INTRODUCTION
Vestibular evoked myogenic potential (VEMP) is an electromyographic response derived from the vestibular labyrinth evoked by sound, vibration, or electrical stimulation[1]. VEMP is a clinical test of the otolith organs, sensors of linear acceleration. The otolith organs in human are consisted of the saccule and the utricle. VEMP was first reported by Colebatch and Halmagyi in 1992[2]. Since 1992 many papers concerning VEMP have been published all over the world. At first, VEMP, which was recorded on the sternocleidomastoid muscle (SCM), was performed as a test of otolith-collic reflex[3]. Later, another method, recording around the eyes, has been also adopted as a test of otolith-ocular reflex[4,5]. The former is called cVEMP (cervical VEMP), and the latter is called oVEMP (ocular VEMP). These tests provide different information concerning the vestibular labyrinth from a caloric test and a head-impulse test (HIT)[6], which are tests of the semicircular canals. In this review I will present fundamentals concerning VEMP.
RESPONSIBILITY TO SOUND OF THE MAMMALIAN VESTIBULAR LABYRINTH
For understanding VEMP, responsiveness to sound of the mammalian vestibular labyrinth must be addressed. In 1977 Young et al[7] reported responses of the vestibular labyrinth to air-conducted sound (ACS) and bone-conducted vibration (BCV) using squirrel monkeys. They showed vestibular afferents could respond to ACS and BCV. Concerning ACS, saccular afferents showed lower thresholds than other end-organs. Didier et al[8] showed that the inferior branch of the vestibular nerve (the inferior vestibular nerve) could respond to sound stimulation using guinea pigs which had destroyed cochlea but preserved vestibular labyrinth by amikacin injection. In 1990s, McCue et al[9,10] using cats and Murofushi et al[11-13] using guinea pigs showed sound-sensitivity of the mammalian otolith organs using single-unit recording technique. McCue et al[10] reported that saccular afferents responded to sound stimulation and that best frequencies of responses were between 500 and 1000 Hz. Murofushi et al[12] showed that sound-sensitive otolith afferents could be also tilt-sensitive and were in the caudal part of the superior vestibular nerve as well as the inferior vestibular nerve. Both groups reported that irregularly firing units, which are from type I hair cells, responded to sound well. Their studies confirmed that hair cells in the saccular macula, especially type I cells could respond to sound. McCue et al[10] also suggested that preferred frequencies of the saccule were between 500 and 1000 Hz while Murofushi et al[13] suggested that the utricle might be also respond to sound. Curthoys et al[14] studied responsibilities to BCV. Their study showed that irregularly firing otolith afferents also responded to BCV well.
RECORDING METHODS AND NORMAL RESPONSES OF VEMP
cVEMP
Surface electrodes are used for recording. Active electrodes are placed on the belly of the sternocleidomastoid muscle (SCM) with reference electrodes on the lateral end of the upper sternum. The ground electrode is placed on the nasion. Nowadays 500 Hz short tone bursts (STB) (rise/fall time = 1 ms, plateau time = 2 ms, 125-130 dBSPL, ACS) are usually used as stimuli. Clicks are also applicable. The repetition rate of stimulation presentation is 5 Hz, and the time window for analysis is -20-80 ms. Signals are bandpass-filtered (20-2000 Hz) and 100 responses are averaged. BCV can be also used. Subjects must be instructed to keep contracting their SCM during recording. As methods for contraction, rotation of the neck or raising the head from the pillow is recommended.
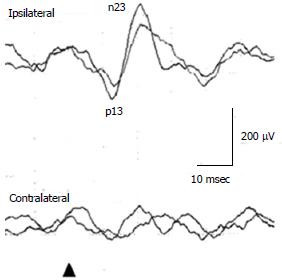
In a healthy subject, to 125 dBSPL 500 Hz ACS STB, the first positive deflection, of which the peak is around 15 ms is recorded in the ipsilateral SCM to the stimulated ear, followed by the negative deflection, of which the peak is around 23 ms (Figure 1). Conventionally, the first positive peak and the following negative peak are called p13 and n23 respectively. Absence of responses, large interaural asymmetry of p13-n23 amplitudes, prolonged peak latencies, and abnormal thresholds of responses are regarded as abnormal responses[1]. Concerning cVEMP, a published guideline should be referenced[5].
Figure 1 Cervical vestibular evoked myogenic potential waveforms in a healthy subject.
For assessment of amplitudes, correction of amplitudes using background muscle activities is desirable. For assessment of interaural asymmetry of amplitudes, percent cVEMP asymmetry has been used[15,16]. Percent cVEMP asymmetry = 100 (CAcu - CAca)/(CAcu + CAca)CAcu(a) = corrected amplitude of p13-n23 on the unaffected (affected) side. The upper limit of percent cVEMP asymmetry in our laboratory is 41.6. The above-mentioned guideline indicated 50.0 as a strict standard of the upper limit of percent cVEMP asymmetry[15]. As latencies can be affected by recording conditions, each laboratory should set their own normal range. The upper limit of p13 latency in our laboratory is 17.7 msec (125 dBSPL 500 Hz STB ACS)[16]. Thresholds lower than 95 dBSPL (500 Hz STB ACS) are definitely abnormal.
It should be taken into consideration that subjects with conduction problems in the middle ear would show absence of responses even though they had normal vestibular function. Air-bone gap more than 15dB makes recording of cVEMP to ACS useless.
oVEMP
Surface electrodes are used for recording. Active electrodes are placed just beneath the lower eye lids with reference electrodes 2 cm below active electrodes[16]. The ground electrode is placed on the nasion. STB of 500 Hz (rise/fall time = 1 ms, plateau time = 2 ms, 125-130 dBSPL, ACS) are standard stimuli. Clicks are not used because healthy subjects frequently show absence of responses[5]. Instead, BCV are used more frequently for recording oVEMP than cVEMP[17]. The repetition rate of stimulation is 5 Hz, and the time window for analysis is -20-80 ms. Signals are bandpass-filtered (20-2000 Hz) and 100 responses are averaged. Other ranges of bandpass-filter may be used (e.g., 5-500 Hz)[17]. Subjects must be instructed to keep upward gaze during recording (approx. 20 deg).
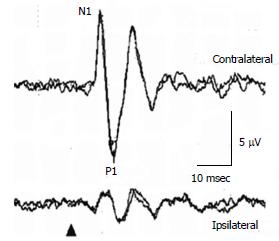
In a healthy subject, to 125 dBSPL 500 Hz ACS STB, the first negative deflection, of which the peak is around 11 msec, is recorded beneath the contralateral eye to the stimulated ear, followed by the positive deflection, of which the peak is around 15 ms (Figure 2)[16]. Conventionally, the first negative peak and the following positive peak are called N1 and P1. Absence of responses, large interaural asymmetry of N1-P1 amplitudes, prolonged peak latencies, and abnormal thresholds of responses are regarded as abnormal responses. For assessment of interaural asymmetry of amplitudes, percent oVEMP asymmetry has been used[16]. The formula is basically the same as percent cVEMP asymmetry. The upper limit of percent oVEMP asymmetry in our laboratory is 44.3. The upper limit of N1 latency in our laboratory is 13.6 msec (125 dBSPL 500 Hz STB ACS)[16]. As latencies can be affected by recording conditions, each laboratory should set their own normal range. Thresholds lower than 105 dBSPL (500 Hz STB ACS) are abnormal.
Figure 2 Ocular vestibular evoked myogenic potential waveforms in a healthy subject.
Pathways of VEMP
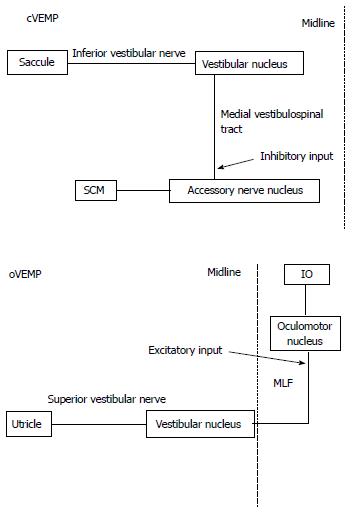
Main pathways related to VEMP are considered as follow (Figure 3). The main neural pathway of cVEMP is uncrossed in the brainstem. cVEMP mainly reflects sacculo-collic reflexes to sound stimulation. Saccular afferents project to the vestibular nucleus through the inferior vestibular nerve. Neurons in the vestibular nucleus (inhibitory) project to the motoneurons in the ipsilateral accessory nerve nucleus through the ipsilateral medial vestibulo-spinal tract[1,18].
Figure 3 Supposed neural pathway of Cervical vestibular evoked myogenic potential and ocular vestibular evoked myogenic potential.
cVEMP : Cervical vestibular evoked myogenic potential; oVEMP: Ocular vestibular evoked myogenic potential; SCM: Sternocleidomastoid muscle; IO: Inferior oblique muscle; MLF: Medial longitudinal fasciculus.
The main neural pathway of oVEMP is crossed in the brainstem[5]. oVEMP mainly reflects utriculo-ocular reflexes to sound stimulation[16,19]. Utricular afferents project to the vestibular nucleus through the superior vestibular nerve. Neurons in the vestibular nucleus (excitatory) project to the contralateral oculomotor nucleus through the contralateral medial longitudinal fasciculus (MLF)[20]. oVEMP responses are mainly from the inferior oblique muscle[21]. However, the pathway of oVEMP is still somewhat controversial.
Clinical application of VEMP
VEMP has been clinically applied to various diseases or conditions which might have abnormal findings in the vestibular system.
Vestibular neuritis
Conventional diagnostic criteria of Vestibular neuritis (VN) were as follow: (1) a single attack of acute spontaneous vertigo lasting at least several hours without accompanying auditory symptoms; (2) absence of other cranial nerve or central nervous system symptoms or signs; and (3) severe canal paresis (CP) on caloric testing (CP more than 50%)[22]. These criteria are good for detection of acute deafferentation of the superior vestibular nerve, but they cannot detect deafferentation of the inferior vestibular nerve. In VN patients diagnosed according to these conventional diagnostic criteria, cVEMPs were absent or decreased in amplitudes in one third to half of patients[1,22], while oVEMPs were abnormal in most patients[16]. Patients with absent or highly decreased caloric responses and abnormal cVEMP responses can be regarded as superior and inferior (total) VN, while patients with absent or highly decreased caloric responses but normal cVEMP responses can be regarded as superior VN with spared inferior vestibular nerve functions. This classification lead to a new clinical entity, inferior VN with spared superior vestibular nerve functions[23]. According to retrospective study by Chihara et al[23], at the period when 24 patients were diagnosed as total VN and 34 patients were diagnosed as superior VN, 13 patients were regarded as inferior VN. Patients with inferior VN showed tendency of milder and shorter symptoms than patients with total or superior VN. Clinical application of cVEMP enabled us to diagnose patients as having inferior VN. These patients, otherwise, would be left as undiagnosed.
Meniere’s disease
One of distinct features of VEMP in Meniere’s disease (MD) patients is a shift of a preferred frequency in cVEMP[24,25]. Healthy subjects show the largest amplitudes and the lowest thresholds in ACS cVEMP to stimulation of STB around 500 Hz. On the other hand, MD patients frequently showed a shift of a preferred frequency to 1000 Hz. Node et al[26] found that the preferred frequency shift was normalized by dehydration using furosemide. Probably, the frequency shift was caused by endolymphatic hydrops in the saccule. Endolymphatic hydrops in the saccule can be also detected by glycerol-VEMP test. Murofushi et al[27] found that 50% of MD patients with abnormal cVEMP prior to glycerol administration showed significant improvement of VEMP responses in 3 h after oral administration of glycerol (1.3 mg/kg body weight) on the affected side.
Benign paroxysmal positional vertigo
The whole aspects of VEMP findings in patients with Benign paroxysmal positional vertigo (BPPV) are not clarified yet. Some investigators reported unilaterally abnormal oVEMP in patients with posterior canal BPPV[28]. Abnormal oVEMPs could be decrease or augmentation of responses. Seo et al[28] assumed that reduced responses on the affected side might be from partial degeneration of the utricular hair cells and that augmented responses might be from hypermobility of stereocilia due to detachment of otoconia. On the other hand, Nakahara et al[29] reported bilaterally abnormal oVEMP in patients with posterior canal BPPV. They assumed that bilaterally abnormal (= absent) oVEMP might reflect utricular degeneration as a background of BPPV. Further study is required concerning VEMP in BPPV.
Vestibular schwannoma
Majority of patients with Vestibular schwannoma (VS) showed absent or decreased responses on the affected side, while some had prolonged latencies[30,31]. According to Ushio et al[31], sensitivity of cVEMP was 80.0%, while specificity was 52.7%. Although it is expected that combined application of cVEMP with caloric tests might be useful for prediction of the nerve origin of VS, Ushio et al[32] did not find correlation of results of these tests to the nerve origin. However, Murofushi et al[33] reported that a patient with very small VS from the inferior vestibular nerve showed abnormal cVEMP to clicks with normal cVEMP to 500 Hz ACS STB, normal caloric responses, and normal auditory brainstem responses (ABR). Study concerning prediction of the nerve origin of VS using physiological tests should be focused on cases with very small mass. Kinoshita et al[34] and Murofushi et al[35] reported that oVEMP might be also useful for diagnosis of VS. However, diagnostic values of oVEMP are remained to be clarified.
Superior canal dehiscence syndrome
Dehiscence of the bone overlying the superior (anterior) semicircular canal was first described in 1998 by Minor et al[36]. It has been reported that this condition (SCDS) manifests as various vestibular and/or auditory symptoms[36-38]. While detection of dehiscence with computed tomography scans is essential for definite diagnosis, it has been also reported that augmentation of VEMP responses, especially oVEMP to ACS is marked[37,39,40]. ACS oVEMP might be useful for screening of SCDS in dizzy patients. Zuniga et al[40] reported that an n10 (N1 in this paper) amplitude of greater than 9.3 μV and a peak-to-peak amplitude (N1-P1 in this study) of greater than 17.1 μV exhibited 100% sensitivity and specificity for SCDS.
Idiopathic otolithic vertigo
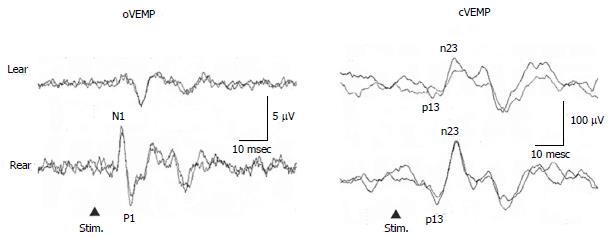
Murofushi et al[41,42] reported that some patients complained of lateral tilting sensation in the roll plane, or tilting or translational sensation in the pitch plane without rotatory vertigo. Majority of patients with these symptoms had absent or decreased responses of oVEMP and/or cVEMP (Figure 4). Patients with tilting sensation in the roll plane had tendency to show abnormal oVEMP, while patients with tilting or translational sensation in the pitch plane had tendency to show abnormal cVEMP. Murofushi et al[41,42] proposed “idiopathic otolithic vertigo” as a new clinical entity, because the otolith organs are sensors of linear acceleration and dysfunction of them could result in illusion of linear movement[43]. Abnormal VEMP findings may be essential for diagnosis of otolithic vertigo. As a next step, pathophysiology of idiopathic otolithic vertigo should be clarified.
Figure 4 Vestibular evoked myogenic potential responses of a 57-year-old man with episodic lateral tilt sensation diagnosed as having idiopathic otolithic vertigo.
He showed absent oVEMP to the left ear stimulation. cVEMP : Cervical vestibular evoked myogenic potential; oVEMP: Ocular vestibular evoked myogenic potential.
Sensorineural hearing loss
Sensorineural hearing loss itself does not affect cVEMP or oVEMP. Patients with total hearing loss showed normal responses[1,3,44,45].
VEMP is a still developing technique and new discovery is expected. I hope that many clinicians and researchers may be interested in it.