Published online Feb 28, 2012. doi: 10.5319/wjo.v2.i1.1
Revised: November 2, 2011
Accepted: February 27, 2012
Published online: February 28, 2012
AIM: To study the effects of adeno-associated virus (AAV) delivered short hairpin RNAs (shRNAs) on adult CD-1 mouse cochlea damaged by aminoglycoside antibiotic kanamycin.
METHODS: Three different shRNAs were designed (p27 Kip1, p53 and p27 Kip1+p53) and tested in COS cells. A total of 20 adult CD-1 mice were used in the experiment. Mice were divided into five different groups (four animals/group) depending on the AAV-shRNA construct they received and whether they received kanamycin or not. Saline and AAV-EGFP injected animals were used as controls. All constructs were injected through the round window membrane (RWM) into the cochlea. Cochleae were harvested after 1 mo. Apoptosis was detected with Tunel labeling from paraffin-embedded cochlear tissue sections.
RESULTS: AAV2/2-p27 Kip1-shRNA and AAV2/2-p53-shRNA were tested in COS cells. Western blotting analysis confirmed that both constructs silenced their target genes effectively in the cell culture. AAV2/2-shRNA constructs were injected into the cochlea of CD-1 mice through the intact RWM. Cotransduction of individual AAV2/2-shRNAs with AAV2/2-EGFP resulted in EGFP expression in the organ of Corti. Kanamycin treatment had no effect on the expression pattern of the EGFP. AAV2/2-shRNA treated mice (either with p53 or p27Kip1and p53 together) showed fewer apoptotic hair cells in the cochlea than the control group (P < 0.05; AAV2/2-p53-shRNA vs saline P = 0.00014; AAV2/2-p27+p53-shRNA vs saline P = 0.0011). AAV2/2-p27-shRNA injected cochleae showed no significant difference in the number of apoptotic cells when compared to the saline injected cochleae.
CONCLUSION: Silencing of p53 protein in the kanamycin treated ears may decrease cell death in the organ of Corti.
- Citation: Pietola L, Jero J, Jalkanen R, Kinnari TJ, Jero O, Frilander M, Pajusola K, Salminen M, Aarnisalo AA. Effects of p27Kip1- and p53- shRNAs on kanamycin damaged mouse cochlea. World J Otorhinolaryngol 2012; 2(1): 1-7
- URL: https://www.wjgnet.com/2218-6247/full/v2/i1/1.htm
- DOI: https://dx.doi.org/10.5319/wjo.v2.i1.1
Specific genes can be efficiently silenced with RNA interference (RNAi). Fire et al[1] showed in 1998 that double-stranded RNA (dsRNA) can be used in post-transcriptional gene silencing in animals. They also noticed that dsRNA was more effective at producing gene silencing when compared to single-stranded antisense or sense RNA. RNAi can be accomplished by introducing chemically synthesized small interfering RNAs (siRNAs) or vector-based short hairpin RNAs (shRNAs) into living cells[2,3]. Adenovirus and adeno-associated virus (AAV) vector based shRNAs have been shown to induce RNAi in neural tissues in vivo[4]. shRNAs can be delivered into the inner ear by cationic liposomes or different viral vectors (lentivirus, adenovirus or AAV). Transduction efficiency with cationic liposomes is known to be low and the effect is transient[5,6], long-term transfection is possible with adeno-associated and lentiviral vectors[7-9]. Long-term, stable expression of shRNAs can be achieved by using RNA polymerase III promoter U6[10,11]. The unique sequence specificity of shRNAs makes them suitable to be applied to the inner ear diseases caused by gain of function mutations[12].
