Published online Aug 28, 2013. doi: 10.5319/wjo.v3.i3.100
Revised: June 27, 2013
Accepted: July 23, 2013
Published online: August 28, 2013
Processing time: 112 Days and 14.1 Hours
AIM: To investigate a novel pharmacological intervention to mitigate cisplatin ototoxicity using a selective adenosine A1 receptor agonist adenosine amine congener (ADAC).
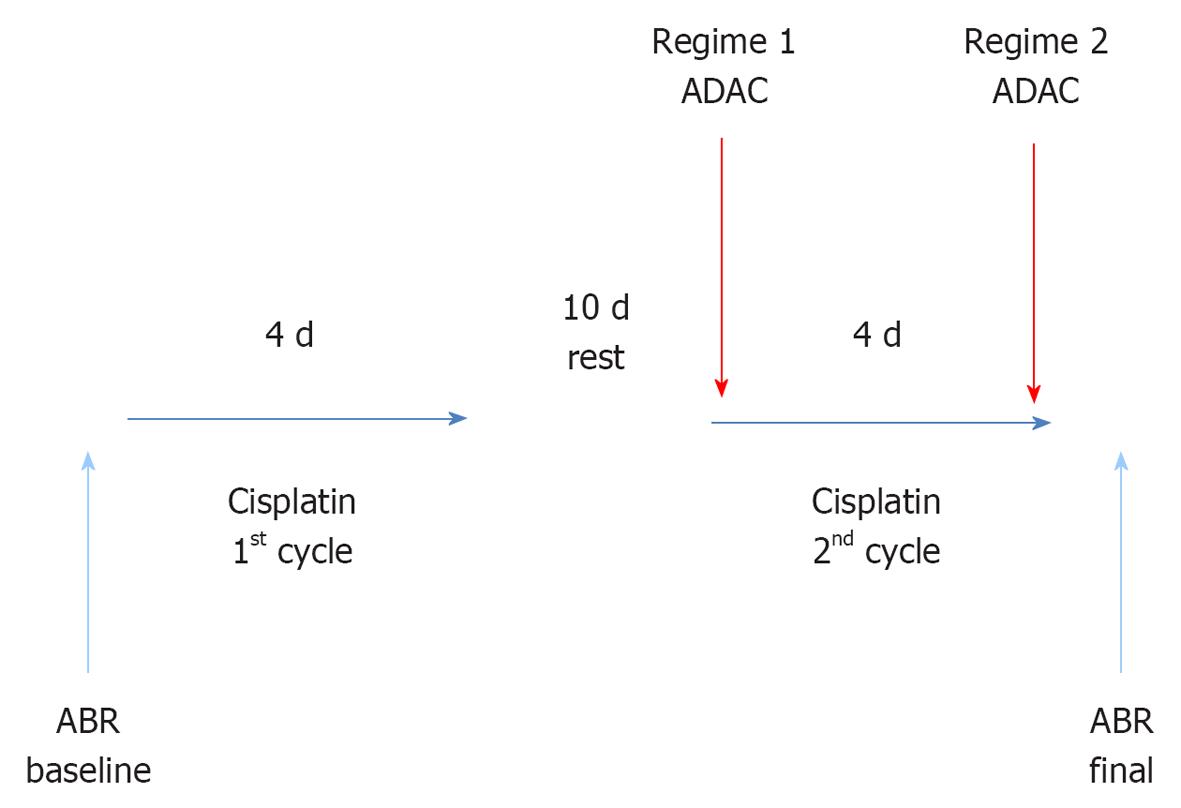
METHODS: Male Wistar rats (8-10 wk) were exposed to a two-cycle cisplatin treatment similar to clinical course of cancer chemotherapy. Each cycle comprised 4 d of intraperitoneal cisplatin injections (1 mg/kg twice daily) separated by 10 d of rest. ADAC (100 μg/kg) or drug vehicle solution (control) was administered intraperitoneally for 5 d at 24 h intervals during the second cisplatin cycle (Regime 1), or upon completion of the cisplatin treatment (Regime 2). Hearing thresholds were measured using auditory brainstem responses (ABR) before cisplatin administration (baseline) and 7 d after the end of cisplatin treatment. Histological analysis of cochlear tissues included hair cell counting and qualitative assessment of apoptosis using terminal deoxynucleotidyl transferase mediated dUTP nick end labelling (TUNEL) staining.
RESULTS: ABR threshold shifts in cisplatin-treated Wistar rats ranged from 5-29 dB across the frequency range used in the study (4-24 kHz). Higher frequencies (16-24 kHz) were mostly affected by cisplatin ototoxicity (mean threshold shift 25-29 dB). ADAC treatment during the second cisplatin cycle reduced cisplatin-induced threshold shifts by 12-16 dB (P < 0.01) at higher frequencies compared to control vehicle-treated rats. However, the treatment was ineffective if ADAC administration was delayed until after the completion of the cisplatin regime. Functional recovery was supported by increased survival of hair cells in the cochlea. Qualitative analysis using TUNEL staining demonstrated reduced apoptosis of the outer hair cells and marginal cells in the stria vascularis in animals treated with ADAC during the second cisplatin cycle.
CONCLUSION: A1 adenosine receptor agonist ADAC mitigates cisplatin-induced cochlear injury and hearing loss, however its potential interference with antineoplastic effects of cisplatin needs to be established.
Core tip: This study investigated a novel pharmacological intervention to mitigate cisplatin ototoxicity using systemic administration of a selective adenosine A1 receptor agonist adenosine amine congener (ADAC). Our study demonstrates that systemic administration of ADAC confers partial protection from cisplatin-induced ototoxicity. In rats exposed to cisplatin, ADAC ameliorated high frequency hearing loss, improved the survival of the outer hair cells and reduced apoptosis of the outer hair cells and marginal cells in the stria vascularis. This study provides support for the otoprotective role of ADAC with potential clinical benefits extending from noise-induced hearing loss to cisplatin ototoxicity.
- Citation: Gunewardene N, Guo CX, Wong AC, Thorne PR, Vlajkovic SM. Adenosine amine congener ameliorates cisplatin-induced hearing loss. World J Otorhinolaryngol 2013; 3(3): 100-107
- URL: https://www.wjgnet.com/2218-6247/full/v3/i3/100.htm
- DOI: https://dx.doi.org/10.5319/wjo.v3.i3.100
Two major classes of therapeutic agents can induce sensorineural hearing loss: aminoglycoside antibiotics and platinum-containing chemotherapy agents[1]. These drugs primarily target the outer hair cells in the basal region of the cochlea and cause high frequency sensorineural hearing loss. Oxidative stress, triggering downstream cell death signalling pathways, appears to be the common mechanism of ototoxicity[1,2].
Platinum-containing agents, such as cisplatin, carboplatin and oxaliplatin, are widely used to treat malignancies ranging from testicular, ovarian and bladder cancers to lung, head and neck malignancies[3]. These platinum complexes cause cross-linking of DNA and proteins and formation of adducts which ultimately trigger apoptosis in tumor cells[1,2]. The use of these anti-cancer drugs is limited by serious side effects, which include nephrotoxicity, neurotoxicity, gastrointestinal toxicity, leukopenia, thrombocytopenia and ototoxicity[4]. Cisplatin is considered the most ototoxic among platinum-containing agents, but it is generally more effective than carboplatin and oxaliplatin against different forms of cancer[4]. Cisplatin ototoxicity affects most patients, and is mainly manifested as tinnitus and bilateral high-frequency hearing loss, in the absence of the vestibular symptoms[4]. With prolonged cisplatin treatment, hearing loss extends to lower frequencies necessary for speech perception, and the spiral ganglion neurons degenerate concomitantly with the loss of hair cells[5]. Platinated DNA has been detected in the nuclei of the outer hair cells, marginal cells of the stria vascularis and the fibrocytes of the spiral ligament[6]. Cross-linking of DNA by cisplatin may lead to p53-mediated apoptosis of the outer hair cells and the lateral wall tissues, the spiral ligament and stria vascularis[7-9]. A reduction in the endocochlear potential, probably resulting from a dysfunctional stria vascularis, often precedes outer hair cell loss in the acute model of cisplatin ototoxicity[10].
Cisplatin reacts with the outer hair cells to form the highly reactive monohydrate complexes[1], and these complexes activate the NOX3 isoform of NADPH oxidase, which in turn generates superoxide[11-13]. This leads to formation of more toxic reactive oxygen species (ROS), such as hydroxyl radicals and peroxynitrite[2]. Excessive ROS production can overwhelm endogenous anti-oxidant mechanisms (e.g., glutathione, superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase), and activate c-Jun N-terminal kinase signalling pathways in cochlear tissues, leading to apoptosis via caspase-dependent mechanisms[1]. ROS can also activate nuclear factor κB (NF-κB), which regulates the expression of pro-inflammatory cytokines such as interleukin-1β and tumor necrosis factor-α[14] and triggers the inflammatory cascade.
Cisplatin ototoxicity can be ameliorated by various protective agents targeting oxidative stress, inflammation and apoptosis[15]. Enhancing the endogenous antioxidant system of the cochlea and administration of free radical scavengers has been the main approach to reduce ototoxic effects of cisplatin[2,16]. Systemic antioxidant administration, however, can interfere with the anti-tumour activity of cisplatin, whilst local (intratympanic) administration is an invasive procedure with its own limitations[2,16]. There is no ideal protective agent for clinical use at present, and finding safe and effective treatments for cisplatin ototoxicity would significantly improve the quality of life of many cancer sufferers. In this study, we investigated a novel pharmacological intervention to mitigate cisplatin ototoxicity using systemic administration of a selective adenosine A1 receptor agonist adenosine amine congener (ADAC).
It has been shown that cisplatin treatment induces a fivefold increase in adenosine A1 receptor expression in the chinchilla cochlea[17], suggesting a potential role of these receptors in cochlear response to cisplatin. Other studies have reported that the local administration of adenosine A1 receptor agonists R-phenylisopropyladenosine (R-PIA) or 2-chloro-N6-cyclopentyladenosine (CCPA) to the round window membrane of the cochlea reduces cisplatin-induced auditory threshold shifts[18]. The capacity of A1 receptor agonists to protect the cochlea from cisplatin opened a new realm of therapeutic strategies to combat cisplatin ototoxicity.
Systemic administration of A1 receptor agonists is generally limited by their cardiovascular side effects[19]. However, a selective A1 receptor agonist ADAC is characterized by reduced cardiovascular side effects (bradycardia, hypotension and hypothermia) compared to other drugs acting on adenosine A1 receptors[20,21]. This suggests that ADAC can be administered systemically, avoiding the surgical procedures required to deliver drugs to the inner ear. Our previous studies have shown that ADAC can attenuate noise-induced hearing loss and ameliorate cochlear injury in instances of acute and extended noise exposure[22]. The improvement of hearing thresholds was supported by increased survival of sensory hair cells and reduced expression of oxidative stress markers in the cochlea. Here, we demonstrate that ADAC ameliorates cochlear injury and partially prevents cisplatin-induced hearing loss, providing further support for the role of A1 receptors in cochlear protection from ototoxic anti-cancer drugs.
Male Wistar rats (8-10 wk) were used in this study. Animals with pre-existing hearing loss or abnormalities in the external or middle ear were excluded from the study. All procedures complied with international guidelines for the ethical use of animals and were approved by the University of Auckland Animal Ethics Committee.
A two-cycle cisplatin treatment resembling a clinical course of chemotherapy was used in this study. This model has a low mortality rate, and provides an excellent tool to study cisplatin ototoxicity and its prevention[23]. Each cycle consists of 4 d of cisplatin injections (1 mg/kg ip twice daily) separated by 10 d of rest. Each rat received a total of 16 mg/kg of cisplatin. Cisplatin (Sigma Aldrich) was dissolved in saline (0.5 mg/mL), aliquoted and stored at -20 °C. Cisplatin aliquots were heated in a 37 °C water bath before administration.
ADAC treatment was initiated concomitantly with the second cycle of cisplatin administration (Regime 1) or immediately after completion of cisplatin administration (Regime 2) (Figure 1). In both studies, ADAC was given as five daily injections (100 μg/kg per day ip) at 24 h intervals. This is the same dosing schedule that was previously used to mitigate noise-induced hearing loss[22]. In the control group, injections of the drug vehicle (200 μL/100 g per day ip) were administered at the same intervals as ADAC. ADAC (Sigma-Aldrich) was dissolved in 1 mol/L HCl and then in 0.1 mol/L phosphate buffered saline (PBS; pH 7.4) to prepare a 50 μg/mL stock solution, as described previously[22]. The stock solution was then aliquoted and stored at -20 °C. Light-protected ADAC aliquots were heated in a 37 °C water bath for 30 min before administration.
Auditory brainstem responses (ABR) were measured before cisplatin administration (baseline) and 7 d after the end of cisplatin treatment. The acoustic stimuli for ABR were produced and the responses recorded using a digital signal processing package and associated BioSig software (Tucker Davis Technologies, Alachua, FL, United States). ABR measurements were conducted in a double walled sound attenuating chamber (Shelburg Acoustics, Pty Ltd., Croydon North, Australia). The rats were anesthetized with a mixture of ketamine (75 mg/kg) and xylazine (10 mg/kg), and placed on a heating pad to maintain body temperature at 37 °C. To obtain ABR responses, fine Grass F-E3 stainless steel electrodes were placed subdermally at the vertex (reference), at the mastoid region of the ear of interest (active electrode) and the ground electrode was inserted at the mastoid region of the opposite ear. The electrodes were attached to a TDT Bioamp head stage and amplified 100000 times in a TDT DB4 amplifier. Sound stimuli were supplied via a DT 48 Beyerdynamic transducer connected to a 10 cm plastic tubing placed into the external auditory canal of the animal’s ear. Rats were tested by applying a series of tone pips (5 ms duration, 1.5 ms rise and fall times) at varying intensities to determine the auditory threshold at the set frequency (4-24 kHz). The threshold of the ABR complex (waves i-v) were determined by progressively attenuating the sound intensity in 5 dB steps until the wave i-v complex of the averaged ABR waveforms was no longer distinguishable from noise floor in recorded traces. The ABR threshold was defined as the lowest intensity (to the nearest 5 dB) at which a response could be visually detected above the noise floor. Repeat waveforms were analysed at each frequency to determine the consistency of the responses and to identify the recurring peaks.
After the last ABR measurement, rats from the first ADAC treatment group (Regime 1, see Figure 1) were euthanised with an overdose of anaesthetic (Pentobarbitone, 100 mg/kg ip) and cochleae removed for histological analysis. After the overnight fixation in 4% paraformaldehyde (PFA), the cochleae were decapsulated and the organ of Corti removed. The surface preparation of the organ of Corti was separated into the apical, middle and basal turns, and the tissues were permeabilized with 1% Triton-X 100 for 1 h. Alexa Fluor 488 phalloidin (Invitrogen) dissolved in 0.1 mol/L PBS (pH 7.4) was used to stain F-actin in the hair cells and their stereocilia. Tissues were incubated in 1% phalloidin (2 U/mL) for 40 min, washed with PBS for 30 min, and mounted onto glass slides using Citifluor AF1 antifading mounting medium (Agar Scientific, London, United Kingdom). The slides were visualised using a Zeiss epifluorescence microscope equipped with an Axiocam camera and Axiovision v3.1 software. Images were taken for the entire length of the cochlea, and the number of missing hair cells was counted for each turn and presented as a percentage of total number of hair cells in that turn.
Apoptosis in the cisplatin-treated rat cochleae was identified by terminal deoxynucleotidyl transferase (TdT) mediated dUTP nick end labelling (TUNEL) using a commercial In Situ Cell Death Detection Kit (Roche Diagnostics GmbH, Mannheim, Germany). After fixation with 4% PFA, decalcification in 5% EDTA solution for 7 d and overnight cryoprotection in 30% sucrose, the cochleae were embedded in Tissue-Tek optimal cutting temperature compound (OCT, Miles Laboratories, Elkhart, IN, United States), snap-frozen in isopentane, and stored at -80 °C. Mid-modiolar cochlear cryosections (30 μm) from ADAC- and vehicle-treated rats were permeabilised with 1% Triton X-100 and blocked with 5% normal goat serum in PBS for 1 h. The sections were washed and incubated with the TUNEL reaction mixture (fluorescein nucleotide label solution and TdT enzyme solution at 1:10 dilution) for 2 h at 37 °C in a dark humidified chamber. Negative controls were incubated with the label solution only. The sections were rinsed several times in PBS, mounted in Citifluor, and visualised using a laser scanning confocal microscope (FluoView™ FV1000, Olympus) and processed with Olympus FluoView v.1.6a software. The cochleae obtained from the ADAC treatment Regime 1 were analysed and the images representative of at least five individual experiments are shown.
Results were presented as the mean ± SE (n = 8 per group). The comparison of ABR thresholds was performed using one-way analysis of variance followed by a Holm-Sidak pairwise multiple comparison and hair cell loss was analysed using a Student’s unpaired t-test assuming unequal variances. The α level was set at 0.05.
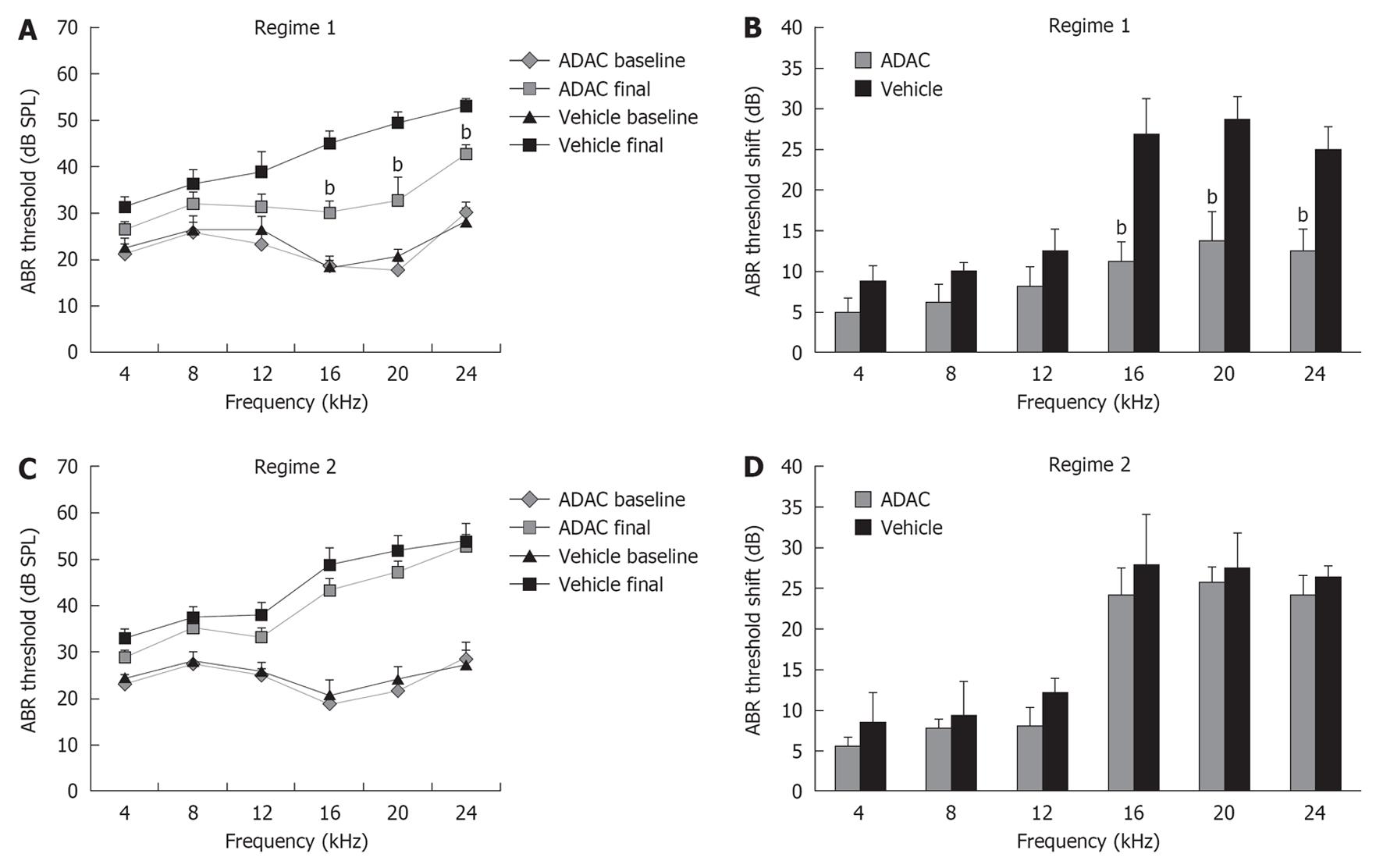
ABR were used in this study as a standard functional method of assessing cochlear function in animals. ABR thresholds were measured prior to the start of the first cisplatin cycle and 7 d after the completion of cisplatin treatment, and the threshold shift was calculated for each animal as a difference between these two measurements. Baseline ABR thresholds before cisplatin administration were similar in all groups of animals (Figure 2A and C). Intraperitoneal administration of cisplatin caused significant elevation of ABR thresholds in all animals (Figure 2), which was more substantial at higher frequencies (16-24 kHz; mean threshold shift 25-29 dB). ADAC treatment during the second cycle of cisplatin (Regime 1) reduced ABR threshold shifts by 12-16 dB at higher frequencies (P < 0.01) compared to control vehicle-treated rats (Figure 2B). However, when ADAC treatment was delayed until after the completion of both cisplatin cycles (Regime 2), there was no improvement of ABR thresholds (Figure 2D).
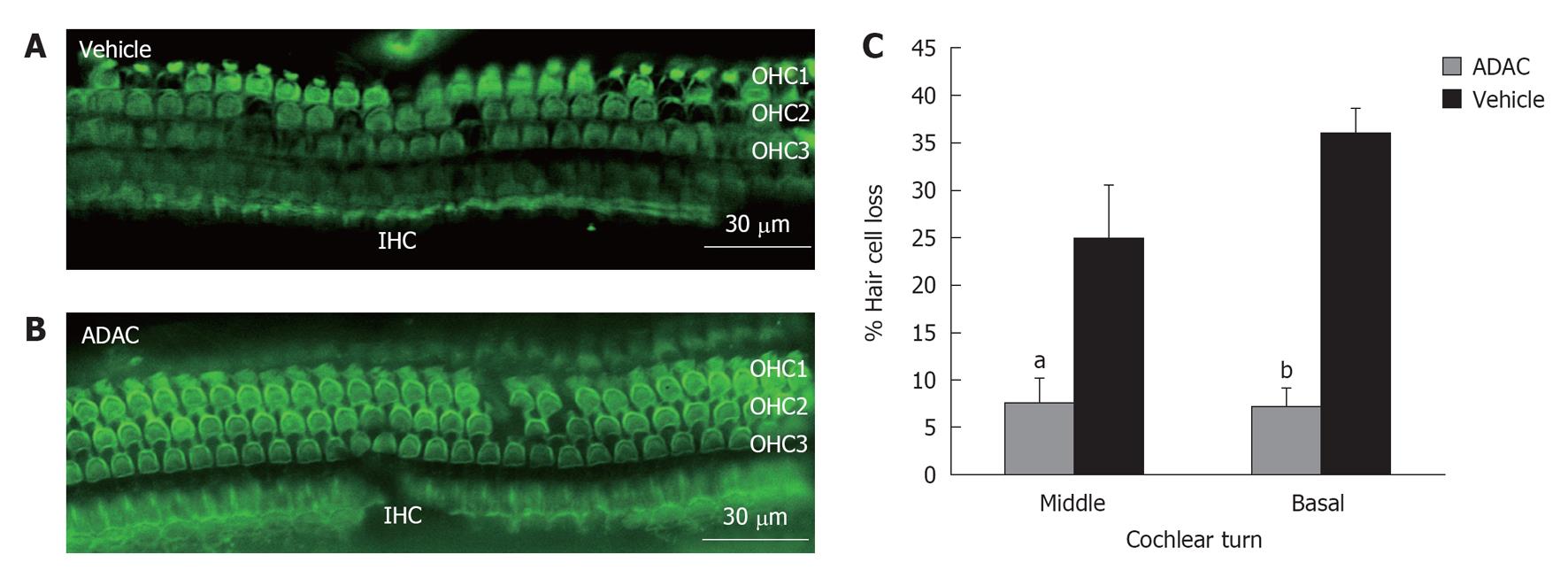
To determine the extent of hair cell loss with ADAC treatment during the second cycle of cisplatin administration (Regime 1), the outer hair cells were counted in the basal, middle and apical turns of the cochlea. Figure 3A is a representative image of the surface preparation of the middle cochlear turn showing cisplatin-induced loss of the outer hair cells in the vehicle-treated cochlea. The inner hair cells were mostly unaffected by exposure to cisplatin. The survival of the outer hair cells was improved in the ADAC-treated cochlea (Figure 3B), suggesting a cytoprotective effect of this compound. Quantitative assessment of the cisplatin-induced hair cell loss in the vehicle-treated cochleae (Figure 3C) demonstrated a high percentage of missing hair cells in the basal and middle turns (36% and 25%, respectively), whilst the hair cell loss in the apical turn was less than 5% (data not shown). ADAC treatment during the second cisplatin cycle significantly (P < 0.05 for the middle turn and P < 0.001 for the basal turn) reduced hair cell loss in the basal and middle turns of the cochlea (Figure 3C).
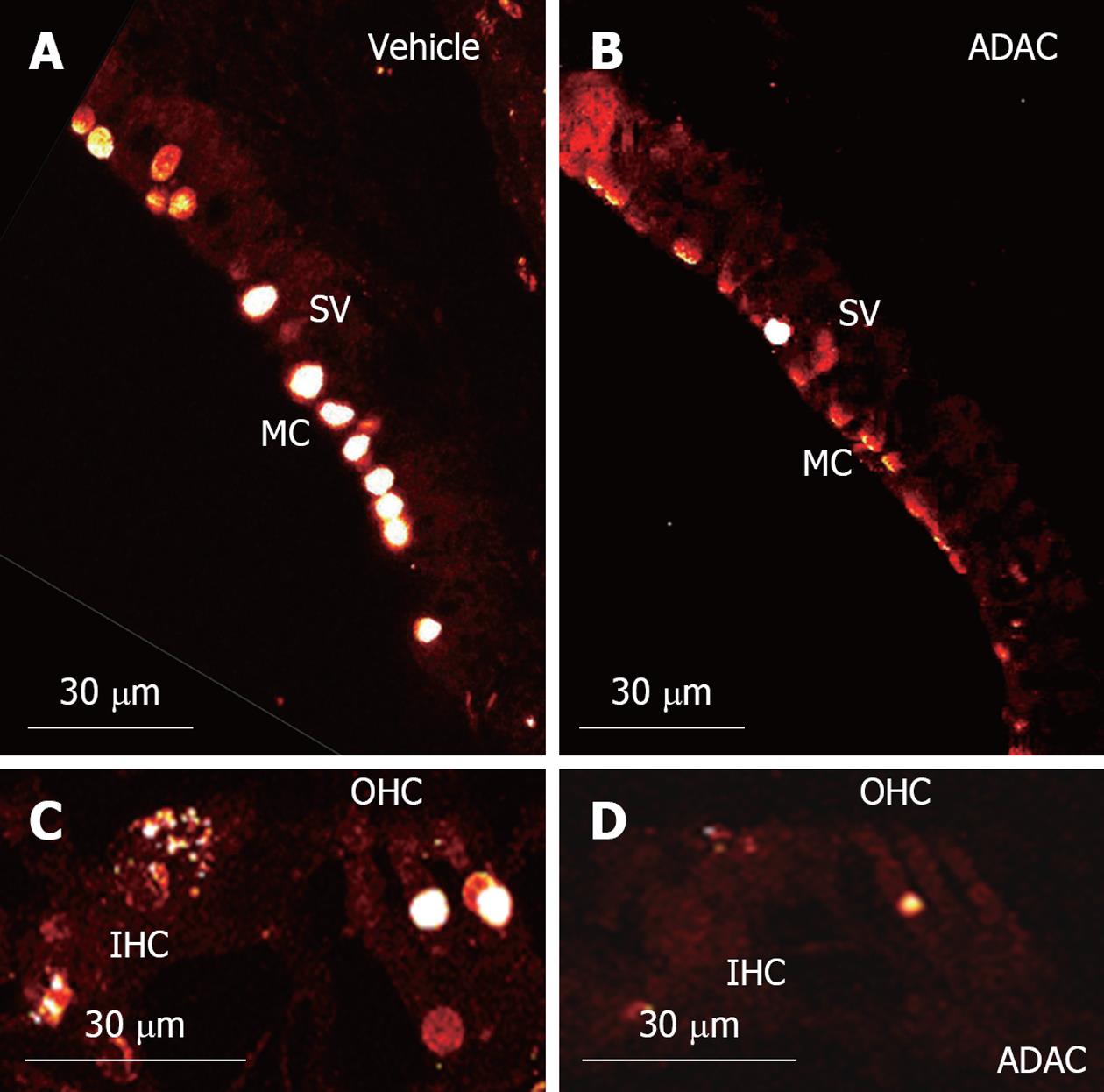
TUNEL staining is a technique used for detection of apoptosis at a single cell level, based on labeling of fragmented DNA in the nuclei of apoptotic cells. In this study, TUNEL staining was used for qualitative assessment of apoptosis in the midmodiolar cross-sections of the cisplatin-treated cochlea (Figure 4). This study was performed on cochlear tissues of animals treated with ADAC or vehicle solution during the second cycle of cisplatin treatment (Regime 1). TUNEL staining was mostly limited to the marginal cells of the stria vascularis and the outer hair cells, whilst the inner hair cells were occasionally positive for TUNEL staining (Figure 4A and C). As expected, the number of apoptotic cells was the highest in the basal turn, slightly lower in the middle turn and minimal in the apical turn. In the basal and middle turns, we mostly observed 1 out of 3 or 2 out of 3 TUNEL-positive outer hair cells, whilst in the apical turn TUNEL-positive cells were observed only occasionally. Supporting cells were mostly unaffected except in the basal turn, where some TUNEL-positive Deiters’ cells were observed. Figure 4C shows two TUNEL-positive outer hair cells and an inner hair cell in an advanced stage of apoptosis, judged by diffuse TUNEL staining which correlates with disrupted chromatin and cellular disintegration. In all turns, however, there were more advanced stage outer hair cells than inner hair cells, and in extreme cases complete disintegration of the outer hair cells was observed. TUNEL staining in the spiral ganglion was limited to satellite cells, whilst the neurons appeared unstained (data not shown). ADAC treatment during the second cycle of cisplatin regime consistently decreased TUNEL staining in the stria vascularis and the organ of Corti in the basal and middle turns of the cochlea (Figure 4B and D).
Our study demonstrates that systemic administration of ADAC, a selective A1 adenosine receptor agonist, confers partial protection from cisplatin-induced ototoxicity. In rats exposed to cisplatin, ADAC ameliorated high frequency hearing loss and improved the survival of the outer hair cells. As a qualitative outcome, ADAC treatment reduced apoptosis of the outer hair cells and marginal cells in the stria vascularis.
In this study we have taken advantage of the two-cycle model of cisplatin treatment, which mimics the clinical course of chemotherapy[23] and avoids a high mortality rate known to exist in other animal models of cisplatin toxicity[24,25]. A previous study using the two-cycle model[23] established that hearing loss and the loss of outer hair cells occurs after the second cycle of cisplatin injections, suggesting that this period should be therapeutically targeted to reduce cisplatin ototoxicity. Indeed, ADAC was most effective when administered concomitantly with cisplatin during the second cycle. After the completion of cisplatin treatment, the ototoxic effects of cisplatin were irreversible and the hearing loss was permanent. These results suggest that the role of ADAC is to facilitate the recovery process of the hair cells and other cochlear tissues at early stages of cochlear injury.
Previous studies have shown that apoptotic cell death is the main mechanism of cisplatin ototoxicity[2,4]. In the present study, ADAC reduced the loss of outer hair cells and apoptosis in the organ of Corti and stria vascularis, cochlear tissues particularly vulnerable to cisplatin ototoxicity[2,26]. Our results thus suggest that ADAC improves the survival of sensory and secretory tissues critical for normal cochlear functioning.
It is possible that ADAC targets multiple mechanisms of cochlear injury, reducing oxidative stress and apoptosis upon stimulation of adenosine A1 receptors. It has been established that A1 receptors exert a strong cytoprotective role in the cochlea[18,22,27-29], most likely by inducing the activation of antioxidant enzymes[30,31]. A1 adenosine receptors are up-regulated after local cisplatin administration to the cochlea, and this is considered to be a compensatory mechanism to counter excessive ROS production[17]. Local administration of R-PIA (A1 adenosine receptor agonist) to the round window membrane can increase the production of antioxidant enzymes superoxide dismutase and glutathione peroxidase and significantly reduce the levels of malondialdehyde, a marker of lipid peroxidation[32]. In addition, selective A1 adenosine receptor agonists, such as CCPA and ADAC, reduce the production of a toxic metabolite nitrotyrosine (marker of oxidative stress) in the noise-exposed cochlea[22,29], providing further support for antioxidant actions of adenosine A1 receptor agonists. Anti-apoptotic activity of A1 receptors has also been established. A1 receptors are positively coupled to extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases[33], known to mediate pro-survival signals in tissues[34]. In addition, the increased expression of adenosine receptors in response to oxidative stress is regulated by transcription factors NF-κB and activator protein 1, which facilitate cell survival in tissues exposed to oxidative stress[35].
A1 adenosine receptors have been previously shown to reduce cisplatin-induced auditory threshold shifts after local administration of adenosine A1 receptor agonists, such as R-PIA or CCPA, onto the round window membrane of the cochlea[18]. The potential advantage of ADAC in comparison with other adenosine A1 receptor agonists is the possibility of systemic administration due to reduced cardiovascular effects[20-22]. However, translation from animal models to clinical practice is essential to assess the effectiveness of ADAC. Further studies are required to assess potential side effects, optimal dose and route of drug administration, and bioavailability. Another important caveat is to establish whether systemic administration of ADAC interferes with anti-cancer effects of cisplatin. Nevertheless, this study provides further support for the otoprotective role of ADAC with potential clinical benefits extending from noise-induced hearing loss to cisplatin ototoxicity.
Cisplatin is one of the most commonly used chemotherapeutic agents highly effective in treatment of various malignancies. The principal dose-limiting side effects of cisplatin include ototoxicity, neurotoxicity and nephrotoxicity. Cisplatin ototoxicity affects most patients, and is manifested as tinnitus and bilateral high-frequency hearing loss.
Cisplatin ototoxicity can be ameliorated by various protective agents targeting oxidative stress, inflammation and apoptosis. There is no ideal protective agent for clinical use at present, and finding safe and effective treatments for cisplatin ototoxicity would significantly improve the quality of life of many cancer sufferers.
Cisplatin-induced cochlear injury and hearing loss can be reduced after local administration of adenosine A1 receptor agonists. The advantage of adenosine amine congener (ADAC) in comparison with other adenosine A1 receptor agonists is the possibility of systemic (e.g., oral) administration due to reduced cardiovascular effects of ADAC at the therapeutic dose.
This study may lead to therapeutic management of ototoxic side effects in patients receiving cisplatin anti-cancer therapy. An important caveat is to establish whether systemic administration of ADAC interferes with anti-cancer effects of cisplatin.
ADAC is a selective A1 adenosine receptor agonist. Adenosine acts as a cytoprotective substance released from tissues in response to stress. Released adenosine may account for tissue protection and regeneration in a range of tissues via adenosine A1 receptors.
The authors present compelling evidence for the ability of ADAC to reduce loss of hearing function and loss of cochlear hair cells induced by cisplatin in Wistar rats, a recognized model of cisplatin ototoxicity. These data are of particular interest due to the possibility of systemically treating humans undergoing cisplatin cancer therapy with ADAC, which has reduced cardiovascular side effects compared to other adenosine agonists.
P- Reviewers Coling D, Gouveris H, Nakashima T S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72:931-935. [PubMed] |
| 2. | Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157-167. [PubMed] |
| 3. | Boulikas T, Vougiouka M. Recent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs (review). Oncol Rep. 2004;11:559-595. [PubMed] |
| 4. | Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec (Hoboken). 2012;295:1837-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 5. | van Ruijven MW, de Groot JC, Klis SF, Smoorenburg GF. The cochlear targets of cisplatin: an electrophysiological and morphological time-sequence study. Hear Res. 2005;205:241-248. [PubMed] |
| 6. | van Ruijven MW, de Groot JC, Hendriksen F, Smoorenburg GF. Immunohistochemical detection of platinated DNA in the cochlea of cisplatin-treated guinea pigs. Hear Res. 2005;203:112-121. [PubMed] |
| 7. | Devarajan P, Savoca M, Castaneda MP, Park MS, Esteban-Cruciani N, Kalinec G, Kalinec F. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hear Res. 2002;174:45-54. [PubMed] |
| 8. | Zhang M, Liu W, Ding D, Salvi R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience. 2003;120:191-205. [PubMed] |
| 9. | Jamesdaniel S, Ding D, Kermany MH, Davidson BA, Knight PR, Salvi R, Coling DE. Proteomic analysis of the balance between survival and cell death responses in cisplatin-mediated ototoxicity. J Proteome Res. 2008;7:3516-3524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Tsukasaki N, Whitworth CA, Rybak LP. Acute changes in cochlear potentials due to cisplatin. Hear Res. 2000;149:189-198. [PubMed] |
| 11. | Mukherjea D, Whitworth CA, Nandish S, Dunaway GA, Rybak LP, Ramkumar V. Expression of the kidney injury molecule 1 in the rat cochlea and induction by cisplatin. Neuroscience. 2006;139:733-740. [PubMed] |
| 12. | Mukherjea D, Jajoo S, Kaur T, Sheehan KE, Ramkumar V, Rybak LP. Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid Redox Signal. 2010;13:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB, Park C, Park BH, Lee HK, Chung SY. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J Neurosci. 2010;30:3933-3946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 14. | Chung WH, Boo SH, Chung MK, Lee HS, Cho YS, Hong SH. Proapoptotic effects of NF-kappaB on cisplatin-induced cell death in auditory cell line. Acta Otolaryngol. 2008;128:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Waissbluth S, Daniel SJ. Cisplatin-induced ototoxicity: transporters playing a role in cisplatin toxicity. Hear Res. 2013;299:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Rybak LP, Mukherjea D, Jajoo S, Ramkumar V. Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J Exp Med. 2009;219:177-186. [PubMed] |
| 17. | Ford MS, Nie Z, Whitworth C, Rybak LP, Ramkumar V. Up-regulation of adenosine receptors in the cochlea by cisplatin. Hear Res. 1997;111:143-152. [PubMed] |
| 18. | Whitworth CA, Ramkumar V, Jones B, Tsukasaki N, Rybak LP. Protection against cisplatin ototoxicity by adenosine agonists. Biochem Pharmacol. 2004;67:1801-1807. [PubMed] |
| 19. | Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247-264. [PubMed] |
| 20. | Von Lubitz DK, Lin RC, Paul IA, Beenhakker M, Boyd M, Bischofberger N, Jacobson KA. Postischemic administration of adenosine amine congener (ADAC): analysis of recovery in gerbils. Eur J Pharmacol. 1996;316:171-179. [PubMed] |
| 21. | Von Lubitz DK, Lin RC, Bischofberger N, Beenhakker M, Boyd M, Lipartowska R, Jacobson KA. Protection against ischemic damage by adenosine amine congener, a potent and selective adenosine A1 receptor agonist. Eur J Pharmacol. 1999;369:313-317. [PubMed] |
| 22. | Vlajkovic SM, Lee KH, Wong AC, Guo CX, Gupta R, Housley GD, Thorne PR. Adenosine amine congener mitigates noise-induced cochlear injury. Purinergic Signal. 2010;6:273-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Minami SB, Sha SH, Schacht J. Antioxidant protection in a new animal model of cisplatin-induced ototoxicity. Hear Res. 2004;198:137-143. [PubMed] |
| 24. | Campbell KC, Rybak LP, Meech RP, Hughes L. D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res. 1996;102:90-98. [PubMed] |
| 25. | Li G, Sha SH, Zotova E, Arezzo J, Van de Water T, Schacht J. Salicylate protects hearing and kidney function from cisplatin toxicity without compromising its oncolytic action. Lab Invest. 2002;82:585-596. [PubMed] |
| 26. | Thomas JP, Lautermann J, Liedert B, Seiler F, Thomale J. High accumulation of platinum-DNA adducts in strial marginal cells of the cochlea is an early event in cisplatin but not carboplatin ototoxicity. Mol Pharmacol. 2006;70:23-29. [PubMed] |
| 27. | Hu BH, Zheng XY, McFadden SL, Kopke RD, Henderson D. R-phenylisopropyladenosine attenuates noise-induced hearing loss in the chinchilla. Hear Res. 1997;113:198-206. [PubMed] |
| 28. | Vlajkovic SM, Housley GD, Thorne PR. Adenosine and the auditory system. Curr Neuropharmacol. 2009;7:246-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Wong AC, Guo CX, Gupta R, Housley GD, Thorne PR, Vlajkovic SM. Post exposure administration of A(1) adenosine receptor agonists attenuates noise-induced hearing loss. Hear Res. 2010;260:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Maggirwar SB, Dhanraj DN, Somani SM, Ramkumar V. Adenosine acts as an endogenous activator of the cellular antioxidant defense system. Biochem Biophys Res Commun. 1994;201:508-515. [PubMed] |
| 31. | Ramkumar V, Nie Z, Rybak LP, Maggirwar SB. Adenosine, antioxidant enzymes and cytoprotection. Trends Pharmacol Sci. 1995;16:283-285. [PubMed] |
| 32. | Ford MS, Maggirwar SB, Rybak LP, Whitworth C, Ramkumar V. Expression and function of adenosine receptors in the chinchilla cochlea. Hear Res. 1997;105:130-140. [PubMed] |
| 33. | Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527-552. [PubMed] |
| 34. | Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905-2927. [PubMed] |
| 35. | Ramkumar V, Jhaveri KA, Xie X, Jajoo S, Toth LA. Nuclear Factor κB and Adenosine Receptors: Biochemical and Behavioral Profiling. Curr Neuropharmacol. 2011;9:342-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |