Published online Nov 28, 2013. doi: 10.5320/wjr.v3.i3.67
Revised: August 26, 2013
Accepted: September 3, 2013
Published online: November 28, 2013
Processing time: 148 Days and 14.7 Hours
Chronic obstructive pulmonary disease (COPD) and lung cancer are two important smoking related conditions. However, COPD has been shown to be an independent risk factor for lung cancer regardless of smoking history, suggesting that COPD and lung cancer may share a common pathogenesis. This review summarizes the epidemiology of lung cancer and COPD briefly, as well as discussing the potential for shared genetic risk, and shared genomic mechanisms, such as epigenetic changes or DNA damage induced by smoking. How key areas of COPD pathogenesis, such as inflammation, oxidative stress and protease imbalance may contribute to subsequent development of cancer will also be covered. Finally the possibility that consequences of COPD, such as hypoxia, influence carcinogenesis will be reviewed. By understanding the pathogenesis of COPD and lung cancer in detail it is possible that new treatments may be developed and the risk of lung cancer in COPD may be reduced.
Core tip: Chronic obstructive pulmonary disease (COPD) has been shown to be an independent risk factor for lung cancer regardless of smoking history, suggesting that COPD and lung cancer may share a common pathogenesis. Chronic inflammation and oxidative stress are the most likely mechanistic links between COPD and lung cancer. Further analysis and elucidation of the molecular mechanisms involved in the pathogenesis of COPD and lung cancer should provide us with new treatment modalities and perhaps a key to understanding how the risk of lung cancer in COPD patients may be reduced.
- Citation: Green CE, Turner AM. Role of chronic obstructive pulmonary disease in lung cancer pathogenesis. World J Respirol 2013; 3(3): 67-76
- URL: https://www.wjgnet.com/2218-6255/full/v3/i3/67.htm
- DOI: https://dx.doi.org/10.5320/wjr.v3.i3.67
Chronic obstructive pulmonary disease (COPD) is a condition characterized by airflow obstruction which is not normally fully reversible with the use of bronchodilators and is progressive over time[1]. The airflow obstruction usually occurs as a result of smoking but genetic factors (e.g., alpha 1 antitrypsin deficiency), the burning of biomass fuels in developing countries and occupational factors also play a role[1,2]. Damage in both the airways and lung parenchyma contribute to the airway obstruction[1,3]. COPD is common. One study found a 10.1% prevalence of COPD (with forced expiratory volume in one second (FEV1) < 80%) in adults over 40 years in 12 different countries worldwide[4]. COPD is an important cause of morbidity and mortality and resulted in over 3 million deaths in 2005 globally[5].
Lung cancer is the leading cause of cancer morbidity in North America, and is amongst the top 5 cancers ranked by disability adjusted life years lost (DALYs) in all regions of the world, except Sub-Saharan Africa[6]. It causes 626 DALYs/100000 heads of population worldwide[6] and is the most common cause of cancer death accounting for 1.18 million deaths in 2002[7]. 90% of lung cancers are caused by smoking, although other environmental factors such as asbestos exposure also play a role[8]. Lung cancers are classified by histology into small cell and non-small lung cell cancers (NSCLC); the latter are the more common and include squamous and adenocarcinomas. Generally small cell tumours have the poorest prognosis, with overall survival being 9 mo when managed by chemotherapy[9]. Even in NSCLC prognosis is generally poor unless surgery can be offered, and a recent systematic review reported mean survival of just over 7 mo if left untreated[10].
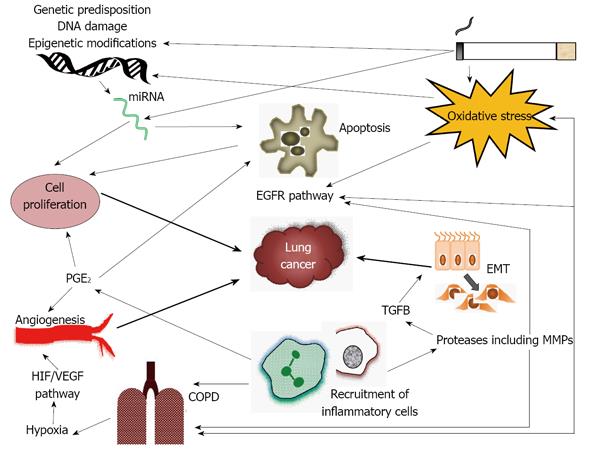
The relative risk of lung cancer in COPD is over twice that of the general population[11]. Longitudinal studies have shown that after approximately 15 years up to 33% of patients with COPD will develop lung cancer[12-14]. COPD is also a common co-morbidity in lung cancer patients occurring in 40%-70% of lung cancer cases[15]. As both diseases are related to smoking it could be assumed that the relationship between COPD and lung cancer might be related to smoking alone. However, in recent years it has been shown that patients with COPD, or CT diagnosed emphysema, have a higher risk for lung cancer even when adjusting for smoking history[15-18]. One study demonstrated that patients with COPD were six times more likely to develop lung cancer when compared to matched smokers without COPD[15]. Furthermore increased severity of airway obstruction is associated with a correspondingly elevated risk of lung cancer[18]. Emphysema in non-smokers is also a risk for lung cancer further reinforcing that smoking, alone, cannot explain the relationship between COPD and lung cancer[19,20]. In addition stopping smoking does not completely reduce the risk of lung cancer in patients with COPD[12]. Taken together this evidence suggests that there is a link between COPD and lung cancer other than smoking; this article will review the current evidence for common pathogenesis of the two conditions. The key concepts are summarized in Figure 1.
Only 20%-30% of smokers develop COPD and 10%-15% develop lung cancer[21]. This epidemiological evidence, together with familial patterns of disease, and many subsequent genetic association studies have demonstrated that for both diseases a proportion of the risk of developing them is due to genetic factors. In fact, there is evidence that COPD has a heritability of 40%-77% and lung cancer a heritability of 15%-25%[21]. Familial linkage studies and genome-wide association studies (GWAS) have been performed for COPD, lung function and lung cancer and there is overlap in the chromosome areas identified, which demonstrates shared genetic risk[21,22]. Most of the primary studies have been in patients with either COPD or lung cancer and others have then sought areas of commonality. Key regions of shared susceptibility are shown in Table 1.
| Chromosomal region | Possible gene(s) | Ref. |
| 2q | GSS | [23] |
| 4q31 | HHIP, GYPA | [21] |
| 5q33 | HTR4, ADAM19 | [21] |
| 6p | GCLCL | [23] |
| 6q | SMOC2 | [22,24,25] |
| 10q | GSTO2 | [23] |
| 11p | MMP1, MMP12, RRM1 | [23,26] |
| 12p | GSTM1 | [27,28] |
| 15q25 | CHRNA3-5, IREB2 | [21] |
| 19q | ERCC1 | [23] |
In common with genetic epidemiology work, most of the studies that have linked COPD and lung cancer at a genomic level have been in one disease or the other, and similarities or common themes have been noted later. Some studies have taken advantage of resections performed for lung cancer in patients who have COPD to get data on both diseases. For example, Wang et al[29] looked at the gene expression pattern in lung samples from COPD patients and demonstrated that genes involved in extracellular membrane synthesis and apoptosis were up-regulated, whilst genes involved in the anti-inflammatory response were down-regulated. It was also shown that urokinase plasminogen activator, its receptor and thrombospondin were expressed which are involved in transforming growth factor-β1 (TGF-β) and matrix metalloprotease (MMP) activation[29]. Growth factors and MMPs may be responsible for promoting malignant transformation of the bronchial epithelium[30-32]. Another study looking at gene expression in squamous cell carcinoma found that in patients with co-existing COPD there was a more frequent loss of 5q or a low expression of genes on 5q than in patients without co-existing COPD[33]. Cystatin A (CSTA), an intracellular protease inhibitor, has been shown to be up-regulated in NSCLC. CSTA is also expressed to a greater level in patients with COPD compared to smokers with normal lung function[34]. This suggests that pathways leading to lung tumorigenesis may vary between COPD patients and smokers with normal lung function.
Smoking has also been shown to alter gene expression - specifically it increases expression of xenobiotic genes, antioxidants, electron transport and oncogenes. Reduced levels of inflammatory regulator genes and tumor suppressor genes have also been seen in smokers[35,36]. Some of these genetic changes reverse on smoking cessation, but others including changes in oncogenes and tumor suppressor genes do not[35]. Wistuba et al[37] also demonstrated genetic alterations in the form of microsatellite deletions and loss of heterozygosity in normal epithelium of both current and ex-smokers. Irreversibility of genetic alterations or gene expression may explain why ex-smokers remain at an increased risk of developing lung cancer. It has also been shown that these changes in gene expression are associated with changes in protein expression[38].
Smoking, occupational toxins and air pollution may result in damaging mutations which have potential to induce dysplastic and neoplastic changes in the lung parenchyma due to alterations in cell differentiation, growth and death[19]. Cigarette smoke contains many carcinogens which can be activated by cytochrome P450 enzymes; inhalation of these, pollutants and micro-organisms can cause damage directly or due to oxidative stress[19]. Repeated insults such as in COPD results in increased amounts of reactive oxygen species (ROS) which interact with DNA in the epithelium causing mutations. This results in DNA adducts which in turn may cause mutations if they are not repaired[39]. Commonly these mutations are in oncogenes, but they may also affect inflammatory pathways[40]. Mostly these mutations are repaired, but when there is a high rate of damage due to ROS cells are likely to be transformed to a malignant phenotype[40]. Studies have shown that there is impairment in DNA repair in COPD due to low levels of Ku 86, a protein involved in DNA repair[41]. This suggests that oxidant induced damage in COPD patients is more likely to result in carcinogenesis.
It is also possible that processes which control DNA repair may influence a patient’s risk of developing lung cancer[42] and that such processes may be altered in COPD. For example, there is evidence that acetylation of histone H3 on lysine 56 (H3K56) is important in DNA repair[43]. Deacetylation of H3K56 is controlled by histone deactylases (HDACs) 1 and 2 and sirtuin (SIRT) 1[44]. As there are low levels of HDAC2 and SIRT1 in COPD[45] this may reduce the protection against DNA breakage caused by environmental factors further increasing lung cancer risk.
Epigenetics is the regulation of gene expression by heritable mechanisms that do not make direct changes to DNA itself. Examples of epigenetic mechanisms include histone acetylation and methylation; these may silence genes without changing their coding sequence and regulate pro-inflammatory gene expression in COPD and lung cancer[19]. The degree of histone acetylation in promoters of pro-inflammatory genes in COPD is related to disease severity and reversed by HDACs[46]. Since HDAC2 levels are low in COPD this could result in hyperacetylation of histones. SIRT1 acts similarly to HDACs and there are variable SIRT1 levels in lung cancer. However tumor suppressor genes, including p53 may be rendered inactive in patients with low SIRT1 levels, including COPD patients[47,48]. Mouse models suggest this is a relevant pathway leading to lung tumors[48].
Genome-wide demethylation with site-specific hypermethylation is seen in lung cancer[19]. NA methylation is usually associated with gene silencing[49] and is associated with tumorigenesis and recurrence of NSCLC[50]. Methylation of the promoter of p16 (a tumor suppressor gene) is seen in the sputum of COPD patients and aberrant methylation of p16 can be also seen in the sputum of patients with NSCLC suggesting this too may be a shared mechanism of disease[51]. Hypermethylation of p16, CDH13, RASSF1A and APC have been associated with recurrence in lung cancer[50], but have not yet been reported in COPD.
These are small non-coding RNAs which act to regulate protein expression and immune response by acting on mRNA synthesis or translation. They can act as oncogenes or tumor suppressor genes and promote a range of functions including cell proliferation and apoptosis, both of which are relevant to tumor growth[52]. The function of micro-RNA (miRNA) may be altered by single nucleotide polymorphisms (SNPs), some of which are associated with poor survival in NSCLC[52].
miRNA expression is down-regulated with smoke exposure in both animal lungs and bronchial epithelium in man. In the rat model it was seen that many of the miRNAs involved in the activation of the NF-κB pathway were down-regulated[53]. Other studies have also demonstrated unique miRNA profiles in COPD[54] and lung cancer[55]. In COPD miR-223 and miR-1274a were the most markedly different from healthy smokers, and there were smaller changes in some of the miRNA associated with cancer, such as miR-10a and miR-451[54]. In NSCLC miR-21, miR-30d, miR-451, miR-10a, miR-30e-5p, miR-126*, miR-126 and miR-145 were differentially regulated and it was possible for some of their signatures to be picked up in the circulating blood[55]. These signatures could be used to aid prognostication in lung cancer or potentially to diagnose cancer earlier in high risk groups, although such strategies have not yet been evaluated in clinical studies.
There is a wealth of evidence of systemic inflammation in COPD, as shown by increased levels of chemokines, cytokines and acute phase reactants[56]. Smoking can cause inflammation, but the degree seen in COPD is higher than in smokers alone and persists despite smoking cessation[57]. Inflammation in COPD also appears to be greater in severe disease[58], although its variability over time and with exacerbations has thwarted attempts to find biomarkers in the blood that relate consistently to clinical features[59]. Interleukin-6 (IL-6), C-reactive protein (CRP), IL-8 and surfactant protein D (SP-D)[60,61] levels are typically high in COPD and are important in the recruitment of inflammatory cells, although fibrinogen seems the most reliable biomarker to date[56,59]. Fibrinogen levels are associated with increased exacerbation rates and poorer outcomes[62,63], however it is a non-specific marker and as such has inherent weaknesses. More specific related markers, such as AaVal360 may prove more useful in the future[64].
There is an accumulation of inflammatory cells in COPD lungs, including macrophages, neutrophils, B cells and CD4+ and CD8+ T cells[65]. Macrophages release multiple inflammatory mediators including reactive oxygen species, cytokines, chemokines, extracellular matrix proteins and matrix metalloproteinases (MMPs). In COPD their function may be impaired, for instance they show impaired phagocytosis of bacteria, which may result in an increased inflammatory response to bacteria in the lower airways[66]. Neutrophils also produce reactive oxygen species, elastase and cytokines which play a role in emphysema and COPD development. Lymphocytes, including both B and T cells, are also found in high numbers in COPD lung[67] and may be involved in immune activation, leading to perpetuation of inflammation and ongoing parenchymal destruction. Such a reaction is typical of autoimmune disease, and characteristics of autoimmunity have been reported in COPD[68] although whether they are cause or effect is a matter of debate[69].
There is evidence that carcinogenesis occurs at sites of chronic inflammation[70]. For example, hepatocellular carcinoma can occur in patients with chronic hepatitis and colon cancer in the setting of colitis[71]. There is some evidence that increased inflammation may also be associated with the development of lung cancer. Epidemiologically, a cohort study of 7081 patients showed an increased risk of lung cancer in patients with a CRP of > 3 mg/dL[72]. Furthermore, a mouse model of chronic inflammation showed increased lung tumorigenesis[73]. However clinical studies of anti-inflammatory drugs, such as inhaled corticosteroids, have shown inconsistent results. A cohort study has shown lower rates of lung cancer compared to patients not taking inhaled corticosteroids[74], whilst a randomized controlled trial (RCT) did not[75]. Whether targeting pulmonary inflammation to prevent lung cancer will be beneficial therefore remains uncertain.
One way inflammation may lead to the development of lung cancer is by activation of the epithelial growth factor receptor (EGFR) cascade (Figure 1). This is activated in response to oxidative stress, neutrophil elastase and other proteases[76], thus might be expected to be overactive in COPD; recent evidence suggests this may be the case[77]. Overexpression of EGFR has been associated with a high risk of developing lung cancer and can occur years after smoking cessation[78]. The arachidonic acid metabolic pathway may also be related to COPD and lung cancer development. Inflammatory cells release arachidonic acid metabolites including prostaglandins - this is mediated by cyclooxygenase enzymes (COX) including COX-2. Prostaglandin E2, the product of COX-2, regulates the inflammatory response, but also has effects on cell proliferation, apoptosis and angiogenesis[70] and therefore may have a role in cancer development. Whilst this concept has been focused on far more in other cancers than in the lung there is some evidence it is relevant to cancer risk in COPD. Increased levels of COX-2 occur in COPD and are inversely proportional to FEV1[79]. Raised COX-2 levels relate to survival in NSCLC[80], inhibition of COX-2 reduces lung cancer in animal models[81] and patients who regularly take COX-2 inhibitors have reduced rates of lung cancer[82]. Finally, carriers of a polymorphism of the COX-2 gene have an increased risk of lung cancer[83].
The normal metabolism of oxygen results in the development of ROS - these are usually removed from the cell by enzymes or anti-oxidants[84]. If the balance between the formation and removal of ROS is disturbed oxidative stress can occur; this may activate intracellular pathways which modulate the inflammatory response, as well as causing DNA damage (discussed above), and therefore have a role in the development of COPD and lung cancer[84]. Oxidative stress is well recognized in COPD and is particularly elevated during exacerbations[85].
Cigarette smoke is a key driver of oxidative stress. It contains noxious chemicals which are metabolized to benign and/or toxic metabolites, the latter of which can damage tissue and predispose to disease. Differences in metabolism between individuals can contribute to the risk of developing COPD or lung cancer[86,87]. An example of a metabolic enzyme involved in both diseases is Microsomal epoxide hydrolase (EPHX1)[88,89]. Many of the early studies of antioxidant genes such as this were hampered by low power; and most have not been borne out by GWAS, but there is other biological evidence (e.g., in vitro work) delineating their role in pathogenesis, which is covered in the primary genetic papers referenced in Table 2.
| Gene | Polymorphism | COPD risk | Lung cancer risk | Ref. |
| EPHX1 | Rs2234922 (A139G) and haplotypes | ↑Asians ↔ Caucasians | ↑ | [88,89] |
| GSTM1 | Null genotype | ↑ | ↑ | [28,90] |
| SOD3 | Rs1799896 | ↑ | In vitro evidence of SOD3 ↑risk | [90-92] |
Nuclear factor erythroid 2-related factor 2 (Nrf2) is the main transcription factor that regulates phase II detoxifying antioxidant enzymes and therefore plays an important role in defence against carcinogens in smoke[93]. Nrf2 is negatively regulated by Kelch-like ECH-associated protein 1 (Keap1); mutations in either of these genes can predispose to malignancy including NSCLC[94,95]. Defective Nrf2 occurs in COPD and this may also predispose patients with COPD to lung cancer due to increased oxidative stress[96]. Oxidative stress also increases p21 expression. p21 is acyclin-dependent kinase inhibitor whose levels are raised in alveolar epithelial cells and macrophages exposed to smoke[97], and in patients with COPD and lung cancer[98]. Elevation of p21 promotes the cell cycle to move from G1 to G2/M phase resulting in hyperproliferation and carcinogenesis[99].
The balance between anti-proteinases and proteinases is an important determinant of emphysema that occurs in COPD[100]. Proteinases are also important in lung cancer development as they release growth factors TGFβ and VEGF, which can lead to tumorigenesis[19]. Proteinases common to both diseases are summarized in Table 3. A good clinical example of the importance of proteinase balance is alpha1-antitrypsin deficiency (AATD); this is a genetically determined anti-proteinase deficiency which predisposes to COPD[101]. Patients who are AATD carriers also have a 70% increased risk of developing lung cancer compared to healthy controls[102]. Drugs targeting MMPs, which are recognized in the pathogenesis of both COPD and lung cancer have been tested in early phase trials in COPD[103] and are at a more advanced stage of development in NSCLC[104].
| Signal pathway | Downstream effects | Role in COPD | Role in lung cancer | Ref. |
| NFκβ | ↑MMPs, ↑TNFα, ↓apoptosis, ↑angiogenesis | ↑inflammation | ↑cell proliferation, ↓cell death, metastasis | [111] |
| PI3K | Activation and migration of leukocytes | ↑inflammation | ↑cell proliferation, ↓cell death | [112,113] |
| P38 MAPK | Block JNK/c-Jun, ↑TNFα | ↑inflammation | Metastasis, ↓cell death | [114] |
| PPARγ | ↓MMP9, ↓TNFα, ↓TGFβ | ↓inflammation | ↑cell differentiation, ↓cell proliferation | [115,116] |
Fibrotic processes are recognized in the small airway in COPD and are thought to be driven by the TGFβ1/MMP12 pathway, as TGFβ1 levels are raised in relation to the severity of airway obstruction[105]. MMP12 is normally inhibited by the binding of αvβ6 to TGFβ resulting in TGFβ activation. αvβ6 is a transmembrane receptor of the integrin family which is present on the surface of epithelial cells and is up-regulated in lung inflammation[19]. Loss of αvβ6 helps to preserve normal lung architecture and homeostasis and if it is removed in mice airspace enlargement results, suggesting it is important in the development of emphysema as well as fibrosis[106]. TGFβ also drives epithelial cell mesenchymal transition (EMT) which is a recognized pre-malignant change capable of enhancing invasion and thus predisposing to cancer development and progression[107] (Figure 1). Fibrosis due to integrins and TGFβ is regulated by galectin 3. There are raised levels of galectin 3 in COPD lung[108] and increased levels are also associated with poor prognosis in NSCLC[109].
A number of intracellular signaling pathways, often directing processes such as inflammation, oxidative stress and protease balance, are dysregulated in both COPD and lung cancer. They are summarized in Table 3. NF-κB is particularly important due to its role in chronic inflammation. It is a transcription factor activated in inflammatory cells and in the lower airways of COPD[110] and lung cancer patients.
Parenchymal destruction in COPD may ultimately result in hypoxaemia, which may activate transcription factors and result in the expression of pro-inflammatory genes[117] (Figure 1).This leads to hypoxia-inducible factor (HIF) release, VEGF expression and angiogenesis[118]. The induction of HIF is reduced in emphysema and levels of VEGF are low in emphysematous lungs which results in low levels of angiogenesis[119]. Low VEGF levels can also cause apoptosis and airspace enlargement[120]. Conversely VEGF can be increased in chronic bronchitis[118] such that the consequences in airway predominant compared to emphysema predominant COPD might differ. Hypoxia and HIF activation can also occur in lung tumors that are increasing in size and can result in progression and metastasis of lung cancer through induction of VEGF and MMPs in an animal model[121]. Circulating VEGF is associated with a poor prognosis in operated lung cancer patients as it predicts recurrence[122].
Patients with COPD often reduce their physical activity levels due to breathlessness, and have markedly reduced activity levels compared to those without airflow obstruction[123]. Physical inactivity is associated with lung cancer incidence[124] and appears to remain so even after adjustment for smoking and other lifestyle factors[125]. The mechanism behind this association is not yet clear.
Chronic inflammation and oxidative stress are the most likely mechanistic links between COPD and lung cancer. Further analysis and elucidation of the molecular mechanisms involved in the pathogenesis of COPD and lung cancer should provide us with new treatment modalities and perhaps a key to understanding how the risk of lung cancer in COPD patients may be reduced.
P-Reviewers: Garfield D, Matteo F, Necati S- Editor: Song XX L- Editor: A E- Editor: Wang CH
| 1. | Available from: http://www.goldcopd.com. |
| 2. | Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 802] [Cited by in RCA: 872] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 3. | Kim WJ, Silverman EK, Hoffman E, Criner GJ, Mosenifar Z, Sciurba FC, Make BJ, Carey V, Estépar RS, Diaz A. CT metrics of airway disease and emphysema in severe COPD. Chest. 2009;136:396-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, Crapo RO, Jensen RL, Burney PG. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD. 2005;2:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. World Health Organization Statistics [database on the Internet]. Available from: http: //www.who.int/respiratory/copd/burden/en/index.html. |
| 6. | Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, Bray F. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 427] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 7. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13555] [Article Influence: 677.8] [Reference Citation Analysis (1)] |
| 8. | Tyczynski JE, Bray F, Parkin DM. Lung cancer in Europe in 2000: epidemiology, prevention, and early detection. Lancet Oncol. 2003;4:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 153] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Rossi A, Di Maio M, Chiodini P, Rudd RM, Okamoto H, Skarlos DV, Früh M, Qian W, Tamura T, Samantas E. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol. 2012;30:1692-1698. [PubMed] |
| 10. | Wao H, Mhaskar R, Kumar A, Miladinovic B, Djulbegovic B. Survival of patients with non-small cell lung cancer without treatment: a systematic review and meta-analysis. Syst Rev. 2013;2:10. [PubMed] |
| 11. | Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One. 2011;6:e17479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 12. | Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 870] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 13. | de Torres JP, Marín JM, Casanova C, Cote C, Carrizo S, Cordoba-Lanus E, Baz-Dávila R, Zulueta JJ, Aguirre-Jaime A, Saetta M. Lung cancer in patients with chronic obstructive pulmonary disease-- incidence and predicting factors. Am J Respir Crit Care Med. 2011;184:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 14. | Kornum JB, Sværke C, Thomsen RW, Lange P, Sørensen HT. Chronic obstructive pulmonary disease and cancer risk: a Danish nationwide cohort study. Respir Med. 2012;106:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34:380-386. [PubMed] |
| 16. | Koshiol J, Rotunno M, Consonni D, Pesatori AC, De Matteis S, Goldstein AM, Chaturvedi AK, Wacholder S, Landi MT, Lubin JH. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PLoS One. 2009;4:e7380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, Pueyo JC, Villanueva A, Lozano MD, Montes U. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132:1932-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 324] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 18. | Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax. 2005;60:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Rooney C, Sethi T. The epithelial cell and lung cancer: the link between chronic obstructive pulmonary disease and lung cancer. Respiration. 2011;81:89-104. [PubMed] |
| 20. | Brenner DR, Boffetta P, Duell EJ, Bickeböller H, Rosenberger A, McCormack V, Muscat JE, Yang P, Wichmann HE, Brueske-Hohlfeld I. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol. 2012;176:573-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Young RP, Hopkins RJ, Whittington CF, Hay BA, Epton MJ, Gamble GD. Individual and cumulative effects of GWAS susceptibility loci in lung cancer: associations after sub-phenotyping for COPD. PLoS One. 2011;6:e16476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Schwartz AG, Ruckdeschel JC. Familial lung cancer: genetic susceptibility and relationship to chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | de Andrade M, Li Y, Marks RS, Deschamps C, Scanlon PD, Olswold CL, Jiang R, Swensen SJ, Sun Z, Cunningham JM. Genetic variants associated with the risk of chronic obstructive pulmonary disease with and without lung cancer. Cancer Prev Res (Phila). 2012;5:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Bailey-Wilson JE, Amos CI, Pinney SM, Petersen GM, de Andrade M, Wiest JS, Fain P, Schwartz AG, You M, Franklin W. A major lung cancer susceptibility locus maps to chromosome 6q23-25. Am J Hum Genet. 2004;75:460-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 194] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Joost O, Wilk JB, Cupples LA, Harmon M, Shearman AM, Baldwin CT, O’Connor GT, Myers RH, Gottlieb DJ. Genetic loci influencing lung function: a genome-wide scan in the Framingham Study. Am J Respir Crit Care Med. 2002;165:795-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Hunninghake GM, Cho MH, Tesfaigzi Y, Soto-Quiros ME, Avila L, Lasky-Su J, Stidley C, Melén E, Söderhäll C, Hallberg J. MMP12, lung function, and COPD in high-risk populations. N Engl J Med. 2009;361:2599-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 27. | DeMeo DL, Celedón JC, Lange C, Reilly JJ, Chapman HA, Sylvia JS, Speizer FE, Weiss ST, Silverman EK. Genome-wide linkage of forced mid-expiratory flow in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Carlsten C, Sagoo GS, Frodsham AJ, Burke W, Higgins JP. Glutathione S-transferase M1 (GSTM1) polymorphisms and lung cancer: a literature-based systematic HuGE review and meta-analysis. Am J Epidemiol. 2008;167:759-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Wang IM, Stepaniants S, Boie Y, Mortimer JR, Kennedy B, Elliott M, Hayashi S, Loy L, Coulter S, Cervino S. Gene expression profiling in patients with chronic obstructive pulmonary disease and lung cancer. Am J Respir Crit Care Med. 2008;177:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Wang RD, Wright JL, Churg A. Transforming growth factor-beta1 drives airway remodeling in cigarette smoke-exposed tracheal explants. Am J Respir Cell Mol Biol. 2005;33:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling. Am J Respir Cell Mol Biol. 2008;38:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 32. | Lechapt-Zalcman E, Prulière-Escabasse V, Advenier D, Galiacy S, Charrière-Bertrand C, Coste A, Harf A, d’Ortho MP, Escudier E. Transforming growth factor-beta1 increases airway wound repair via MMP-2 upregulation: a new pathway for epithelial wound repair? Am J Physiol Lung Cell Mol Physiol. 2006;290:L1277-L1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Boelens MC, Gustafson AM, Postma DS, Kok K, van der Vries G, van der Vlies P, Spira A, Lenburg ME, Geerlings M, Sietsma H. A chronic obstructive pulmonary disease related signature in squamous cell lung cancer. Lung Cancer. 2011;72:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Butler MW, Fukui T, Salit J, Shaykhiev R, Mezey JG, Hackett NR, Crystal RG. Modulation of cystatin A expression in human airway epithelium related to genotype, smoking, COPD, and lung cancer. Cancer Res. 2011;71:2572-2581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, Palma J, Brody JS. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci USA. 2004;101:10143-10148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 451] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 36. | Beane J, Sebastiani P, Liu G, Brody JS, Lenburg ME, Spira A. Reversible and permanent effects of tobacco smoke exposure on airway epithelial gene expression. Genome Biol. 2007;8:R201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | Wistuba II, Lam S, Behrens C, Virmani AK, Fong KM, LeRiche J, Samet JM, Srivastava S, Minna JD, Gazdar AF. Molecular damage in the bronchial epithelium of current and former smokers. J Natl Cancer Inst. 1997;89:1366-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 315] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 38. | Steiling K, Kadar AY, Bergerat A, Flanigon J, Sridhar S, Shah V, Ahmad QR, Brody JS, Lenburg ME, Steffen M. Comparison of proteomic and transcriptomic profiles in the bronchial airway epithelium of current and never smokers. PLoS One. 2009;4:e5043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Tuder RM, Yun JH, Graham BB. Cigarette smoke triggers code red: p21CIP1/WAF1/SDI1 switches on danger responses in the lung. Am J Respir Cell Mol Biol. 2008;39:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2292] [Cited by in RCA: 2401] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 41. | Caramori G, Adcock IM, Casolari P, Ito K, Jazrawi E, Tsaprouni L, Villetti G, Civelli M, Carnini C, Chung KF. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax. 2011;66:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 42. | Popanda O, Schattenberg T, Phong CT, Butkiewicz D, Risch A, Edler L, Kayser K, Dienemann H, Schulz V, Drings P. Specific combinations of DNA repair gene variants and increased risk for non-small cell lung cancer. Carcinogenesis. 2004;25:2433-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 43. | Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, Jackson SP. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol. 2010;17:1144-1151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 513] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 44. | Vempati RK, Jayani RS, Notani D, Sengupta A, Galande S, Haldar D. p300-mediated acetylation of histone H3 lysine 56 functions in DNA damage response in mammals. J Biol Chem. 2010;285:28553-28564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 45. | Nakamaru Y, Vuppusetty C, Wada H, Milne JC, Ito M, Rossios C, Elliot M, Hogg J, Kharitonov S, Goto H. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J. 2009;23:2810-2819. [PubMed] |
| 46. | Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352:1967-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 669] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 47. | Tseng RC, Lee CC, Hsu HS, Tzao C, Wang YC. Distinct HIC1-SIRT1-p53 loop deregulation in lung squamous carcinoma and adenocarcinoma patients. Neoplasia. 2009;11:763-770. [PubMed] |
| 48. | Iskandar AR, Liu C, Smith DE, Hu KQ, Choi SW, Ausman LM, Wang XD. β-cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev Res (Phila). 2013;6:309-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2672] [Cited by in RCA: 2594] [Article Influence: 152.6] [Reference Citation Analysis (0)] |
| 50. | Brock MV, Hooker CM, Ota-Machida E, Han Y, Guo M, Ames S, Glöckner S, Piantadosi S, Gabrielson E, Pridham G. DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med. 2008;358:1118-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 441] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 51. | Palmisano WA, Divine KK, Saccomanno G, Gilliland FD, Baylin SB, Herman JG, Belinsky SA. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000;60:5954-5958. [PubMed] |
| 52. | Hu Z, Chen J, Tian T, Zhou X, Gu H, Xu L, Zeng Y, Miao R, Jin G, Ma H. Genetic variants of miRNA sequences and non-small cell lung cancer survival. J Clin Invest. 2008;118:2600-2608. [PubMed] |
| 53. | Izzotti A, Calin GA, Arrigo P, Steele VE, Croce CM, De Flora S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J. 2009;23:806-812. [PubMed] |
| 54. | Ezzie ME, Crawford M, Cho JH, Orellana R, Zhang S, Gelinas R, Batte K, Yu L, Nuovo G, Galas D. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax. 2012;67:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 55. | Markou A, Sourvinou I, Vorkas PA, Yousef GM, Lianidou E. Clinical evaluation of microRNA expression profiling in non small cell lung cancer. Lung Cancer. 2013;81:388-396. [PubMed] |
| 56. | Agustí A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, Vestbo J, Lomas DA, Calverley PM, Wouters E. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7:e37483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 590] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 57. | Babusyte A, Stravinskaite K, Jeroch J, Lötvall J, Sakalauskas R, Sitkauskiene B. Patterns of airway inflammation and MMP-12 expression in smokers and ex-smokers with COPD. Respir Res. 2007;8:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Thorleifsson SJ, Margretardottir OB, Gudmundsson G, Olafsson I, Benediktsdottir B, Janson C, Buist AS, Gislason T. Chronic airflow obstruction and markers of systemic inflammation: results from the BOLD study in Iceland. Respir Med. 2009;103:1548-1553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 59. | Dickens JA, Miller BE, Edwards LD, Silverman EK, Lomas DA, Tal-Singer R. COPD association and repeatability of blood biomarkers in the ECLIPSE cohort. Respir Res. 2011;12:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 60. | Sin DD, Leung R, Gan WQ, Man SP. Circulating surfactant protein D as a potential lung-specific biomarker of health outcomes in COPD: a pilot study. BMC Pulm Med. 2007;7:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Lomas DA, Silverman EK, Edwards LD, Locantore NW, Miller BE, Horstman DH, Tal-Singer R. Serum surfactant protein D is steroid sensitive and associated with exacerbations of COPD. Eur Respir J. 2009;34:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 62. | Celli BR, Locantore N, Yates J, Tal-Singer R, Miller BE, Bakke P, Calverley P, Coxson H, Crim C, Edwards LD. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 315] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 63. | Thomsen M, Ingebrigtsen TS, Marott JL, Dahl M, Lange P, Vestbo J, Nordestgaard BG. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309:2353-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 64. | Carter RI, Mumford RA, Treonze KM, Finke PE, Davies P, Si Q, Humes JL, Dirksen A, Piitulainen E, Ahmad A. The fibrinogen cleavage product Aα-Val360, a specific marker of neutrophil elastase activity in vivo. Thorax. 2011;66:686-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2584] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 66. | Taylor AE, Finney-Hayward TK, Quint JK, Thomas CM, Tudhope SJ, Wedzicha JA, Barnes PJ, Donnelly LE. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35:1039-1047. [PubMed] |
| 67. | van der Strate BW, Postma DS, Brandsma CA, Melgert BN, Luinge MA, Geerlings M, Hylkema MN, van den Berg A, Timens W, Kerstjens HA. Cigarette smoke-induced emphysema: A role for the B cell? Am J Respir Crit Care Med. 2006;173:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 224] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 68. | Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 403] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 69. | Sapey E, Wood AM. Auto-antibodies and inflammation. A case of the chicken and the egg? Am J Respir Crit Care Med. 2011;183:959-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18:3831-3852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 291] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 71. | Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology. 2012;143:550-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 297] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 72. | Siemes C, Visser LE, Coebergh JW, Splinter TA, Witteman JC, Uitterlinden AG, Hofman A, Pols HA, Stricker BH. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24:5216-5222. [PubMed] |
| 73. | Moghaddam SJ, Li H, Cho SN, Dishop MK, Wistuba II, Ji L, Kurie JM, Dickey BF, Demayo FJ. Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am J Respir Cell Mol Biol. 2009;40:443-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 74. | Parimon T, Chien JW, Bryson CL, McDonell MB, Udris EM, Au DH. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:712-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 75. | van den Berg RM, van Tinteren H, van Zandwijk N, Visser C, Pasic A, Kooi C, Sutedja TG, Baas P, Grünberg K, Mooi WJ. The influence of fluticasone inhalation on markers of carcinogenesis in bronchial epithelium. Am J Respir Crit Care Med. 2007;175:1061-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Bergin DA, Greene CM, Sterchi EE, Kenna C, Geraghty P, Belaaouaj A, Taggart CC, O’Neill SJ, McElvaney NG. Activation of the epidermal growth factor receptor (EGFR) by a novel metalloprotease pathway. J Biol Chem. 2008;283:31736-31744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 77. | Ganesan S, Unger BL, Comstock AT, Angel KA, Mancuso P, Martinez FJ, Sajjan US. Aberrantly activated EGFR contributes to enhanced IL-8 expression in COPD airways epithelial cells via regulation of nuclear FoxO3A. Thorax. 2013;68:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 78. | Lapperre TS, Sont JK, van Schadewijk A, Gosman MM, Postma DS, Bajema IM, Timens W, Mauad T, Hiemstra PS. Smoking cessation and bronchial epithelial remodelling in COPD: a cross-sectional study. Respir Res. 2007;8:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Chen Y, Chen P, Hanaoka M, Droma Y, Kubo K. Enhanced levels of prostaglandin E2 and matrix metalloproteinase-2 correlate with the severity of airflow limitation in stable COPD. Respirology. 2008;13:1014-1021. [PubMed] |
| 80. | Cathcart MC, Gray SG, Baird AM, Boyle E, Gately K, Kay E, Cummins R, Pidgeon GP, O’Byrne KJ. Prostacyclin synthase expression and epigenetic regulation in nonsmall cell lung cancer. Cancer. 2011;117:5121-5132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Setia S, Vaish V, Sanyal SN. Chemopreventive effects of NSAIDs as inhibitors of cyclooxygenase-2 and inducers of apoptosis in experimental lung carcinogenesis. Mol Cell Biochem. 2012;366:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1194] [Cited by in RCA: 1109] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 83. | Campa D, Zienolddiny S, Maggini V, Skaug V, Haugen A, Canzian F. Association of a common polymorphism in the cyclooxygenase 2 gene with risk of non-small cell lung cancer. Carcinogenesis. 2004;25:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 160] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 84. | Lawless MW, O’Byrne KJ, Gray SG. Oxidative stress induced lung cancer and COPD: opportunities for epigenetic therapy. J Cell Mol Med. 2009;13:2800-2821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 85. | Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 520] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 86. | DeMeo DL, Hersh CP, Hoffman EA, Litonjua AA, Lazarus R, Sparrow D, Benditt JO, Criner G, Make B, Martinez FJ. Genetic determinants of emphysema distribution in the national emphysema treatment trial. Am J Respir Crit Care Med. 2007;176:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 87. | Gresner P, Gromadzinska J, Wasowicz W. Polymorphism of selected enzymes involved in detoxification and biotransformation in relation to lung cancer. Lung Cancer. 2007;57:1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 88. | Hu G, Shi Z, Hu J, Zou G, Peng G, Ran P. Association between polymorphisms of microsomal epoxide hydrolase and COPD: results from meta-analyses. Respirology. 2008;13:837-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 89. | Liu H, Li HY, Chen HJ, Huang YJ, Zhang S, Wang J. EPHX1 A139G polymorphism and lung cancer risk: a meta-analysis. Tumour Biol. 2013;34:155-163. [PubMed] |
| 90. | Castaldi PJ, Cho MH, Cohn M, Langerman F, Moran S, Tarragona N, Moukhachen H, Venugopal R, Hasimja D, Kao E. The COPD genetic association compendium: a comprehensive online database of COPD genetic associations. Hum Mol Genet. 2010;19:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 91. | Svensk AM, Soini Y, Pääkkö P, Hiravikoski P, Kinnula VL. Differential expression of superoxide dismutases in lung cancer. Am J Clin Pathol. 2004;122:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Teoh-Fitzgerald ML, Fitzgerald MP, Jensen TJ, Futscher BW, Domann FE. Genetic and epigenetic inactivation of extracellular superoxide dismutase promotes an invasive phenotype in human lung cancer by disrupting ECM homeostasis. Mol Cancer Res. 2012;10:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 93. | Kosmider B, Messier EM, Janssen WJ, Nahreini P, Wang J, Hartshorn KL, Mason RJ. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir Res. 2012;13:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 94. | Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 860] [Cited by in RCA: 866] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 95. | Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci USA. 2008;105:13568-13573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 603] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 96. | Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 97. | Marwick JA, Kirkham P, Gilmour PS, Donaldson K, MacNEE W, Rahman I. Cigarette smoke-induced oxidative stress and TGF-beta1 increase p21waf1/cip1 expression in alveolar epithelial cells. Ann N Y Acad Sci. 2002;973:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Cebulska-Wasilewska A, Wierzewska A, Nizankowska E, Graca B, Hughes JA, Anderson D. Cytogenetic damage and ras p21 oncoprotein levels from patients with chronic obstructive pulmonary disease (COPD), untreated lung cancer and healthy controls. Mutat Res. 1999;431:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 99. | Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1195] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 100. | Stockley RA. Neutrophils and protease/antiprotease imbalance. Am J Respir Crit Care Med. 1999;160:S49-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 178] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 101. | Wood AM, Stockley RA. Alpha one antitrypsin deficiency: from gene to treatment. Respiration. 2007;74:481-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Yang P, Sun Z, Krowka MJ, Aubry MC, Bamlet WR, Wampfler JA, Thibodeau SN, Katzmann JA, Allen MS, Midthun DE. Alpha1-antitrypsin deficiency carriers, tobacco smoke, chronic obstructive pulmonary disease, and lung cancer risk. Arch Intern Med. 2008;168:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 103. | Dahl R, Titlestad I, Lindqvist A, Wielders P, Wray H, Wang M, Samuelsson V, Mo J, Holt A. Effects of an oral MMP-9 and -12 inhibitor, AZD1236, on biomarkers in moderate/severe COPD: a randomised controlled trial. Pulm Pharmacol Ther. 2012;25:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 104. | Douillard JY, Peschel C, Shepherd F, Paz-Ares L, Arnold A, Davis M, Tonato M, Smylie M, Tu D, Voi M. Randomized phase II feasibility study of combining the matrix metalloproteinase inhibitor BMS-275291 with paclitaxel plus carboplatin in advanced non-small cell lung cancer. Lung Cancer. 2004;46:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, Okada Y, Yamasawa F, Nakahara K, Umeda A. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. 2001;163:1476-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 269] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 106. | Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 380] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 107. | 109 Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 656] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 108. | Pilette C, Colinet B, Kiss R, André S, Kaltner H, Gabius HJ, Delos M, Vaerman JP, Decramer M, Sibille Y. Increased galectin-3 expression and intra-epithelial neutrophils in small airways in severe COPD. Eur Respir J. 2007;29:914-922. [PubMed] |
| 109. | Szoke T, Kayser K, Baumhakel JD, Trojan I, Furak J, Tiszlavicz L, Horvath A, Szluha K, Gabius HJ, Andre S. Prognostic significance of endogenous adhesion/growth-regulatory lectins in lung cancer. Oncology. 2005;69:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 110. | Di Stefano A, Caramori G, Oates T, Capelli A, Lusuardi M, Gnemmi I, Ioli F, Chung KF, Donner CF, Barnes PJ. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J. 2002;20:556-563. [PubMed] |
| 111. | Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 622] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 112. | Gustafson AM, Soldi R, Anderlind C, Scholand MB, Qian J, Zhang X, Cooper K, Walker D, McWilliams A, Liu G. Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci Transl Med. 2010;2:26ra25. [PubMed] |
| 113. | Popkie AP, Zeidner LC, Albrecht AM, D’Ippolito A, Eckardt S, Newsom DE, Groden J, Doble BW, Aronow B, McLaughlin KJ. Phosphatidylinositol 3-kinase (PI3K) signaling via glycogen synthase kinase-3 (Gsk-3) regulates DNA methylation of imprinted loci. J Biol Chem. 2010;285:41337-41347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 114. | Matsuo Y, Amano S, Furuya M, Namiki K, Sakurai K, Nishiyama M, Sudo T, Tatsumi K, Kuriyama T, Kimura S. Involvement of p38alpha mitogen-activated protein kinase in lung metastasis of tumor cells. J Biol Chem. 2006;281:36767-36775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 115. | Li MY, Yuan H, Ma LT, Kong AW, Hsin MK, Yip JH, Underwood MJ, Chen GG. Roles of peroxisome proliferator-activated receptor-alpha and -gamma in the development of non-small cell lung cancer. Am J Respir Cell Mol Biol. 2010;43:674-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 116. | Huang TH, Razmovski-Naumovski V, Kota BP, Lin DS, Roufogalis BD. The pathophysiological function of peroxisome proliferator-activated receptor-gamma in lung-related diseases. Respir Res. 2005;6:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 117. | Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199-208. [PubMed] |
| 118. | Siafakas NM, Antoniou KM, Tzortzaki EG. Role of angiogenesis and vascular remodeling in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2:453-462. [PubMed] |
| 119. | Michaud SE, Ménard C, Guy LG, Gennaro G, Rivard A. Inhibition of hypoxia-induced angiogenesis by cigarette smoke exposure: impairment of the HIF-1alpha/VEGF pathway. FASEB J. 2003;17:1150-1152. [PubMed] |
| 120. | Edirisinghe I, Yang SR, Yao H, Rajendrasozhan S, Caito S, Adenuga D, Wong C, Rahman A, Phipps RP, Jin ZG. VEGFR-2 inhibition augments cigarette smoke-induced oxidative stress and inflammatory responses leading to endothelial dysfunction. FASEB J. 2008;22:2297-2310. [PubMed] |
| 121. | Karoor V, Le M, Merrick D, Fagan KA, Dempsey EC, Miller YE. Alveolar hypoxia promotes murine lung tumor growth through a VEGFR-2/EGFR-dependent mechanism. Cancer Prev Res (Phila). 2012;5:1061-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 122. | Tang XP, Li J, Yu LC, Chen YC, Shi SB, Zhu LR, Chen P. Clinical significance of survivin and VEGF mRNA detection in the cell fraction of the peripheral blood in non-small cell lung cancer patients before and after surgery. Lung Cancer. 2013;81:273-279. [PubMed] |
| 123. | Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33:262-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 445] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 124. | Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 300] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 125. | Albanes D, Blair A, Taylor PR. Physical activity and risk of cancer in the NHANES I population. Am J Public Health. 1989;79:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 5.4] [Reference Citation Analysis (0)] |