Published online Jul 28, 2015. doi: 10.5320/wjr.v5.i2.126
Peer-review started: August 8, 2014
First decision: December 26, 2014
Revised: February 25, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: July 28, 2015
Processing time: 361 Days and 7.5 Hours
Caveolae are flask-shaped invaginations of cell membrane that play a significant structural and functional role. Caveolae harbor a variety of signaling molecules and serve to receive, concentrate and transmit extracellular signals across the membrane. Caveolins are the main structural proteins residing in the caveolae. Caveolins and another category of newly identified caveolae regulatory proteins, named cavins, are not only responsible for caveolae formation, but also interact with signaling complexes in the caveolae and regulate transmission of signals across the membrane. In the lung, two of the three caveolin isoforms, i.e., cav-1 and -2, are expressed ubiquitously. Cavin protein family is composed of four proteins, named cavin-1 (or PTRF for polymerase I and transcript release factor), cavin-2 (or SDPR for serum deprivation protein response), cavin-3 (or SRBC for sdr-related gene product that binds to-c-kinase) and cavin-4 (or MURC for muscle restricted coiled-coiled protein or cavin-4). All the caveolin and cavin proteins are essential regulators for caveolae dynamics. Recently, emerging evidence suggest that caveolae and its associated proteins play crucial roles in development and progression of pulmonary hypertension. The focus of this review is to outline and discuss the contrast in alteration of cav-1 (cav-1),-2 and cavin-1 (PTRF) expression and downstream signaling mechanisms between human and experimental models of pulmonary hypertension.
Core tip: Pulmonary hypertension is a disease condition that is associated with a wide range of underlying medical conditions and environmental exposures. Currently, the exact molecular mechanisms underlying the pathogenesis of pulmonary hypertension remain unclear. This review is to outline and discuss the current understandings on the novel roles of a group of cell surface proteins, cav-1, -2 and cavin-1, on the development of pulmonary hypertension and vascular remodeling.
- Citation: Chettimada S, Yang J, Moon HG, Jin Y. Caveolae, caveolin-1 and cavin-1: Emerging roles in pulmonary hypertension. World J Respirol 2015; 5(2): 126-134
- URL: https://www.wjgnet.com/2218-6255/full/v5/i2/126.htm
- DOI: https://dx.doi.org/10.5320/wjr.v5.i2.126
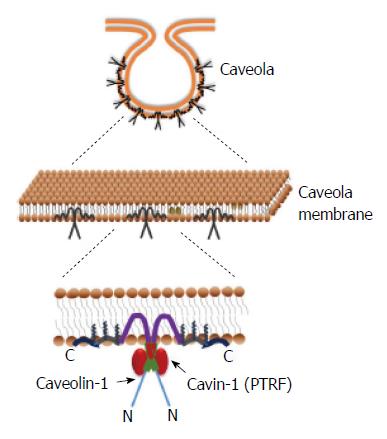
The cell membrane is a dynamic, fluid structure containing lipids and proteins that are asymmetrically distributed between the outer and inner leaflets of the membrane. It functions not only as a protective boundary to the cell, but also aids in selective molecular transport and transduction of signals across the membrane. These processes are facilitated by membrane proteins that form macromolecular complexes and are highly organized with respect to time and position. In some cases, these complexes are localized to specific regions of the cell membrane, such as lipid rafts and caveolae[1]. Caveolae are (Ω)- or flask- shaped invaginations of the plasma membrane. While the surrounding plasma membrane contains mostly lipids with kinked-unsaturated fatty acids, the caveolae have a high concentration of saturated straight chain fatty acids and cholesterol, which makes the structure rigid and highly organized[1]. This organization is maintained by proteins called caveolin, lining the inner leaflet; sometimes referred to as “caveolin-coat”. Three distinct caveolin proteins have been identified in humans: caveolin-1 (cav-1), caveolin-2 (cav-2) and caveolin-3 (cav-3)[2]. Cav-3 is mostly expressed in skeletal and smooth muscle cells, while cav-1 and -2 are widely expressed in many cell types.
In addition to the coat protein caveolin, caveolae also have an inner lining of adapter proteins called cavins. The cavin family consists of four members (cavin-1 to -4) with common structural features including leucine zipper motifs, PEST (Pro-Glu-Ser-Thr-rich) sequence and phosphoregulatory sites[3]. Among the different cavins, cavin-1 is the most abundantly expressed and extensively studied.
As discussed above, signaling protein complexes are organized and concentrated in the caveolae which serve to receive, concentrate and transmit extracellular signals across the cell. And since caveolae are covered with a “caveolin-coat”, it is imperative that caveolin plays an important role in transmitting these signals via interaction with the signaling protein complexes.
In this review we will discuss the role of cav-1 and cavin-1 as a regulator in lung disease, specifically pulmonary hypertension.
Pulmonary hypertension is a chronic and progressive disease characterized by high mean pulmonary arterial pressure (> 25 mmHg at rest). Common symptoms include shortness of breath, dizziness, edema and fatigue. Right heart catheterization and six-minute walk test are often performed to diagnose the disease. However, due to the non-specific nature of symptoms of the disease, by the time patients are diagnosed, frequently, they are at an advanced stage of the disease. Endothelial dysfunction, pulmonary vasoconstriction and vascular remodeling are the common features of pulmonary hypertension of different etiologies. Dysfunctional endothelial cells in PH patients have an altered production of endothelial vasoactive mediators such as NO, endothelin-1, prostacyclin, thromboxane and serotonin[4]. Pulmonary vasoconstriction in response to airway hypoxia is a physiological response to redirect blood flow from poorly ventilated regions of the lungs to well oxygenated regions[5]. Pulmonary vascular remodeling refers to a process that causes thickening of the arterial wall, wherein phenotypic and morphological changes occur in all three layers of the vessel wall: intima, media and adventitia. In more severe forms of PH, such as that in idiopathic and heritable PAH, the additional formation of complex cellular and fibrotic neointimal and plexiform lesions in distal pulmonary arteries is often identified, which involve the proliferation of both PASMCs and PAECs[6-8]. Without treatment, these conditions lead to right ventricular hypertrophy, right heart failure and premature death. Currently, the exact molecular mechanisms underlying the development of pulmonary vasoconstriction and pulmonary arterial remodeling are still unclear. This is mostly due to the fact that pulmonary hypertension is a disease condition that is associated with a wide range of underlying medical conditions and environmental exposures[9]. Interestingly, many of the signaling proteins implicated in the pathobiology of PH such as eNOS, VEGF receptor and prostacyclin receptors are known to interact with the membrane protein, cav-1 in caveolae of endothelial cells[10-13], as reviewed below in detail.
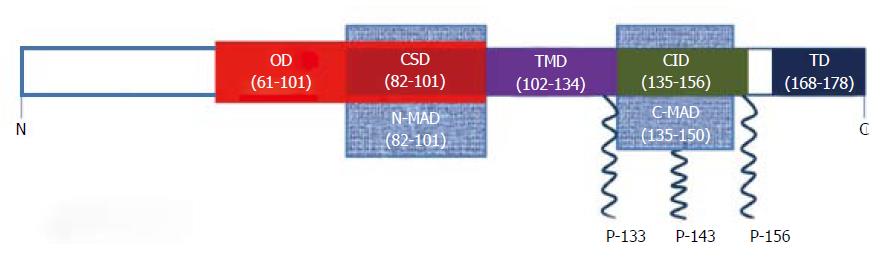
Cav-1 is a 22 kDa phosphoprotein present in many cell types in the lung including endothelial cells, type I epithelial cells, airway and vascular smooth muscle cells, fibroblasts, macrophages and neutrophils[14]. The CAV1 gene on chromosome 7 encodes a 178 amino acid protein[15]. Cav-1 protein exists in two isoforms: cav-1α and cav-1β that are derived from the use of two distinct transcription initiation sites[16]. Pulse-chase analysis studies have shown that soon after the cav-1 protein is synthesized in the ER, it forms homo-oligomers of approximately 14-16 monomers before being translocated to the plasma membrane[17,18]. The oligomerization is brought about by a series of 40 amino acids from residues 61-101 of cav-1. This is called the oligomerization domain (OD). The OD also contains the caveolin scaffolding domain (CSD) which spans from residues 82-101 (Figure 1). The CSD interacts with several other membrane proteins, most of which are signaling proteins that have a cav-1 binding motif[19]. Cav-1 also has a membrane spanning segment (residues 102-134), also called the transmembrane domain (TMD), formed by a hydrophobic loop configuration exposing the N- and C-termini to the cytoplasm[17,20]. The TMD is flanked by membrane attachment domains on each side called the N-terminal membrane attachment domain (N-MAD, residues 82-101) and C-terminal membrane attachment domain (C-MAD, residues 135-150) (Figure 1). Interestingly, N-MAD and C-MAD are minimal regions required to mediate attachment to the membrane. While the N-MAD targets cav-1 attachment to caveolae membrane, C-MAD facilitates trans-golgi targeting[21]. Additionally, the C-terminus has three palmitoylation sites on cysteine residues (133, 143 and 156)[22], and the N-terminus has a phosphorylation site on tyrosine-14[23] (Figure 1).
Various studies have shown that cav-1 plays an important role in the formation of caveolae (Figure 2). Most importantly: (1) Mice lacking the CAV1 gene (CAV1-/-) do not have caveolae on the plasma membrane[24]; (2) Cells that do not have detectable cav-1 and endogenous caveolae on their membranes, were able to form plasma membrane invaginations de novo, upon transient expression of cav-1 in these cells[25,26]. Cav-1 also aids in cellular transport namely, transcytosis[27,28], endocytosis[29,30] and exocytosis[31]. Caveolins have also been implicated in cholesterol homeostasis. Since its discovery, cav-1’s association with cholesterol has been demonstrated in several studies[32-34]. Cholesterol binds to cav-1 in the ER and is then transported to caveolae where it can be either released outside the cell or added to the plasma membrane layer[35,36]. The three palmitoylation sites on C-terminus are required for cholesterol binding of cav-1 and transport to caveolae[37] (Figure 2). Numerous plasma membrane-signaling protein complexes have been reported to concentrate in caveolae in different cell types. These proteins have caveolin binding sequence motif that allows them to interact with CSD on cav-1. Some of the cav-1-associated proteins that have been reported are: endothelial-NO synthase (eNOS), G-protein α-subunits, insulin receptor, Rho A and TGFβ receptors[34,38-41].
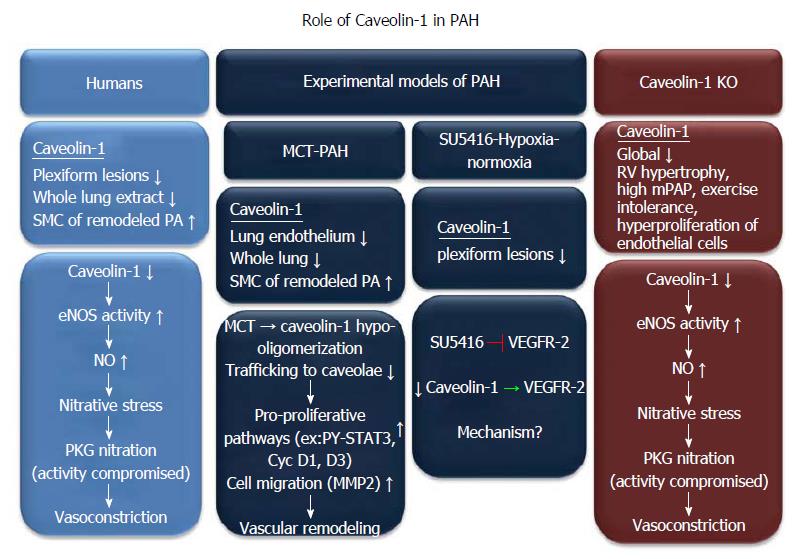
Geraci et al[42] in 2001, first reported a decrease in cav-1 mRNA level, in lung tissue samples from severe PH patients, in a gene expression profiling study. Further, immunohistochemical studies in lungs from severe PH patients show a lack of cav-1 staining in complex plexiform lesions and muscularized pre-capillary arterioles. Cav-2, a protein that normally co-localizes with cav-1 also shows decreased expression in plexiform lesions[43]. Plexiform lesions are mainly composed of highly proliferative endothelial cells and are characteristic features of pulmonary vascular remodeling[44,45]. Dysfunctional endothelial cells of pulmonary arteries play a key role in initiation and progression of PAH[46,47]. Interestingly, endothelial and smooth muscle cells of the surrounding normal-appearing vessels in the severe PH lungs express cav-1 ubiquitously[43]. In contrast, the increase in cav-1 staining is specifically seen in the vascular smooth muscle cells lining the remodeled arteries[48]. In whole lung tissue extract, cav-1 expression (by immunoblotting) is lower in IPAH lungs as compared to normal lungs[11]. This could be due to the fact that human IPAH is a complex disease and the changes in cav-1 expression are cell-specific. Endothelial cells are the majority cell type in the lung expressing abundant cav-1[49]. Therefore, the decreased cav-1 level in whole lung tissue extract reflects mainly the level of cav-1 in endothelial cells. In the pulmonary artery smooth muscle cells (PASMC) of IPAH patients, increase in cav-1 and caveolae formation enhances the capacitative calcium entry and [Ca2+]i which is attributable to the up- regulation of TRPC channels and localization to caveolae[48,50,51]. A sustained increase in [Ca2+]i is known to trigger vasoconstriction in PASMCs and also stimulate cell growth[48,52]. Therefore cav-1 up-regulation in PASMCs may also contribute to increase in pulmonary vascular resistance and pulmonary vascular remodeling.
Another interesting observation in the lungs of IPAH patients is the high level of eNOS derived NO[11] (Figure 3). NO can have beneficial or adverse effect in a disease setting like PH depending on the relative amounts of NO and reactive oxygen species (ROS)[53]. eNOS activity in the lungs of IPAH patients is substantially increased because of the reduction in cav-1 levels. Under basal conditions, cav-1 interacts with eNOS and inhibits NO production. eNOS binds to Cav-1 at a specific amino acid stretch (90-99 residues) in the caveolin scaffolding domain and inhibits its activity[54]. Loss of cav-1 in the lungs of IPAH patients leads to high NO levels and hypoxia-independent ROS production which causes peroxynitrite formation. This induces nitration of PKG at tyrosine (residues 345, 549) which impairs its kinase activity[11], subsequently, leads to pulmonary vasoconstriction and vascular remodeling of the pulmonary arteries. Taken together, the decrease of cav-1 expression in endothelial cells and increase of cav-1 level in smooth muscle cells may both contribute to the development of severe pulmonary vascular remodeling in the pathogenesis of human PAH.
Involvement of cav-1 in pulmonary hypertension has also been demonstrated in different rodent models of severe pulmonary hypertension. Decreased cav-1 and cav-2 expression has been reported in SU5416-hypoxia-normoxia[43,55] and myocardial infarction[56] models. SU5416 {3-[(2,4 dimethylpyrrol-5-yl) methylidenyl]-indolin-2-one}, is a vascular endothelial growth factor receptor-2 (VEGFR-2/Flk-1/KDR) inhibitor[57]. In the SU5416- hypoxia-normoxia model, many complex plexiform lesions show diminished immunohistochemical staining for cav-1 (Figure 3). However, in chronic hypoxia model of PH (without adding SU5416), there is no diminution in cav-1 expression[58]. Given that the plexiform lesions are prominent features in SU5416-hypoxia-normoxia models, this observation suggests that cav-1 may play an essential role in regulating pulmonary vascular remodeling. Interestingly, cav-1 has been shown to regulate the activity of VEGFR-2. Cav-1 forms a complex with VEGFR-2 in the caveolae of endothelial cells and inhibits its basal activity[12]. Similarly, overexpression of cav-1 in endothelial cells blocks VEGF-dependent activation of Elk-1 promoter activity[41]. Conversely, treatment of endothelial cells with VEGF causes a marked decrease in cav-1 protein expression[41]. Furthermore, endothelial cells isolated from cav-1 knockout mice, as compared to WT endothelial cells, show a robust and sustained increase in the tyrosine phosphorylation of VEGFR-2 upon stimulation with VEGF[59]. Apparently, although SU5416 and cav-1 depletion have opposite effects on VEGFR-2, both of these factors play a role in development of pulmonary hypertension. Further studies are required in order to dissect the signaling between SU5416 inhibition of VEGFR-2, and role of cav-1 in the SU5416- hypoxia-normoxia model of severe PAH.
In the relatively older monocrotaline (MCT) model of PAH, a decrease in caveolae was first reported in 1988 where, a reduction in percent volume of caveolae was observed in endothelial cells[60]. Later on, another study showed a decrease in cav-1 protein in the caveolae fraction of MCT treated rat lungs[61]. This was accompanied by hyperactivation of the transcription factor STAT3 (PY-STAT3) and an increase in DNA synthesis (Figure 3). As seen in human IPAH lungs, there is an increase in cav-1 expression in smooth muscle cells of remodeled pulmonary arteries[62]. In this report, the authors studied the sequential events occurring after administration of MCT through the progression of PAH up to 4 wk. After administration of MCT, there is a progressive decline in endothelial cav-1, PECAM-1 and soluble guanylate cyclase (sGC) along with a progressive increase in PY- STAT3 and pro-survival protein, Bcl-xl up to 4 wk. However, endothelial vWF and smooth muscle cav-1 remain unchanged until 2 wk post-MCT. With further loss of endothelial cav-1 at 4 wk post-MCT, there is severe endothelial deterioration indicated by loss of vWF. This exposes the smooth muscle cells to shear stress and high pressure prevalent in the hypertensive pulmonary arteries. Further, this leads to an increase in smooth muscle cav-1 expression in 70% of the vWF-lacking arteries, accompanied by an increased expression and activation of MMP2 which facilitates the proliferation and migration of smooth muscle cells, eventually leading to vascular remodeling[62] (Figure 3). eNOS protein expression increases soon (48 h) after MCT administration up to one week and then decreases by about 50% by 3-4 wk post MCT[63,64]. Similarly, NO production in the lung is decreased by about 50% by 3-5 wk post-MCT[65,66]. Mechanistically, MCT treatment in endothelial cells in vitro leads to hypo-oligomerization of cav-1 (< 8-mers) in the Golgi compartment and inhibits its trafficking from the Golgi compartment to caveolae on the plasma membrane[61]. Interestingly, administration of a peptide corresponding to cav-1 scaffolding domain to MCT rats is able to restore cav-1 expression in whole lung extracts and subsequently normalize right ventricular hypertrophy and pulmonary artery medial hypertrophy that is seen in MCT-PAH rats. Hyperactivation of STAT3 and increase in cyclin D1, D3 protein expression is also suppressed in the lungs of MCT treated rats after the administration of this peptide[67].
Cav-1 knockout mice are viable and fertile. The lung parenchyma of these mice show a multilayered, thickened alveolar septa due to increased pulmonary endothelial cell proliferation and fibrosis[24,68] possibly due to the lack of inhibition of mitogenic signals in the absence of cav-1. The highly disorganized alveolar septum comprise of mostly incompletely differentiated cells as evidenced by lack of vWF (differentiated endothelial cell marker) staining and prominent Flk-1 staining (endothelial progenitor marker)[68]. In the absence of cav-1, cav-2 expression is also reduced by up to 95%. Cav-1 is known to hetero-oligomerize with cav-2 and recruit it to the caveolae on plasma membrane. Depletion of cav-1 halts the trafficking of cav-2 to the plasma membrane thereby sequestering the residual cav-2 mostly in the Golgi compartments[24]. However, depletion of cav-2 in the cav-2 knockout mouse does not affect the expression and trafficking of cav-1 to plasma membrane. Also, caveolae formation is not affected by the absence of cav-2. But cav-2 knockout mice demonstrate alveolar septal thickening, endothelial hyperproliferation and exercise intolerance similar to cav-1 knockout mice, without any altered lipid homeostasis and vascular dysfunction[69]. This observation suggests that cav-2 has selective function in lung homeostasis and is independent of caveolae formation. The depletion of cav-1 gene in the knockout mice however, does not affect the presence of clathrin-coated pits in the cells of these animals[24]. This could be one of the work-around mechanisms by which these cells overcome the lack of caveolae that could make the cav-1 knockout mice viable. These cav-1 knockout mice also exhibit remarkable exercise intolerance as assessed by a forced swimming test. Cav-1 knockout mice develop marked right ventricular hypertrophy indicating a chronic elevated pulmonary artery pressure[70]. Indeed pulmonary artery pressure is increased in cav-1 knockout mice by 90% as compared to wild type mice.
As observed in MCT-PAH rat models, cav-1 knockout mice lungs also showed increased tyrosine phosphorylation of STAT3 and a dramatic upregulation of cyclin D1 and D3 levels[56], that could promote cell proliferation and perhaps contribute to structural remodeling in the lung. Unlike in the MCT-PAH model, in cav-1 knockout mice there is 5-fold increase in systemic NO, while in the lungs, eNOS derived NO is increased by at least 3-fold as compared to wild type mice[11,70,71]. However eNOS protein expression is not changed in the heart and lungs of these mice. Mechanistically, the high NO levels from impaired eNOS activity leads to tyrosine nitration of PKG and thereby decreasing PKG bioavailability (figure 3). This could abolish its vasodilatory effect, perhaps contributing to development of PH[11]. This speculation is supported by the fact that NOS inhibition or superoxide scavenging in cav-1 knockout mice is able to rescue the PH phenotype in these mice. Similarly, PKG-1 overexpression in the lungs of these mice significantly decreases the right ventricular systolic pressure and pulmonary vascular resistance. Furthermore, endothelial specific reconstitution of cav-1 in cav-1 knockout mice not only restores eNOS activity to normal levels, it is also able to suppress pulmonary hypertension and right ventricular hypertrophy[72]. Taken together, these observations clearly demonstrate that loss of cav-1 plays an important role in the development of pulmonary hypertension in mouse PH models.
Cavin-1, also called as polymerase I and transcript release factor (PTRF), is known to be expressed mostly in endothelial cells, fibroblasts and epithelial cells in the lungs and also in heart, adipocytes and skeletal muscle[73]. Since its discovery in 1998, cavin-1 was known to aid in the dissociation of paused ternary transcription complexes in the nucleus[3]. However, in recent years cavin-1/PTRF has been associated with caveolae and reported to regulate caveolae membrane curvature by anchoring cav-1 to the cytoskeleton via its C- terminal region[74]. Also, cavin-1 is required to mediate the normal oligomerization of cav-1[73]. More recent evidence suggests that cavin-2 plays an important role in generating caveolae specifically in the endothelial cells of the lung which is supported by the fact that endothelial cells lacking cavin-2 have flattened or shallow caveolae, but show cavin-1 co- localizing with oligomerized cav-1[73]. While cav-1 knockout mice have almost no cavin-1 expression[73,75], cavin-1 knockout mice also have diminished cav-1 expression[73] suggesting that the expression of cav-1 and cavin-1 are interdependent. Cavin-1 knockout mice have increased lung tissue density and show hypertrophic remodeling of pulmonary arteries. They also exhibit symptoms of pulmonary hypertension, i.e., increased RV-to- RV+LV ratio and increased pulmonary artery pressure as assessed by right ventricular systolic pressure[76]. Microarray analysis in new born lungs of cavin-1 knockout mice show changes in Arg1 (arginase 1) and Ddah1 (dimethylarginine dimethylaminohydrolase) genes. Increase in Arg1 and decrease in Ddah1 have been previously implicated in acquired forms of PAH[77,78]. Although the reciprocal changes in Arg1 and Ddah1 are known to limit the increase in NOS activity decreasing NO levels, this study did not measure changes in NO levels after cavin-1 knockout. Interestingly, silencing cavin-1 in endothelial cells in vitro enhances basal NO release from these cells, which could possibly be the effect of subsequent decrease in cav-1 levels[79]. Collectively, these data suggest that cavin-1 plays an important role in caveolae function in the lungs and could contribute to the development of pulmonary hypertension. However, changes in cavin-1 have not yet been studied in human and experimental models of PAH to date and these studies will help us better understand the role of cavin-1, as well as cav-1, in the development of pulmonary hypertension.
As evidenced in several reports summarized here, cav-1 plays a central role in the development and progression of PH and PAH. The signaling pathways that are affected by cav-1 are diverse in different animal models and in humans, while chronic hypoxia-induced PH mice do not show any change in cav-1 levels. Although the cav-1 knockout mouse is not an experimental model of PH, these mice do exhibit symptoms of PH among other abnormalities. Similar effects are seen in cavin-1 knockout mice whose expression is also diminished in cav-1 knockout mice. SU5416-hypoxia-normoxia PAH mice and IPAH lungs in humans show a decrease in cav-1 in plexiform lesions but not in surrounding normal pulmonary arteries. Moreover, there is a robust increase in cav-1 in the medial smooth muscle of remodeled pulmonary arteries in both animal models and in humans. Therefore, the differential expression and function of cav-1 in specific cells (i.e., endothelial cell vs smooth muscle cell) may all together contribute to the development of pulmonary vascular remodeling, subsequently resulting in PH.
P- Reviewer: La Montagna G, Skobel E, Zhang Z S- Editor: Tian YL L- Editor: A E- Editor: Wang CH
| 1. | Boscher C, Nabi IR. Caveolin-1: role in cell signaling. Adv Exp Med Biol. 2012;729:29-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 671] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 3. | Jansa P, Mason SW, Hoffmann-Rohrer U, Grummt I. Cloning and functional characterization of PTRF, a novel protein which induces dissociation of paused ternary transcription complexes. EMBO J. 1998;17:2855-2864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Loscalzo J. Endothelial dysfunction in pulmonary hypertension. N Engl J Med. 1992;327:117-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Ward JP, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol. 2009;9:287-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Majka SM, Skokan M, Wheeler L, Harral J, Gladson S, Burnham E, Loyd JE, Stenmark KR, Varella-Garcia M, West J. Evidence for cell fusion is absent in vascular lesions associated with pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L1028-L1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Hirose S, Hosoda Y, Furuya S, Otsuki T, Ikeda E. Expression of vascular endothelial growth factor and its receptors correlates closely with formation of the plexiform lesion in human pulmonary hypertension. Pathol Int. 2000;50:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Machado RD. The molecular genetics and cellular mechanisms underlying pulmonary arterial hypertension. Scientifica (Cairo). 2012;2012:106576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 881] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 10. | Ibrahim S, McCartney A, Markosyan N, Smyth EM. Heterodimerization with the prostacyclin receptor triggers thromboxane receptor relocation to lipid rafts. Arterioscler Thromb Vasc Biol. 2013;33:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119:2009-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 12. | Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Béliveau R. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell. 2003;14:334-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Spisni E, Griffoni C, Santi S, Riccio M, Marulli R, Bartolini G, Toni M, Ullrich V, Tomasi V. Colocalization prostacyclin (PGI2) synthase--caveolin-1 in endothelial cells and new roles for PGI2 in angiogenesis. Exp Cell Res. 2001;266:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Couet J, Belanger MM, Roussel E, Drolet MC. Cell biology of caveolae and caveolin. Adv Drug Deliv Rev. 2001;49:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Scherer PE, Tang Z, Chun M, Sargiacomo M, Lodish HF, Lisanti MP. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem. 1995;270:16395-16401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 290] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Kogo H, Fujimoto T. Caveolin-1 isoforms are encoded by distinct mRNAs. Identification Of mouse caveolin-1 mRNA variants caused by alternative transcription initiation and splicing. FEBS Lett. 2000;465:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6:911-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 370] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Sargiacomo M, Scherer PE, Tang Z, Kübler E, Song KS, Sanders MC, Lisanti MP. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci USA. 1995;92:9407-9411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 441] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing „preassembled signaling complexes“ at the plasma membrane. J Biol Chem. 1998;273:5419-5422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1176] [Cited by in RCA: 1178] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 20. | Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 743] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 21. | Schlegel A, Lisanti MP. A molecular dissection of caveolin-1 membrane attachment and oligomerization. Two separate regions of the caveolin-1 C-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem. 2000;275:21605-21617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Dietzen DJ, Hastings WR, Lublin DM. Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J Biol Chem. 1995;270:6838-6842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 287] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996;271:3863-3868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 281] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 24. | Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121-38138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 842] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 25. | Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci USA. 1995;92:8655-8659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 481] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 26. | Li S, Galbiati F, Volonte D, Sargiacomo M, Engelman JA, Das K, Scherer PE, Lisanti MP. Mutational analysis of caveolin-induced vesicle formation. Expression of caveolin-1 recruits caveolin-2 to caveolae membranes. FEBS Lett. 1998;434:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Ghitescu L, Bendayan M. Transendothelial transport of serum albumin: a quantitative immunocytochemical study. J Cell Biol. 1992;117:745-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Predescu D, Predescu S, McQuistan T, Palade GE. Transcytosis of alpha1-acidic glycoprotein in the continuous microvascular endothelium. Proc Natl Acad Sci USA. 1998;95:6175-6180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Montesano R, Roth J, Robert A, Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982;296:651-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 313] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217-1232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 692] [Cited by in RCA: 710] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 31. | Minshall RD, Tiruppathi C, Vogel SM, Malik AB. Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function. Histochem Cell Biol. 2002;117:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 148] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1671] [Cited by in RCA: 1755] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 33. | Murata M, Peränen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339-10343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 713] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 34. | Li S, Song KS, Lisanti MP. Expression and characterization of recombinant caveolin. Purification by polyhistidine tagging and cholesterol-dependent incorporation into defined lipid membranes. J Biol Chem. 1996;271:568-573. [PubMed] |
| 35. | Fielding PE, Fielding CJ. Plasma membrane caveolae mediate the efflux of cellular free cholesterol. Biochemistry. 1995;34:14288-14292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 222] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 36. | Fielding CJ, Fielding PE. Intracellular cholesterol transport. J Lipid Res. 1997;38:1503-1521. [PubMed] |
| 37. | Uittenbogaard A, Smart EJ. Palmitoylation of caveolin-1 is required for cholesterol binding, chaperone complex formation, and rapid transport of cholesterol to caveolae. J Biol Chem. 2000;275:25595-25599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Mastick CC, Brady MJ, Saltiel AR. Insulin stimulates the tyrosine phosphorylation of caveolin. J Cell Biol. 1995;129:1523-1531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 184] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Nohe A, Keating E, Underhill TM, Knaus P, Petersen NO. Dynamics and interaction of caveolin-1 isoforms with BMP-receptors. J Cell Sci. 2005;118:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Gingras D, Gauthier F, Lamy S, Desrosiers RR, Béliveau R. Localization of RhoA GTPase to endothelial caveolae-enriched membrane domains. Biochem Biophys Res Commun. 1998;247:888-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Liu J, Razani B, Tang S, Terman BI, Ware JA, Lisanti MP. Angiogenesis activators and inhibitors differentially regulate caveolin-1 expression and caveolae formation in vascular endothelial cells. Angiogenesis inhibitors block vascular endothelial growth factor-induced down-regulation of caveolin-1. J Biol Chem. 1999;274:15781-15785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res. 2001;88:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 193] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Achcar RO, Demura Y, Rai PR, Taraseviciene-Stewart L, Kasper M, Voelkel NF, Cool CD. Loss of caveolin and heme oxygenase expression in severe pulmonary hypertension. Chest. 2006;129:696-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275-285. [PubMed] |
| 45. | Ogata T, Iijima T. Structure and pathogenesis of plexiform lesion in pulmonary hypertension. Chin Med J (Engl). 1993;106:45-48. [PubMed] |
| 46. | Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF. The pathobiology of pulmonary hypertension. Endothelium. Clin Chest Med. 2001;22:405-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol. 1999;155:411-419. [PubMed] |
| 48. | Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, Swaney JS, Niesman IR, Schermuly RT, Pullamsetti SS. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J. 2007;21:2970-2979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 689] [Cited by in RCA: 750] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 50. | Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 906] [Cited by in RCA: 844] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 51. | Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem. 2003;278:27208-27215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 52. | Platoshyn O, Golovina VA, Bailey CL, Limsuwan A, Krick S, Juhaszova M, Seiden JE, Rubin LJ, Yuan JX. Sustained membrane depolarization and pulmonary artery smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2000;279:C1540-C1549. [PubMed] |
| 53. | Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol Rev. 2000;80:1337-1372. [PubMed] |
| 54. | Trane AE, Pavlov D, Sharma A, Saqib U, Lau K, van Petegem F, Minshall RD, Roman LJ, Bernatchez PN. Deciphering the binding of caveolin-1 to client protein endothelial nitric-oxide synthase (eNOS): scaffolding subdomain identification, interaction modeling, and biological significance. J Biol Chem. 2014;289:13273-13283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005;19:1178-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 56. | Jasmin JF, Mercier I, Hnasko R, Cheung MW, Tanowitz HB, Dupuis J, Lisanti MP. Lung remodeling and pulmonary hypertension after myocardial infarction: pathogenic role of reduced caveolin expression. Cardiovasc Res. 2004;63:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99-106. [PubMed] |
| 58. | Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem. 2002;277:44085-44092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Lin MI, Yu J, Murata T, Sessa WC. Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res. 2007;67:2849-2856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Rosenberg HC, Rabinovitch M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am J Physiol. 1988;255:H1484-H1491. [PubMed] |
| 61. | Mathew R, Huang J, Shah M, Patel K, Gewitz M, Sehgal PB. Disruption of endothelial-cell caveolin-1alpha/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation. 2004;110:1499-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Huang J, Wolk JH, Gewitz MH, Mathew R. Caveolin-1 expression during the progression of pulmonary hypertension. Exp Biol Med (Maywood). 2012;237:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Huang J, Wolk JH, Gewitz MH, Mathew R. Progressive endothelial cell damage in an inflammatory model of pulmonary hypertension. Exp Lung Res. 2010;36:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Tyler RC, Muramatsu M, Abman SH, Stelzner TJ, Rodman DM, Bloch KD, McMurtry IF. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am J Physiol. 1999;276:L297-L303. [PubMed] |
| 65. | Yuan P, Wu WH, Gao L, Zheng ZQ, Liu D, Mei HY, Zhang ZL, Jing ZC. Oestradiol ameliorates monocrotaline pulmonary hypertension via NO, prostacyclin and endothelin-1 pathways. Eur Respir J. 2013;41:1116-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 66. | Ou ZJ, Wei W, Huang DD, Luo W, Luo D, Wang ZP, Zhang X, Ou JS. L-arginine restores endothelial nitric oxide synthase-coupled activity and attenuates monocrotaline-induced pulmonary artery hypertension in rats. Am J Physiol Endocrinol Metab. 2010;298:E1131-E1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation. 2006;114:912-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1200] [Cited by in RCA: 1227] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 69. | Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H, Christ GJ, Edelmann W. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22:2329-2344. [PubMed] |
| 70. | Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99:11375-11380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 375] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 71. | Miyawaki-Shimizu K, Predescu D, Shimizu J, Broman M, Predescu S, Malik AB. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290:L405-L413. [PubMed] |
| 72. | Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373-2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 73. | Hansen CG, Shvets E, Howard G, Riento K, Nichols BJ. Deletion of cavin genes reveals tissue-specific mechanisms for morphogenesis of endothelial caveolae. Nat Commun. 2013;4:1831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 74. | Liu L, Pilch PF. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J Biol Chem. 2008;283:4314-4322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 227] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 75. | Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 629] [Cited by in RCA: 579] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 76. | Swärd K, Sadegh MK, Mori M, Erjefält JS, Rippe C. Elevated pulmonary arterial pressure and altered expression of Ddah1 and Arg1 in mice lacking cavin-1/PTRF. Physiol Rep. 2013;1:e00008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088-3098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 78. | Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B, Rossiter S, Anthony S, Madhani M, Selwood D. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 310] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 79. | Dávalos A, Fernández-Hernando C, Sowa G, Derakhshan B, Lin MI, Lee JY, Zhao H, Luo R, Colangelo C, Sessa WC. Quantitative proteomics of caveolin-1-regulated proteins: characterization of polymerase i and transcript release factor/CAVIN-1 IN endothelial cells. Mol Cell Proteomics. 2010;9:2109-2124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |