat model of smoking, it was shown that cigarette smoking leads to excessive sympathetic stimulation, resulting in down-regulation of β2-AR[39]. Propranolol was found to be able to reduce inflammatory cell infiltration in lungs, mucus secretion, Tumor necrosis factor (TNF)-α and IL-8 levels[40]. It also reduced norepinephrine level in the serum and increased airway smooth muscle response to isoprenaline[41]. These studies highlight the feasibility of using β-blockers in obstructive airway disease (Figure 2).
Figure 2 Potential therapeutic benefits by chronic β-blocker usage in obstructive airway diseases observed in animal and clinical studies[12,40,46,47,64,65].
AR: Adrenoceptors; TNF: Tumor necrosis factor; IL: Interlukin.
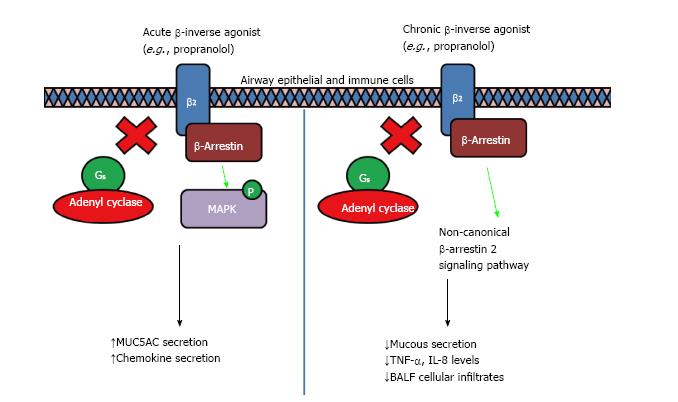
It has been shown that β-inverse agonists such as propranolol inhibit G protein-dependent signaling, but activate MAPK through β-arrestin in mouse embryonic fibroblasts and CHO cells[42,43]. β2-AR have been studied intensively, and depending on the ligand binding site, it can induce differential stabilized conformation which in turn elicits a variety of selectivity toward G-protein-dependent and β-arrestin-dependent signaling[44,45]. It was further proposed that a secondary binding site may be exposed upon adequate conformational state, leading to a different signaling cascade[44]. However, a recent study reveals that chronic propranolol treatment reduced MAPK activation through β-arrestin-dependent signaling, leading to reduced MUC5AC expression and mucus hypersecretion induced by cigarette smoke[46]. The discrepancy could be due to a different models with acute vs chronic treatment with propranolol. It has been reported that acute treatment with nadolol led to an increase in airway resistance to methacholine in a murine asthma model, but chronic administration reduced it together with lower mucin content[47]. In addition, chronic treatment with nadolol in HEK293 cells led to reduced β2-AR degradation and increased protein levels[47]. Therefore the beneficial effects of chronic treatment with β-inverse agonists are worthy of further investigation (Figure 3).
Figure 3 Acute and chronic inverse agonist treatment in obstructive airway diseases.
It was shown in cell and animal models that acute treatment of β-blockers induced a partial agonist response that led to an increase in MUC5AC production viaβ-arrestin2 which serves as a multi-protein scaffold, activating ERK1/2 and p38 mitogen-activated protein kinase (MAPK), resulting in mucus hypersecretion and increased airway resistance response to methacholine. However, chronic treatment of β-blockers led to a reduction in mucus secretion, decreased airway hyperresponsiveness and reduced inflammation, through the non-canonical β-arrestin2-mediated signaling induced by inverse agonism of β2-adrenoceptors[46]. The differential response could be due to the binding of ligand to a shallower secondary binding site exposed only when an adequate conformational state is obtained as proposed by Soriano-Ursúa et al[44], however more work need to be done to validate the mechanism.
Use of β-blocker for cardiovascular protective effects
In 1975, Waagstein et al[48] published the first positive results using a β-blocker to treat congestive cardiac failure (CHF), and this led to the FDA in approving the usage of β-blockers in CHF. Since then, β-blockers have been widely used in treating patients with ischemic heart disease (IHD) and impaired cardiac contractility. However, a significant proportion of patients with IHD also have risk factors for COPD. Reluctance on usage of β-blockers in patients with COPD and asthma has become a major cause of under usage of β-blockers in IHD. In one study, COPD patients had a nearly two-fold increase in cardiovascular disease (CVD) death rates compared to the general population[49]. In fact, impaired lung function seems to be an independent risk factor for arrhythmias, coronary events, and all-cause mortality[50]. Therefore, it seems crucial to explore the potential survival benefit of using β-blockers in obstructive airways disease.
A meta-analysis by Salpeter et al[51] (2005) examined all randomized, blinded and controlled trials from 1966 to 2005, on the effect of single dose or longer duration cardio-selective β-blockers on FEV1 or symptoms in patients of COPD. This meta-analysis demonstrated that cardio-selective β-blockers do not affect the FEV1 or respiratory symptoms compared to placebo. It is also a relief to see that the cardio-selective β-blockers do not blunt the effect of β2-agonists on FEV1[51]. Another recent meta-analysis of observational studies also concluded that non-selective β-blockers can reduce overall mortality risk and exacerbation risk[52]. Over the past decade, the are a plethora of observational trials suggesting that non-selective β-blockers in patients with COPD is not only safe but beneficial in terms of reducing mortality, hospitalization, health-care utilization, and even admissions for respiratory disease including COPD exacerbations[31,34,53-55]. The benefit was not only shown in a wide range of COPD patients with CVD like hypertension, acute myocardial infarction[56,57], congestive heart failure and patients that underwent major vascular surgery[58], but it was also shown in patients without any overt cardiovascular disease[59]. Recent heart failure guidelines published by the Heart Failure Society of America recommend that for the majority of patient with left ventricular systolic dysfunction, cardioselective β-blocker therapy is recommended even in the presence of concomitant COPD[60]. Nevertheless, caution must be exercised as the non-selective β-blockers were associated with an increase rate of hospitalization and emergency room visits in the study by Brook et al[36].
β-blockers beyond cardiovascular protective effects - the new frontier in asthma treatment
There is good evidence to suggest at least the usage of cardio-selective β-blockers in patients with obstructive airway disease with concomitant CVD. However their role beyond cardiovascular protection is still unknown, especially in asthma. Since the publication of the SMART and SNS studies documenting the potential side effects of β2-agonist, several studies have now been undertaken to evaluate the role of chronic β-blocker usage in reducing the long term side effects of β2-agonist, and in asthma control beyond the cardiovascular protection. This is a very bold and exciting development in the field of asthma pharmacotherapy and control.
The safety of β-blockers has also been demonstrated in asthmatic patients. A recent observational study in Scotland investigated the effect of non-selective β-blockers in 1527 asthmatic patients. The study did not find any significant increase in steroid rescue use in β-blocker treatment group[61]. Another meta-analysis study of randomized, blinded, and placebo-controlled trials reveals that acute single dosing with cardioselective β-blockers produced a slight but significant reduction in FEV1 of 7.46% without affecting symptoms, while chronic dosing did not significantly reduce FEV1. In addition, a significant increase in subsequent β2-agonist response was seen upon chronic dosing, indicating that β2-receptor up-regulation might have occurred[34].
In an experimental asthma model, acute administration of β2-agonist salbutamol or alprenolol, a β-blocker without inverse agonist action, reduced airway resistance in mice, but upon chronic use, either drug did not affect the airway resistance response to antigen challenge. On the other hand, acute administration of β2-AR inverse agonist nadolol or carvedilol did not affect airway responsiveness, but after 28 d of treatment, the inverse agonists markedly reduced airway responsiveness to antigen[62]. The beneficial effect may be contributed by an up-regulation β2-AR expression in chronic usage of the β-inverse agonist, as demonstrated by the increased receptor staining in histological lung sections[63]. Furthermore, chronic β-blocker usage also reduces eosinophilic inflammation, cytokine production, and mucin content in a chronic mouse asthma model[12].
These findings in murine models led to the first proof-of-concept open-label study by Hanania et al[64]. Ten patients with mild steroid-naive asthma (mean FEV1 of 90%) were given incremental doses of nadolol from 10 to 40 mg for 9 wk. There was an initial decrease in FEV1, but with chronic dosing this effect tended to ameliorate, and airway hyper-responsiveness to methacholine challenge significantly improved (amounting to 1.8 doubling doses in PC20, the provocative dose of methacholine that leads to a 20% fall in FEV1)[64]. The effect of another β-blocker propranolol was further tested in a randomized control trial conducted by Short et al[65]. Although the primary outcome of the trial was not met, the trial demonstrated the safety of β-blocker in carefully selected steroid-treated stable patients with asthma. The usage of concomitant inhaled steroid may have caused the up-regulation of β2-AR hence reducing the effect of the β-blocker[65]. More trials are warranted in this exciting field.