Hair cells (HC) in the organ of Corti are terminally differentiated and maintenance of the post-mitotic state is essential for HC survival. Almost all cell divisions in the developing auditory epithelium of mammals and birds stop before birth. Damaged auditory epithelium of birds has an innate capacity to evoke proliferation of supporting cells (SC), which leads to regeneration of new HC[13]. HCs in the utricular sensory epithelia of adult guinea pigs and adult humans can proliferate after aminoglycoside damage when maintained in vitro. Also, HCs of vestibular sensory epithelia of young guinea pigs are able to regenerate after in vivo administration of aminoglycoside antibiotics[14]. Despite these observations, HC and SC of the mammalian organ of Corti are quiescent. The majority of mouse cochlear cells become post-mitotic by E14.5[15] and the correct cell cycle withdrawal requires cyclin-dependent kinase inhibitor (CKI) p27Kip1 (also known as Cdkn1b). In newborn and adult mice, p27Kip1 is expressed in cochlear SCs but not in HCs. In SCs, p27Kip1 acts as a negative regulator of the G1-S transition in the cell cycle[16-18]. The deletion of p27Kip1 affects the morphology of cochlear sensory cells and all p27Kip1 knockout mice have severe hearing impairment[17]. However, p27Kip1 deficient mice have mitotically active cells in postnatal organ of Corti[16,17]. p27Kip1 knockout mice also have supernumerary HC in both outer HC (OHC) and inner HC (IHC) rows[16]. While p27Kip1 is required for the maintenance of the post-mitotic state of the SCs, other CKIs such as p19Ink4d, p21Cip1 and the retinoblastoma tumor suppressor protein (pRb) operate in HCs[19-21]. pRb is the primary protein involved in cell cycle regulation in HCs and its function is to repress transcription of genes required for G1-S transition. It is known that inactivation of pRb family members in neurons make the cells re-enter the S-phase. This results in abnormal DNA replication, which is followed by cell death. p53 is a tumor suppressor protein (encoded by the TP53 gene) which has a major role in DNA damage-induced cell death. When a cell’s DNA repair system fails, p53 is phosphorylated and the phosphorylated p53 upregulates Bax, a Bcl-2 family member which takes part in cell death regulation. p53 is also involved in initiating cell death in cochlear and vestibular HC[22]. For now, the mechanisms behind the death of neurons and other post-mitotic cells after cell cycle re-entry are poorly known. A recent study by Sulg et al[23] revealed that misexpression of human papillomavirus-16 E7 oncogene triggered a forced S-phase entry in HCs in explant cultures. They also discovered that DNA damage in OHC following the forced cell cycle entry activated p53 and led to rapid death of the cells. However, utricular cells showed long-term survival after p53 induction and some of the cells progressed into mitosis.
Damage in the mammalian HC is irreversible and leads inevitably to sensorineural hearing loss. Aminoglycoside antibiotics are known to damage primarily the OHCs of the organ of Corti. Aminoglycosides enter the HCs through mechanoelectrical transducer channels. When aminoglycosides are inside HCs, they form aminoglycoside-iron complexes which results in the formation of reactive oxygen species (ROS)[24]. ROS are believed to promote apoptotic cell death. Apoptosis of OHCs begins at the basal region of the cochlea and spreads towards the middle and apical zones. IHC and SC are usually less affected[25]. It is known that deletion of the p53 offers significant protection against cisplatin-induced HC loss[22]. Kaur et al[26] found that siRNA against transient receptor potential vanilloidin 1 also offers protection against HC loss caused by cisplatin.
Here we have performed AAV2/2 vector mediated delivery of p27Kip1 and p53 shRNAs into adult mouse cochlea damaged by aminoglycoside antibiotic kanamycin. We investigated the effects of these constructs on the damaged organ of Corti.
For gene-silencing experiments to produce shRNAs, four different pairs of p27Kip1 sequences were designed from Genbank BC014296 and NM_009875 sequences and synthesized (Oligomer, Finland): (1) p27Kip1 shRNA 1 lower strand: 5'-AATTAAAAACTTACCGCCACACGAGTCTCGGAGCCGCGGATATCACTCGCGGCTCCGAGACTCGCGCGGCGGCAAGGGCC-3' and upper strand: 5'-CTTGCCGCCGCGCGAGTCTCGGAGCCGCGAGTGATATCCGCGGCTCCGAGACTCGTGTGGCGGTAAGTTTTT-3'; (2) p27Kip1 shRNA 2 lower strand: 5'-AATTAAAAACAAACGACCACCCGAGCCGCAGTAGCGCGGATATCACTCGCGCTACTGCGGCTCGGGCGGTCGCTCGGGCC-3' and upper strand: CGAGCGACCGCCCGAGCCGCAGTAGCGCGAGTGATATCCGCGCTACTGCGGCTCGGGTGGTCGTTTGTTTTT-3'; (3) p27Kip1 shRNA 3 lower strand: 5'-AATTAAAAACCTCCTGCCATTCGTATCTGCCCTCCAGGGATATCACTCCTGGAGGGCAGATACGAATGGCAGGAGGGGCC-3' and upper strand: CCTCCTGCCATTCGTATCTGCCCTCCAGGAGTGATATCCCTGGAGGGCAGATACGAATGGCAGGAGGTTTTT-3'; and (4) p27Kip1 shRNA 4 lower strand: 5'-AATTAAAAAGTCGCAGAACTTCGAAGAGGATATCACTCTCTTCGAAGTTCTGCGACGGCC-3' and upper strand: GTCGCAGAACTTCGAAGAGAGTGATATCCTCTTCGAAGTTCTGCGACTTTTT-3'.
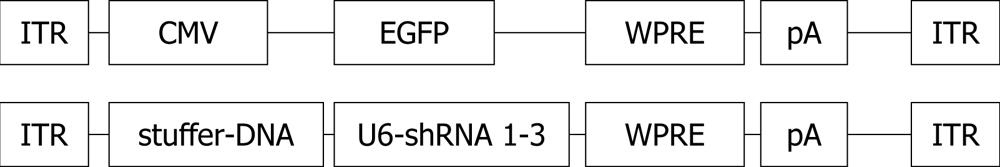
The pairs were annealed and the generated double-stranded DNA fragments were cloned into the pSilencer 1.0-U6 plasmid (Ambion, United States) between ApaI and EcoRI under the U6-promoter. The silencing effect of the different pairs was tested by transfecting 1 μg of shRNA-plasmid into COS-cells in culture with Lipofectamine or Lipofectamine 2000 (Invitrogen, United States) reagents according to the manufacturer’s instructions. Since proliferating COS cells do not express p27Kip1, we cotransfected 1 μg of p27Kip1 cDNA expressing vector together with the shRNA plasmids. Bluescript plasmid (Stratagene, United States) was used as a control. Expression levels of p27Kip1 in COS cells were analyzed from 10 μg of protein from cell lysates with 12% SDS-PAGE acrylamide gel-electrophoresis, Western blotting using Hybond-C Extra membrane (Pharmacia, United States), rabbit polyclonal anti-p27Kip1 antibody (1:1000; Santa Cruz, United States) and Anti-rabbit Ig-HRP visualized by ECL Western blotting substrate (Pierce, United States). The best silencing sequences (shRNAs 2 and 3) with their respective U6 promoters were digested out from the pSilencer 1.0-U6 with BamHI and cloned next to each other into the AAV-vector (serotype 2, AAV2/2) in front of the woodchuck hepatitis virus post-transcriptional element (WPRE)[27] (Figure 1). The CMV promoter present in the AAV-CMV-WPRE construct[27] was removed with EcoRI and XhoI and uncoding stuffer DNA was cloned instead to keep the viral DNA size optimal (Figure 1).
The following p53 shRNA sequences were designed from GenBank: AF051368 and synthesized (Oligomer): p53 lower strand: 5'-AATTAAAAAGGCCCAAGTGAAGCCCTCCGAGTGTCAGATATCACTTAACACTCGAAGGGCTCCACTTAGACCGGCC-3' and upper strand: 5'-GGTCTAAGTGGAGCCCTTCGAGTGTTAAGTGATATCTGACACTCGGAGGGCTTCACTTGGGCCTTTTT-3'. As above, the annealed fragment was cloned in pSilencer 1.0-U6 and tested in COS cells. As controls for Western blotting, mouse embryonic fibroblasts (MEF) from p53 targeted embryos (p53-/- and p53+/-; Jackson laboratory, United States) were grown. Western blotting analysis was performed as above with anti-p53 antibody (1:300; sc-6243 Santa Cruz).
The U6-p53 fragment from pSilencer 1.0-U6 was cloned into the AAV-vector as above either alone or together with the p27Kip1 shRNAs 2 and 3 (Figure 1).
The production of recombinant AAV vectors (Figure 2) was described earlier by Paterna et al[27] in 2000.
Twenty white, 11 wk to 13 wk old male CD-1 mice (weight approx. 30 g, Harlan, Netherlands) were used. The mice were divided into groups (Table 1) according to which AAV2/2-shRNA complex they received: p27Kip1 (AAV-p27-shRNA), p53 (AAV-p53-shRNA), p27Kip1+p53 (AAV-p27+p53-shRNA). These groups were also transduced with the AAV2/2-EGFP. The fourth group received only saline injections. These four groups (16 mice) were pretreated with kanamycin injections 15 d prior to the surgery (Table 1). Each animal received 0.6 mL of kanamycin sulfate (0.02 mL/g daily, 2 × 0.3 mL/d intraperitoneally, i.p., 400 mg/kg, Sigma-Aldrich) daily. The fifth group received only AAV2/2-EGFP injections without kanamycin pretreatment. The animals were anesthetized with an ip injection of ketamine and midazolamine (0.05 mg/10 g body weight). Exposure of the cranial base and middle ear bulla was performed through a ventral paramedian incision as described previously by Jero et al[28] in 2001. AAV-shRNA together with AAV-EGFP or saline was introduced into the inner ear of the mice by injecting directly through the round window membrane (RWM). Saline (1 μL) or AAV-shRNA and AAV-EGFP (0.5 + 0.5 μL) was injected into the inner ear with a glass capillary (inner diameter 0.5 mm, SM100F-15, Harvard apparatus, tip diameter 50 μm) with the help of a microinjector (Narishige, Japan) and a preparation microscope (Olympus SZX12). Virus titer was 1 × 1012 pfu/mL. After the injection, the RWM was sealed with a small piece of thin fascia and the incision was closed in layers. The total operating time was approximately 15-20 min.
| Group | n | Construct | Kanamycin treatment |
| 1 | 4 | AAV-p27-shRNA | Yes |
| 2 | 4 | AAV-p53-shRNA | Yes |
| 3 | 4 | AAV-p27+p53-shRNA | Yes |
| 4 | 4 | Saline | Yes |
| 5 | 4 | AAV-EGFP | No |
Animal care and studies were approved by the Local Ethics Committee of Animal Experiments, University of Helsinki, and by the Provincial State Office of Southern Finland. Animal experiments were conducted in accordance with the European Convention guidelines.
Animals were sacrificed 1 mo after the surgery with an ip overdose of sodium pentobarbital (0.3 mg/10 g, Mebunat, Orion, Finland) and perfused transcardially. Inner ears were harvested and postfixed o/n in 4% PFA and decalcified for 14 d in 0.5 mol/L EDTA. The cochleae were mounted in paraffin.
Paraffin-embedded cochlear tissue sections (5 μm) were dewaxed, mounted in fluorescent Vectashield Mounting Medium (Vector Laboratories, United States) under glass coverslips and analyzed for EGFP expression with Axioplan 2 fluorescence microscope (Zeiss).
Apoptosis in the organ of Corti was evaluated with Tunel staining (Roche Applied Science, Germany). Stained slides were mounted in Vectashield Mounting Medium, fluorescence (Vector Laboratories) under glass coverslips. All stained slides were analyzed with Axioplan 2 microscope (Zeiss).
Four animals per group and twenty sections per animal were studied. Results were evaluated statistically using Student’s t test. Statistical significance was set at P < 0.05.
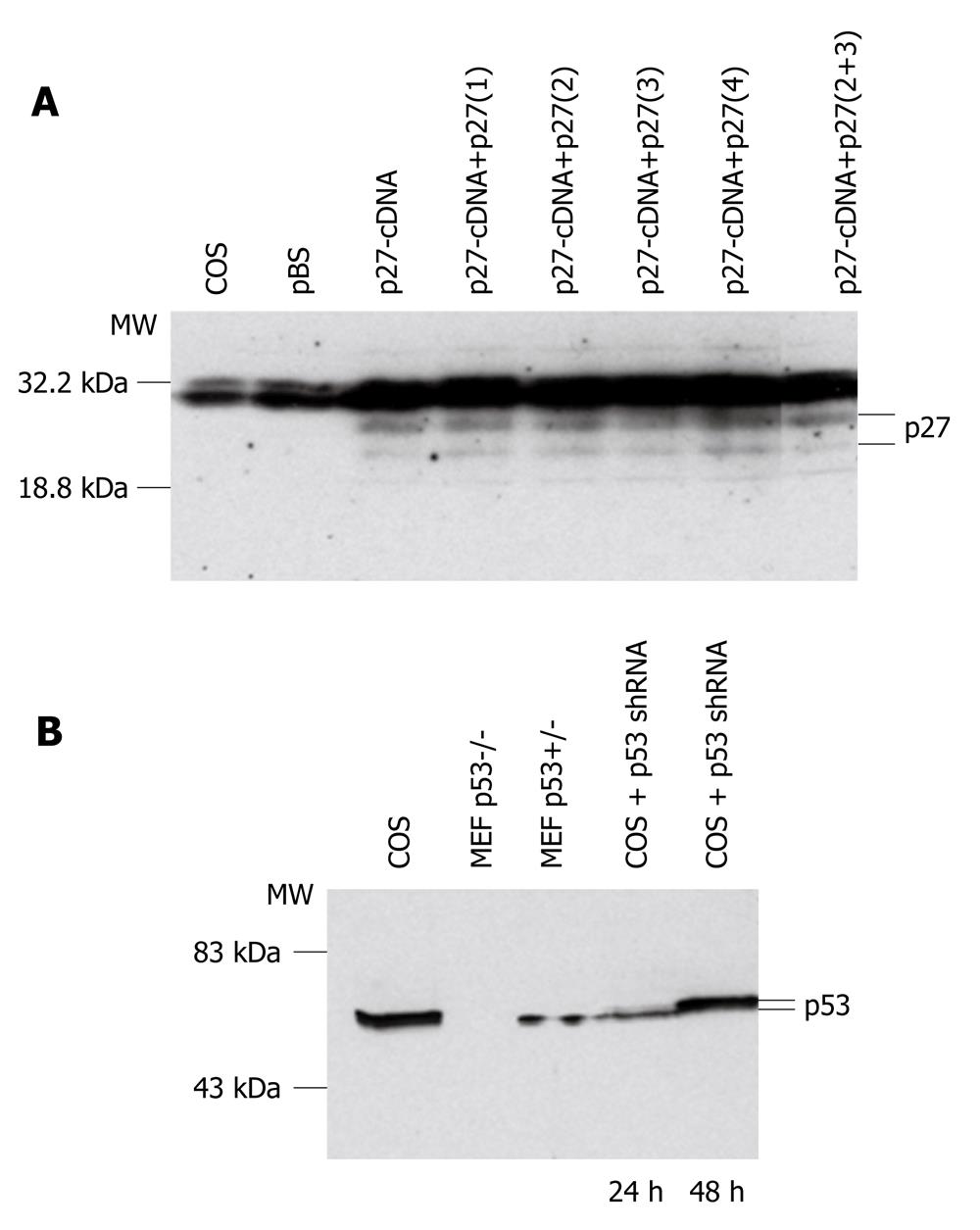
The different pSilencer 1.0-U6 shRNA constructs were transfected into COS cells with Lipofectamine reagents. Cells were collected and proteins extracted after 24 h or 48 h of incubation. To test the p27Kip1 constructs, p27Kip1 cDNA was also cotransfected into COS cells because no p27Kip1 expression was detected in dividing COS cells (Figure 1A). Western blotting analysis with anti-p27Kip1 antibody showed that the best silencing was produced with two p27Kip1-shRNAs 2 and 3. These two shRNAs were first cloned together into the pSilencer 1.0-U6 vector and tested in COS cells (Figure 1A). After successful testing in COS cells, the two p27Kip1-shRNAs were cloned into the AAV2/2 vector[18] to give rise to the AAV-p27-shRNA (Figure 1A).
The p53-shRNA was tested in transfections into COS cells which express p53 (Figure 1B). In Western blotting analysis, proteins were also extracted from MEFs isolated from gene targeted p53 null embryos (p53-/-) and heterozygous embryos (p53+/-). As expected, no p53 was detected in p53-/- MEFs, whereas p53+/- MEFs showed detectable levels of p53 expression (Figure 1B). The p53 expression in the COS cells was clearly decreased after 24 h of the transfection, whereas after 48 h the level appeared nearly normal (Figure 1B). The p53-shRNA fragment was cloned into the AAV2/2 vector either alone to give rise to the AAV-p53-shRNA or together with the two p27Kip1-shRNAs to give rise to AAV-p27+p53-shRNA.
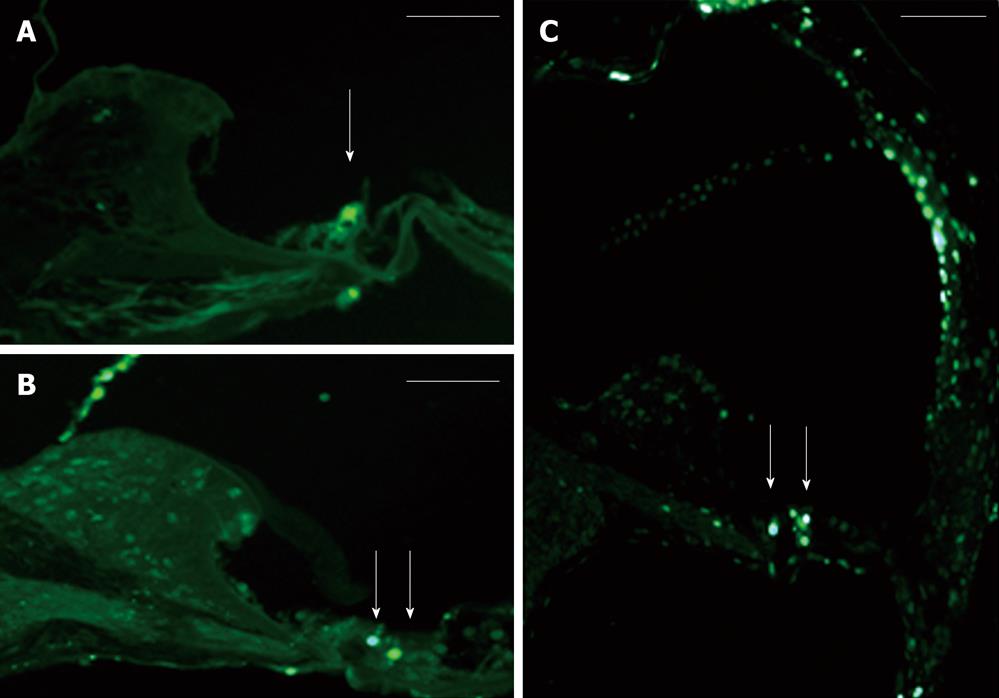
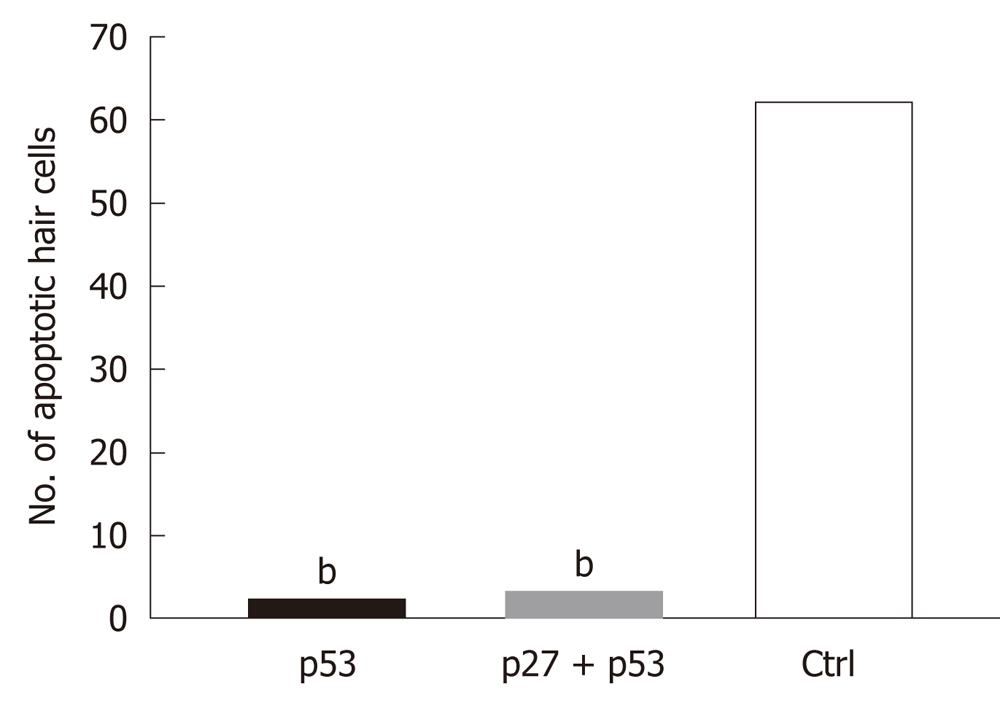
The AAV2/2-shRNA vectors were injected together with AAV2/2-EGFP into kanamycin pretreated mouse cochlea. After a month, EGFP expression was detected in the cochlea of all injected mice (Figure 3A and B). Expression was limited to the organ of Corti, histologically in both IHC and OHC and in SC, and was detected in all cochlear turns. The expression was, however, most intense at the site of injection. One group was injected with AAV2/2-EGFP but did not receive kanamycin injections prior the injections. The expression of EGFP was similar to the other injected mice, so kanamycin had no effect on the expression pattern of the EGFP (data not shown). Apoptosis in the inner ear due to aminoglycoside damage was demonstrated by Tunel staining. Tunel stained cells were detected in all cochlear turns (data not shown). AAV2/2-shRNA injected inner ears showed less apoptotic HC when compared to the contralateral control ear and to the inner ears of saline injected control animals (treated with kanamycin) (Figure 3A-C). AAV-p53-shRNA and AAV-p27+p53-shRNA injected cochleae showed significantly less apoptotic cells compared to saline injected cochleae, P < 0.05 (AAV-p53-shRNA vs saline P = 0.00014, AAV-p27+p53-shRNA vs saline P = 0.0011) (Figure 4, P < 0.001). AAV-p27-shRNA injected cochleae showed no significant difference compared to the saline injected cochleae.
In this study, we evaluated the effects of three AAV2/2-mediated shRNAs on mouse cochlea damaged by aminoglycoside antibiotic kanamycin. AAV2/2 was selected as the carrier for shRNAs based AAV vectors properties. AAV vectors have an ability to transduce non-dividing cells. AAV serotype 2/2 can transduce cells of the organ of Corti, especially both IHC and OHC but also the SC[8]. AAV vectors are generally safe and cause only minimal damage in the inner ear structures[8]. The only limiting factor is the small packaging size of the virus. Approximately only 4.5 kb of foreign DNA can be packaged into the virus capsid. We chose to use AAV2/2 in our study based on our own previous data[8], which had shown that AAV2/2 is suitable for inner ear gene therapy applications. In contrast to adenovirus, AAV generates only minimal immune response and it is primarily a humoral response[29,30]. We chose to do the injections through the RWM in order to be sure that the vector constructs were delivered to scala tympani. The surgery can cause damage to the inner ear and affect hearing, even although a previous study suggests that the direct microinjection through RWM causes only minimal damage and minor changes in the cochlear cytoarchitecture[28]. Possible damage to hearing was not evaluated in this study.
Created shRNAs were first tested in a COS cell culture. Both shRNAs (p27 Kip1 and p53) were successful in silencing their target genes in COS cells. These results suggest that the p27Kip1-shRNAs 2 and 3 can be used in cells in order to silence p27Kip1, and that the p53-shRNA can be used for silencing experiments in cells. Results from cell culture experiments led us to believe that p27Kip1-and p53-shRNAs may also be used in silencing experiments in the cells of auditory epithelium. We hypothesized that silencing of p27Kip1 and p53 could improve cell survival in cochlea. The HC loss caused by kanamycin was evaluated with Tunel staining, which specifically stains apoptotic cells at various stages. Kanamycin injections led to apoptosis in cochlear auditory epithelium. Apoptotic HC were seen throughout the cochlea. Kanamycin has been used with CBA, C57Bl6 and BALB mouse strains[31]. In this study, we used CD-1 mouse strain. With the CD-1 strain, kanamycin caused both IHC and OHC loss and was consistent with earlier studies with other mouse strains[31]. AAV-p53- and AAV-p27Kip1+p53-shRNA treated ears showed less apoptotic HC when compared to the saline injected control ears. AAV-p27-shRNA alone had no significant effect to the number of apoptotic cells in the organ of Corti when compared to contralateral control ear or saline injected (kanamycin treated) ear. The combination of p27Kip1 and p53 shRNAs did not decrease the number of apoptotic cells compared to AAV-p53-shRNA alone. This suggests that silencing of p53 in the kanamycin treated ear may decrease cell death in cochlear auditory epithelium.
The influence of shRNAs to the function of auditory epithelium was not studied. It is currently not known if these shRNAs have an effect on hearing in vivo. This needs to be studied in future.
In conclusion, shRNAs can be used in silencing genes in the cell of inner ear. Silencing of p53 in the kanamycin treated ears seems to decrease cell death in the organ of Corti.
We are grateful to Raija Savolainen for the help with DNA-constructs, Western blottings and cell culture.
Hair cells (HC) in the organ of Corti are terminally differentiated and maintenance of the post-mitotic state is essential for HC survival. Almost all cell divisions in the developing auditory epithelium of mammals and birds stop before birth. The majority of mouse cochlear cells become post-mitotic by embryonic day 14.5 and the correct cell cycle withdrawal requires cyclin-dependent kinase inhibitor p27Kip1 (also known as Cdkn1b). p53 is a tumor suppressor protein which has a major role in DNA damage-induced cell death. p53 is also involved in initiating cell death in cochlear and vestibular HC. For now, the mechanisms behind the death of neurons and other post-mitotic cells after cell cycle re-entry are poorly known. Damage in the mammalian HC is irreversible and leads inevitably to sensorineural hearing loss. Aminoglycoside antibiotics are known to damage primarily the outer HC of the organ of Corti. It is known that deletion of the p53 offers significant protection against cisplatin (chemotherapy drug) induced HC loss.
HC and supporting cells of the mammalian organ of Corti are quiescent. Damage in these cells is irreversible and leads inevitably to sensorineural hearing loss. The main focus of the research is in finding a way to save the HC from trauma, make HC enter the cell cycle again, regenerate HC after cell death, and find a suitable carrier for inner ear gene transfer applications. Cochlear gene therapy studies are, at the moment, focused on the use of different types of vectors (viral and nonviral) as transgene carriers. Each viral vector possesses its own characteristics that limit their use in different applications. At the moment, there is no single vector that would be suitable for each cochlear gene therapy application.
In this study, we evaluated the effects of three adeno-associated virus (AAV)-short hairpin RNAs (shRNAs) on mouse cochlea damaged by aminoglycoside antibiotic kanamycin. Created shRNAs were first tested in a COS cell culture. Both shRNAs (p27 Kip1 and p53) were successful in silencing their target genes in COS cells. These results suggest that both shRNA can be used for silencing experiments in cells. Results from cell culture experiments led us to believe that p27Kip1-and p53-shRNAs may also be used to silence the expression of these genes in the cells of auditory epithelium. The study hypothesized that the silencing of p53 could improve cell survival in cochlea. It found that AAV-p53- and AAV-p27Kip1+p53-shRNA treated ears showed less apoptotic HC when compared to the saline injected control ears. So, shRNAs can be used in silencing genes in the cell of inner ear. Silencing of p53 in the kanamycin treated ears seems to decrease cell death in the organ of Corti.
This study suggests that AAV-shRNAs are promising tools for promoting cell survival in damaged cochlea.
shRNA is a short sequence of RNA that makes a tight hairpin turn that can be used to silence gene expression via RNA interference. AAV: AAV belongs to the genus Dependovirus, which in turn belongs to the family Parvoviridae. It is a small, replication-defective, non-enveloped virus. AAV can infect both dividing and non-dividing cells and may incorporate its genome into that of the host cell. These features make AAV a good candidate for creating viral vectors for gene therapy. p27kip1 is an enzyme that in humans encodes a protein which belongs to the Cip/Kip family of cyclin dependent kinase (Cdk) inhibitor proteins. These proteins control the cell cycle re-entry. It is often called a cell cycle inhibitor protein because its major function is to stop or slow down the cell division cycle. p53 is a tumor suppressor protein. p53 regulates the cell cycle and functions as a tumor suppressor that is involved in preventing cancer. p53 has a role in conserving stability by preventing genome mutation. Kanamycin sulfate: is an aminoglycoside antibiotic, available in oral, intravenous and intramuscular forms. It is used to treat a wide variety of infections. Serious side effects include tinnitus or hearing loss, toxicity to kidneys and allergic reactions to the drug.
The paper is well written and well designed. This study describes a very interesting topic. The effects of kanamycin on the cochlea have been well studied in the current literature, as well as the role of p53 and p27.
Peer reviewers: Alessandro De Stefano, MD, PhD, FAINOT, “G.d’Annunzio” University of Chieti and Pescara, Via di Palma 10, Taranto 74100, Italy; Dr. Anna Eleftheriadou, MD, PhD, Otolaryngologist Head and Neck Surgeon, Department of Otolaryngology, General Hospital of Rethymnon, Trantalidou Str 15-17, 74100 Rethymnon, Greece
S- Editor Wu X L- Editor Roemmele A E- Editor Zheng XM
| 1. | Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10522] [Cited by in RCA: 10115] [Article Influence: 374.6] [Reference Citation Analysis (1)] |
| 2. | Pushparaj PN, Aarthi JJ, Manikandan J, Kumar SD. siRNA, miRNA, and shRNA: in vivo applications. J Dent Res. 2008;87:992-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Rao DD, Vorhies JS, Senzer N, Nemunaitis J. siRNA vs. shRNA: similarities and differences. Adv Drug Deliv Rev. 2009;61:746-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 435] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 4. | Xia H, Mao Q, Paulson HL, Davidson BL. siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol. 2002;20:1006-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 642] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 5. | Jero J, Mhatre AN, Tseng CJ, Stern RE, Coling DE, Goldstein JA, Hong K, Zheng WW, Hoque AT, Lalwani AK. Cochlear gene delivery through an intact round window membrane in mouse. Hum Gene Ther. 2001;12:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Wareing M, Mhatre AN, Pettis R, Han JJ, Haut T, Pfister MH, Hong K, Zheng WW, Lalwani AK. Cationic liposome mediated transgene expression in the guinea pig cochlea. Hear Res. 1999;128:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Han JJ, Mhatre AN, Wareing M, Pettis R, Gao WQ, Zufferey RN, Trono D, Lalwani AK. Transgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vector. Hum Gene Ther. 1999;10:1867-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Aarnisalo AA, Pietola L, Joensuu J, Isosomppi J, Aarnisalo P, Dinculescu A, Lewin AS, Flannery J, Hauswirth WW, Sankila EM. Anti-clarin-1 AAV-delivered ribozyme induced apoptosis in the mouse cochlea. Hear Res. 2007;230:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Pietola L, Aarnisalo AA, Joensuu J, Pellinen R, Wahlfors J, Jero J. HOX-GFP and WOX-GFP lentivirus vectors for inner ear gene transfer. Acta Otolaryngol. 2008;128:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC, Shi Y. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA. 2002;99:5515-5520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 882] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 11. | Yu JY, DeRuiter SL, Turner DL. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci USA. 2002;99:6047-6052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 776] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 12. | Maeda Y, Sheffield AM, Smith RJ. Therapeutic regulation of gene expression in the inner ear using RNA interference. Adv Otorhinolaryngol. 2009;66:13-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 600] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 14. | Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 360] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;Suppl 220:1-22044. [PubMed] |
| 16. | Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581-1590. [PubMed] |
| 17. | Löwenheim H, Furness DN, Kil J, Zinn C, Gültig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci USA. 1999;96:4084-4088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 275] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Harper JW. Protein destruction: adapting roles for Cks proteins. Curr Biol. 2001;11:R431-R435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel MF, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, García-Añoveros J, Hinds PW, Corwin JT, Corey DP. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Mantela J, Jiang Z, Ylikoski J, Fritzsch B, Zacksenhaus E, Pirvola U. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development. 2005;132:2377-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Sulg M, Kirjavainen A, Pajusola K, Bueler H, Ylikoski J, Laiho M, Pirvola U. Differential sensitivity of the inner ear sensory cell populations to forced cell cycle re-entry and p53 induction. J Neurochem. 2010;112:1513-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72:931-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Murillo-Cuesta S, Contreras J, Cediel R, Varela-Nieto I. Comparison of different aminoglycoside antibiotic treatments to refine ototoxicity studies in adult mice. Lab Anim. 2010;44:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Kaur T, Mukherjea D, Sheehan K, Jajoo S, Rybak LP, Ramkumar V. Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis. 2011;2:e180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Paterna JC, Moccetti T, Mura A, Feldon J, Büeler H. Influence of promoter and WHV post-transcriptional regulatory element on AAV-mediated transgene expression in the rat brain. Gene Ther. 2000;7:1304-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Jero J, Tseng CJ, Mhatre AN, Lalwani AK. A surgical approach appropriate for targeted cochlear gene therapy in the mouse. Hear Res. 2001;151:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Xiao X, Li J, McCown TJ, Samulski RJ. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 140] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, Brantly M, Muzyczka N, Byrne BJ, Atkinson M. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci USA. 1998;95:14384-14388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 229] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res. 2001;158:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |