Published online Feb 28, 2016. doi: 10.5319/wjo.v6.i1.19
Peer-review started: October 2, 2015
First decision: November 3, 2015
Revised: December 19, 2015
Accepted: January 16, 2016
Article in press: January 19, 2016
Published online: February 28, 2016
Processing time: 150 Days and 11.8 Hours
X-linked deafness is a rare genetic disorder causing a severe mixed hearing loss. This is due to an abnormal connection between the internal auditory meatus (IAM) and the basal turn of the cochlear leading to a “3rd window effect” and cochlear conductive hearing loss. Patients are traditionally treated with conventional hearing aids however these are often unsatisfactory. Cochlear implantation is a high-risk procedure in such cases due to the risk of inadvertent electrode placement in the IAM. We present three paediatric cases where the hearing loss was managed with a combination of a bone anchored hearing aid in combination with a conventional behind the ear hearing aid. We also present a review of the current literature regarding the management of X-linked deafness.
Core tip: X-linked deafness is a rare genetic disorder causing a severe mixed hearing loss. This is due to an abnormal connection between the internal auditory meatus (IAM) and the basal turn of the cochlear leading to a “3rd window effect” and cochlear conductive hearing loss. Patients are traditionally treated with conventional hearing aids however these are often unsatisfactory. Cochlear implantation is a high-risk procedure in such cases due to the risk of inadvertent electrode placement in the IAM.
- Citation: Kumar S, Mawby T, Sivapathasingam V, Humphries J, Ramsden J. X-linked deafness: A review of clinical and radiological findings and current management strategies. World J Otorhinolaryngol 2016; 6(1): 19-22
- URL: https://www.wjgnet.com/2218-6247/full/v6/i1/19.htm
- DOI: https://dx.doi.org/10.5319/wjo.v6.i1.19
X linked deafness (DFXN3) is a rare genetic disorder associated with a mutation on the POU3F4 gene on the Xq21 chromosome. Due to its X-linked recessive pattern of inheritance, male patients present with a severe hearing loss whilst female patients may present with normal to mild hearing loss[1]. Patient’s present with a mixed progressive hearing loss at a young age, delayed speech and subsequent educational difficulties. They are traditionally treated with conventional hearing aids.
The hearing loss associated with X-linked deafness can be explained by the well-recognised inner ear abnormalities identified. Most notably there is widening of the fundus of the internal auditory meatus (IAM) bilaterally with dilatation of the internal auditory canal. This was first described in the early 1990s across seven pedigrees of patients[2]. In addition there is also an absence of the bony partition between the fundus of the IAM and the basal turn of the cochlear. Abnormalities of the bony modioli, vestibular aqueduct and facial nerve canals have also been described with female patients displaying milder abnormalities compared with the males.
The abnormal connection between the IAM and the basal turn of cochlear acts as a 3rd window and therefore causes both a cochlear conductive loss as well as a progressive profound sensorineural hearing loss. Clinically there is also an association with stapes fixation adding to the conductive component of the hearing loss[1].
The abnormal connection between the CSF filled subararachnoid spaces and the perilymphatic space of the cochlear represents a high risk for surgery such as stapedectomy. This abnormal communication leads to an increased perimlymphatic pressure which in turn leads to perilymph gushing or a “stapes gusher” which is well documented during mobilization of the stapedial footplate[3]. It was suggested therefore that X-linked deafness was an absolute contraindication to stapes surgery due to the risk of gushing[4]. The increased perilymphatic pressure also causes progressive cochlear damage and therefore a progressive sensorineural hearing loss.
Cochlear implantation is a recognized treatment for patients with profound X-linked sensorineural deafness. However there is a risk in such patients of inadvertent electrode placement within the IAM due to the abnormal connection between the basal turn and the IAM. There are several reports of CSF leak during cochleostomy and in some cases minimal auditory benefit[5]. Repeat implantation following wrongful electrode insertion, although possible, is a difficult procedure with many risks including injury to the labarynthine artery[6] and image guided insertion may be useful tool in the future[7].
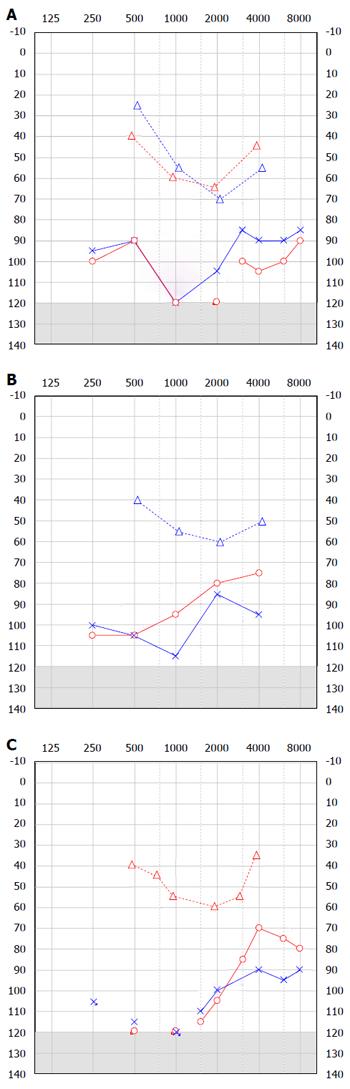
An 11-year-old boy from Bulgaria was referred for a cochlear implant assessment when he moved to the United Kingdom. He became deaf as a baby shortly after gentamicin treatment for meningitis at the age of 1. His parents declined a cochlear implant aged 3 and he subsequently learnt to sign, attended mainstream school and wore bilateral behind-the-ear (BTE) hearing aids. As he grew older his communication became more limited and he was only able to repeat 9% of the AB word list when he used both hearing aids. His audiogram is presented below (Figure 1). His air conduction threshold demonstrated a severe to profound loss whilst his bone conduction threshold confirmed a moderate hearing loss. A CT scan showed a wide connection between the IAM and the basal turn of the cochlear and genetic testing confirmed a mutation in the POU3F4 gene. The patient was fitted with a right bone anchored hearing aid (BAHA), which he wears alongside his BTE hearing aids.
TB was referred to the cochlear implant clinic aged 2 years and 10 mo. His hearing loss was identified through the newborn hearing screen and he had been managed with hearing aids. However his thresholds had deteriorated significantly over the last few months. His speech was significantly delayed. There was no family history of hearing loss. His audiogram demonstrated a down-sloping mild to moderate sensorineural loss and an up-sloping conductive loss (Figure 1). The CT scan showed an outpouching in the area of the vestibular aqueduct and a wide connection between the IAM and the basal turn of the cochlear. There was also evidence of fixation of the malleus hear to the anterior attic wall bilaterally. An examination under anaesthetic of the right ear revealed mobile ossicles and therefore TB was fitted with a left BAHA. The BAHA in combination with bilateral BTE hearing aids allowed TB’s speech and conversational language to progress.
AP is a 7-year-old boy whose hearing loss was identified aged 2 when he lived in India. He was initially treated with conventional hearing aids and a diagnosis of Mondinis malformation of the cochlear was made. Over time he was noted to have a mixed hearing loss and a drop in his low frequency air conduction thresholds (Figure 1). A review of the imaging he underwent in India as a baby has shown the typical appearances of X-linked deafness and he is currently undergoing genetic testing confirmed mutations in POU3F4 gene alongside his younger brother who also has a hearing loss. He was fitted with a BAHA to use with his conventional hearing aids.
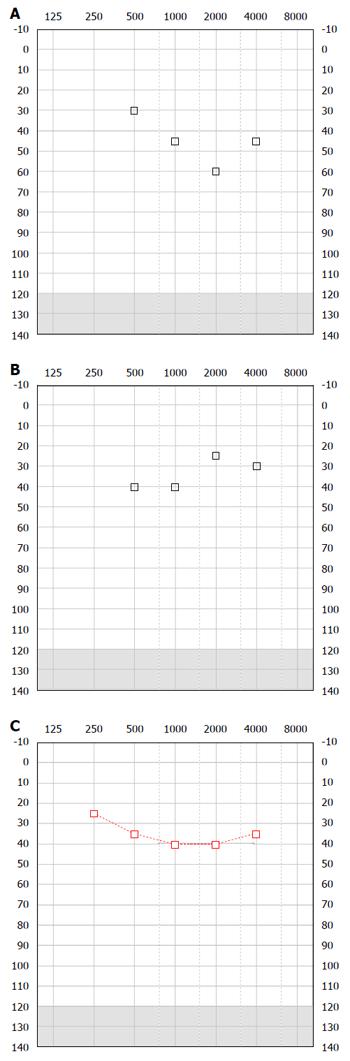
In our three cases we have successfully managed the hearing in these patients with a combination of a BAHA alongside a conventional BTE avoiding the complications of implant surgery (Figure 2). Our series of patients all demonstrated a progressive drop in low frequency air conduction thresholds. BAHAs have the advantage of aiding the low frequency thresholds where conventional air conduction aids may fail. In conjunction with a conventional aid for the high frequency loss our patients have reported good outcomes as stated by parents and school. The bone conduction thresholds in our patients were within the limits at which a BAHA is considered beneficial and even our third patients, AP, whose threshold were borderline, had some perceived auditory benefit. BAHA is a safe, quick and well-tolerated procedure and is licensed in the United Kingdom in children aged 5 and over. Those patients that are younger may use the device on a softband.
An 11-year-old boy from Bulgaria was referred for a cochlear implant assessment when he moved to the United Kingdom; AP is a 7-year-old boy whose hearing loss was identified aged 2 when he lived in India.
As he grew older his communication became more limited and he was only able to repeat 9% of the AB word list when he used both hearing aids; over time he was noted to have a mixed hearing loss and a drop in his low frequency air conduction thresholds.
The hearing loss associated with X-linked deafness can be explained by the well-recognised inner ear abnormalities identified.
All labs were within normal limits.
The computed tomography scan showed an outpouching in the area of the vestibular aqueduct and a wide connection between the internal auditory meatus and the basal turn of the cochlear.
Bone anchored hearing aids have the advantage of aiding the low frequency thresholds where conventional air conduction aids may fail. In conjunction with a conventional aid for the high frequency loss the patients have reported good outcomes as stated by parents and school.
Cochlear implantation is a recognized treatment for patients with profound X-linked sensorineural deafness.
There are several reports of CSF leak during cochleostomy[5] and in some cases minimal auditory benefit[5].
Repeat implantation following wrongful electrode insertion, although possible, is a difficult procedure with many risks including injury to the labarynthine artery[6] and image guided insertion may be useful tool in the future[7].
The paper is well written.
P- Reviewer: Abulezz T S- Editor: Qi S L- Editor: A E- Editor: Jiao XK
| 1. | de Kok YJ, van der Maarel SM, Bitner-Glindzicz M, Huber I, Monaco AP, Malcolm S, Pembrey ME, Ropers HH, Cremers FP. Association between X-linked mixed deafness and mutations in the POU domain gene POU3F4. Science. 1995;267:685-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 290] [Article Influence: 9.7] [Reference Citation Analysis (2)] |
| 2. | Phelps PD, Reardon W, Pembrey M, Bellman S, Luxom L. X-linked deafness, stapes gushers and a distinctive defect of the inner ear. Neuroradiology. 1991;33:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 137] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 3. | Olson NR, Lehman RH. Cerebrospinal fluid otorrhea and the congenitally fixed stapes. Laryngoscope. 1968;78:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Cremers CW, Huygen PL. Clinical features of female heterozygotes in the X-linked mixed deafness syndrome (with perilymphatic gusher during stapes surgery). Int J Pediatr Otorhinolaryngol. 1983;6:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Stankovic KM, Hennessey AM, Herrmann B, Mankarious LA. Cochlear implantation in children with congenital X-linked deafness due to novel mutations in POU3F4 gene. Ann Otol Rhinol Laryngol. 2010;119:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Heman-Ackah SE, Friedmann DR, Cosetti MK, Waltzman SB, Roland JT. Revision cochlear implantation following internal auditory canal insertion. Laryngoscope. 2013;123:3141-3147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Aschendorff A, Maier W, Jaekel K, Wesarg T, Arndt S, Laszig R, Voss P, Metzger M, Schulze D. Radiologically assisted navigation in cochlear implantation for X-linked deafness malformation. Cochlear Implants Int. 2009;10 Suppl 1:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (1)] |