Published online Nov 29, 2024. doi: 10.5319/wjo.v11.i3.25
Revised: September 19, 2024
Accepted: October 28, 2024
Published online: November 29, 2024
Processing time: 278 Days and 20.4 Hours
The main goal of our research is to introduce transoral robotic surgery and laser resection (TLR) as a considerable way of treating patients with recurrent oropha
To develop a foundation of minimally invasive transoral surgical technique for patients with oropharyngeal recurrence.
This study prospectively and retrospectively included patients with recurrent tumors from 2003 to 2018. Subjects were allocated into two groups: (1) Group I; underwent TLR; and (2) Group II (control); underwent open surgeries of varying volume. Evaluation was done with intraoperative blood loss, postoperative infection incidence, and quality of life using the scale for patients with head and neck tumors known as the Functional Assessment of Cancer Therapy-Head & Neck Scale.
One-hundred and forty one patients were included (103 males and 38 females), in 82 cases (85.4%), a recurrent tumor developed earlier than a year after primary tumor therapy; forty-six were in group I and 69 in group II, age ranging from 18 years to 86 years (average: 57.6 years). The first group showed a statistically significant less amount of blood loss and a decreased incidence of infectious complications (P < 0.05). Additionally, there was a significant difference in functional outcomes (quality of life scores) but no significant difference in survival curves.
In properly elected patients, TLR is not just reasonable but tends to be a favorable alternative for recurrent oropharyngeal cancers compared to the outcomes of the open surgery group.
Core Tip: Our study included a large retrospective part and compared two prospective groups of patients, who were subjected to open surgery or transoral robotic surgery and laser resection (TLR). The results show that in properly elected patients, TLR is not just reasonable but has a tendency to be a favorable alternative for recurrent oropharyngeal cancer compared to the outcomes of the open surgery group. The use of TLR in our study was associated with shorter operating times, lower blood loss counts, lower postoperative complication rates, a higher quality of life and a proportionate 2-year survival when compared with open surgery performance rates. As open surgery was thought for decades to be the mainstream treatment approach for recurrent tumors of the oropharyngeal zone, considerable experience has accumulated which indicates that this management bears unfavorable functional outcomes. The results of our comparative study defined a higher quality of life in patients who underwent TLR.
- Citation: Ilkaev K, Gvetadze SR, Roshchina EA, Azizyan RI, Mudunov AM, Bolotin MV, Yang X, Larinov D. Recurrent oropharyngeal cancer: Analysis of surgical treatment outcomes. World J Otorhinolaryngol 2024; 11(3): 25-32
- URL: https://www.wjgnet.com/2218-6247/full/v11/i3/25.htm
- DOI: https://dx.doi.org/10.5319/wjo.v11.i3.25
Malignant tumors of the oral cavity and oropharynx compose around 2%-5% of all malignancies. The current mainstream, first-line treatment for oropharyngeal squamous cell carcinoma (SCC) is function preserving chemoradiation. Neoadjuvant chemoradiotherapy salvage surgery for local recurrent tumor is widely deemed as the only possible treatment strategy that may establish curative effect. The possibilities of life-saving operations in such cases are limited by the complexity of surgical access, high probability of serious complications, proximity to vital organs and structures, and the general health of patients during relapse. Unfortunately, salvage interventions which incorporate open surgical access to the head and neck region are associated with prolonged hospital stays, high intraoperative blood loss counts, decreased survival prognosis, and quality of life. These interventions also may require additional bone resection and/or a reconstructive component, which incorporates a regional pedicle flap or a microsurgical flap.
Recently evolving transoral robotic surgery and transoral laser microsurgical resection (TLMR) have a potential to overcome the morbidities associated with open surgery. Published academic data suggests that a considerable fraction of subjects with recurrent oropharyngeal cancers may benefit from performance of TLMR. Currently very limited data is available on the comparison of surgical, oncological and functional results in patients with recurrent oropharyngeal SCC treated with TLMR and with those treated by traditional open surgical approaches. The purpose of this study was to compare surgical, functional and oncological outcomes in patients undergoing open surgery vs TLMR, and to determine the role of minimally invasive surgical techniques in the management of recurrent oropharyngeal tumors.
According to the medical archive, from 2003 to 2018, 141 patients with recurrent oropharyngeal tumors were observed at the N.N. Blokhin National Research Center of Oncology (BNRCO). The clinical data of the patients were analyzed retrospectively and prospectively.
The following clinical parameters were evaluated in all patients: Sex, age at the time of diagnosis, localization of the primary tumor, method of treatment of the primary tumor, morphological properties of the primary tumor, presence of adjuvant therapy, date of the first progression after treatment of the primary tumor, localization of metastases, systemic chemotherapy for recurrence of the primary tumor and realized metastases, both regional and long-term, overall survival and progression-free survival on the background of treatment. The date of death and disease progression was estimated according to the data provided by the out-patient monitoring department. The date of the visit was established by analyzing outpatient records of the patient's visit to the out-patient department of BNRCO.
To conduct a comparative analysis of the effectiveness of TLMR compared to salvage surgery, a comparison group was identified and included 25 patients. To assess operative outcomes, intraoperative blood loss counts, and postoperative infection incidence were compared between the two study groups. Functional results were evaluated by comparative assessment of the quality of life by the Functional Assessment of Cancer Therapy-Head & Neck Scale in the groups of TLMR and open surgery.
Life expectancy and time to progression were evaluated using the Kaplan-Mayer method and compared by a log-rank test. The χ2 tests and Fisher's exact criterion were used to verify the validity of differences in the values of features in the groups. The differences were considered statistically significant at P < 0.05. The correlation was carried out using the Pearson correlation coefficient and Spearman's rank correlation coefficient. The Cox proportional regression analysis model was used to assess the independence of traits and calculate comparative risk.
The study included 141 patients who underwent treatment at BRNCO and underwent surgery for recurrent oropharyngeal tumors. Twenty-one (14.9%) patients received primary treatment at our institution and 120 (85.1%) patients were treated in other medical institutions. Of the examined patients, 38 (27.0%) were women, 103 (73.0%) were men. The mean age of the enrolled patients was 57.6 (52.0; 66.5) years, minimum 18, maximum 86 years (Table 1).
| Age in years | Woman, n = 23 | Men, n = 73 | Total |
| 30-40 | 2 (5.3) | 9 (8.7) | 11 |
| 41-50 | 7 (18.4) | 12 (11.7) | 19 |
| 51-60 | 10 (26.3) | 43 (41.7) | 53 |
| 61-70 | 13 (34.2) | 35 (34.0) | 48 |
| > 70 | 6 (15.8) | 4 (3.9) | 10 |
| Мe [25%; 75%] | 59.7 ± 2.1 [52.0; 67.0] | 56.8 ± 1.9 [52.0; 66.0] | 57.6 ± 2.0 [52.0; 66.5] |
Chemoradiotherapy, as the initial function preserving treatment mode for oropharyngeal cancers, has shown good effect, however, up to one third of cases demonstrate locoregional relapses. Until recently, the only way to treat such cases was open access surgery. However, oncologic results of such interventions are modest. Five-year survival rate was reported to range between 26% and 49.1% for patients who underwent salvage surgery[1,2].
Advancements in endoscopic surgery have led to the development of minimally invasive techniques that enable transoral surgery as an alternative to transmandibular and or transcervical approaches. Transoral microsurgery laser resection (TLRM) was the first minimally invasive technique to be applied to the oropharynx. High-volume TLRM surgeons have reported favorable oncologic outcomes using TLRM in cases of oropharyngeal recurrence. However, the technical challenges of this method have limited widespread adoption outside of select large academic centers. The target of a transoral approach is different in patients with recurrent tumors of the oropharynx. In these cases, surgery may be the only available means of treatment or a method for treatment intensification. Small-volume recurrent tumors can be managed by a transoral approach without reconstruction. Hence, considering the effect of prior radiation on wound healing and the risk of life threatening complications (bleeding) after transoral surgery, large-volume recurrent oropharyngeal tumors may require simultaneous microvascular reconstruction (Table 2) (Figures 1 and 2A).
| Surgical approach | Transoral laser resection | Median mandibulotomy | Segmental resection | Total |
| Resection of the lateral wall of the pharynx | 1 (3.0) | 7 (20.0) | 4 (15.4) | 12 (17.4) |
| Resection of the base of the tongue | 1 (3.0) | 2 (5,7) | 0 (0.0) | 3 (4.3) |
| Resection of the lateral wall of the pharynx and soft palate | 1 (3.0) | 8 (22.9) | 1 (3.9) | 10 (14.5) |
| Resection of the base of the tongue and the lateral wall of the pharynx | 3 (9.1) | 9 (25.7) | 13 (50.0) | 25 (36.2) |
| Resection of the root of the tongue, lateral wall of the pharynx and cheek | 1 (3.0) | 1 (2.9) | 4 (15.4) | 6 (8.7) |
| Resection of the lateral wall of the pharynx and hard palate | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1 (1.4) |
| Resection of the base of the tongue, lateral wall of the pharynx, cheeks and soft palate | 1 (3.0) | 3 (8.6) | 1 (3.9) | 5 (7.2) |
| Resection of the base of the tongue, resection of the lateral wall of the pharynx, cheeks, soft palate and hard palate | 2 (6.1) | 2 (5.7) | 3 (11.5) | 7 (10.1) |
| Total | 10 (14.5) | 33 (47.8) | 26 (37.7) | 69 (100) |
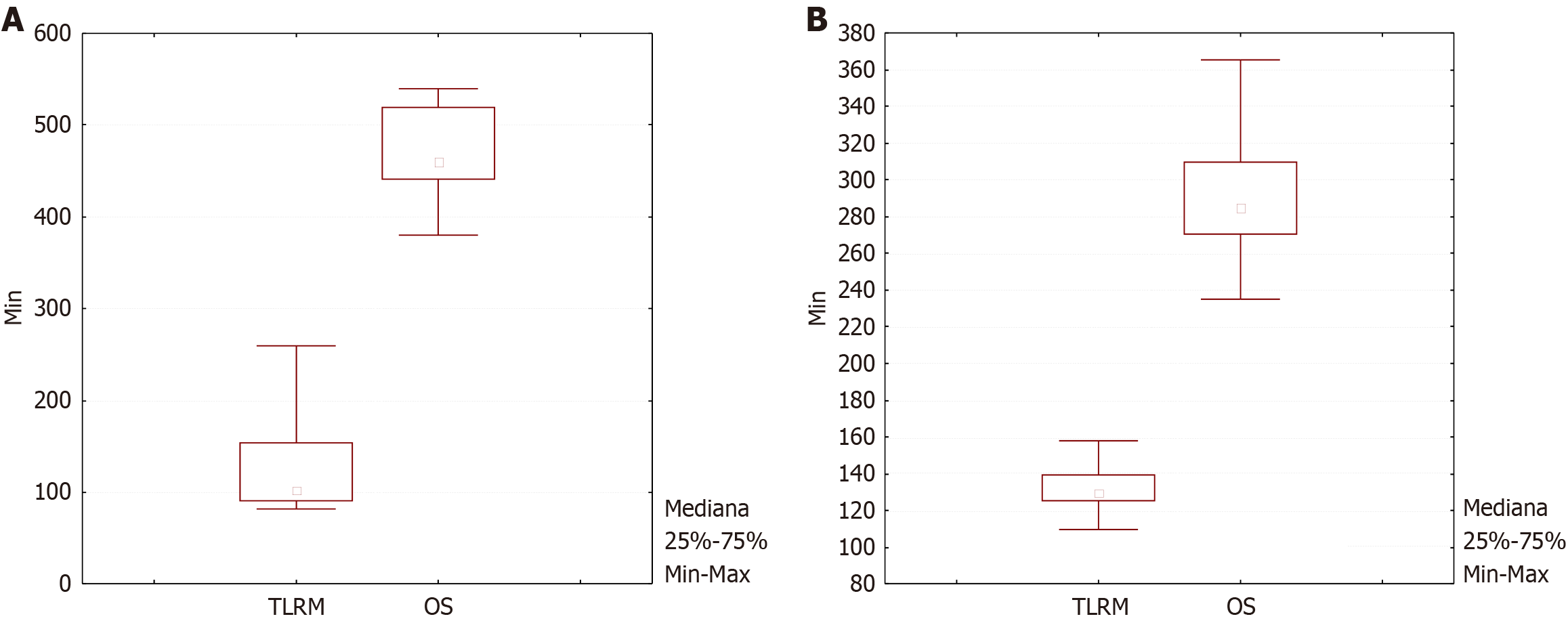
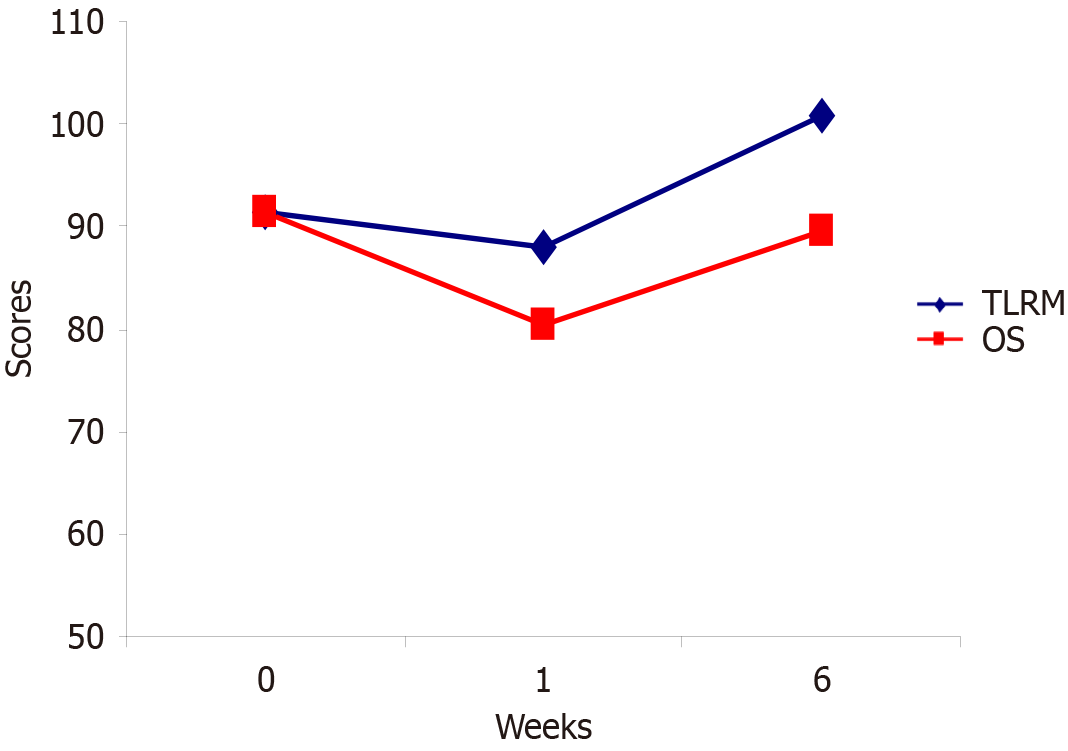
Our study included a large retrospective part and compared two prospective groups of patients, who were subjected to open surgery or TLRM. The results show that in properly elected patients, TLRM is not just reasonable but has a tendency to be a favorable alternative for recurrent oropharyngeal cancers compared to the outcomes of the open surgery group. TLRM in our study was associated with shorter operating time, lower blood loss counts, lower postoperative complication rates, higher quality of life and proportionate 2-year survival when compared with open surgery performance rates. Since open surgery was the mainstream treatment approach for recurrent tumors of the oropharyngeal zone for decades, considerable experience has accumulated which indicates that this management bears unfavorable functional outcomes. The results of our comparative study defined higher quality of life in patients who underwent TLRM. (Figures 2B and 3).
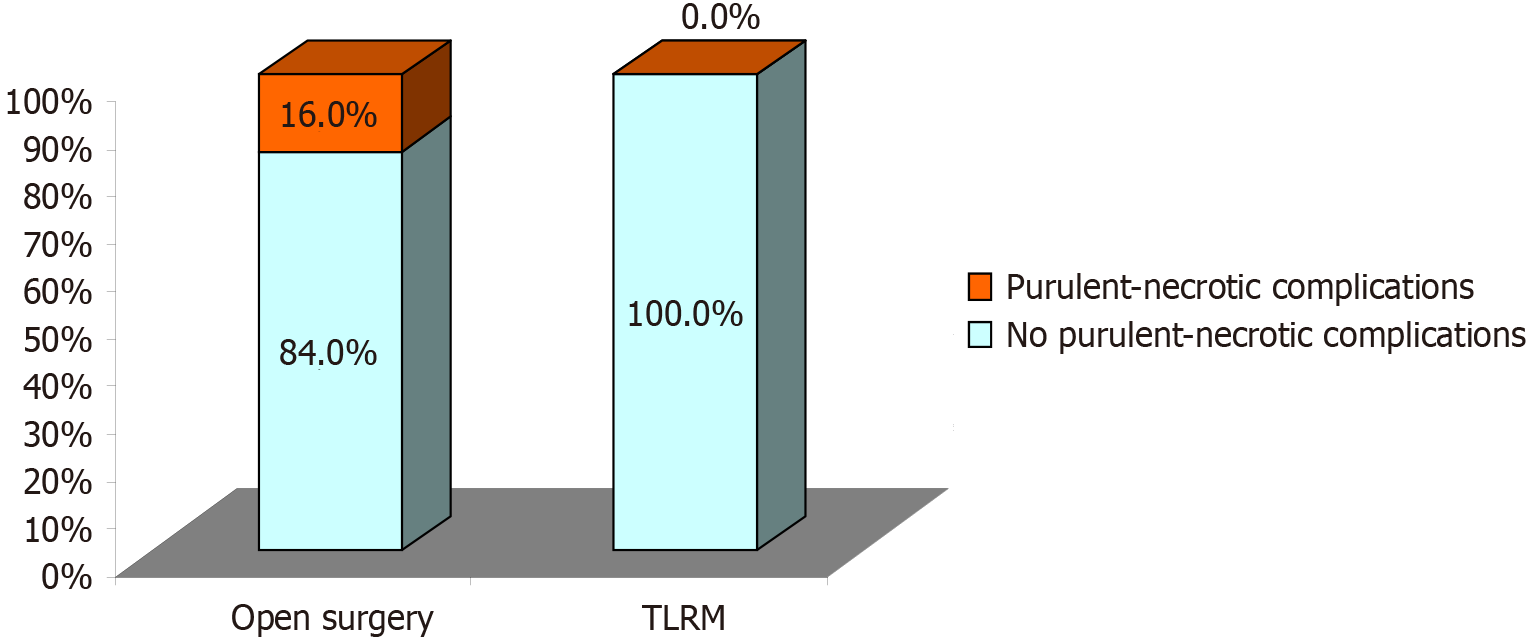
Postoperative surgical complications are observed occasionally after salvage open surgery of recurrent oropharyngeal cancers, these may include infectious complications as well as wound healing problems. This was supported by our observations. The complication rate seen in the TLMR group was lower than for open surgery. Such parameters as blood loss counts, operative time and occurrence of postoperative infectious complications were all lower in the TLRM study group. Considerations that are to be taken in account when planning TLRM as treatment for recurrent oropharyngeal cancers are: The size and exact location of the lesion and personal operator’s experience with this technique (Table 3) (Figure 1, Figure 2A, Figure 3).
| Surgery type | τ Kendall | P value |
| Operations on the mandible | 0.36 | < 0.001a |
| Reconstruction of bone defect | 0.30 | < 0.001a |
| “Open” operations | 0.30 | < 0.001a |
| Reconstruction of a soft tissue defect | 0.28 | < 0.001a |
| Operation volume (total score) | 0.26 | < 0.001a |
| Radiation therapy of the primary tumor | 0.20 | 0.005a |
| Early recurrence tumor | 0.19 | 0.007 |
| Operations on the lymph drainage area | 0.14 | 0.042a |
Surgical treatment alone or in combined approaches are main methods of choice for the treatment of recurrent tumors, if surgery is possible to perform. Many authors pointed out better survival rates in surgical treatment of relapses compared to those in conservative treatment.
Koo et al[3] analyzed the effectiveness of treatment in 23 patients with recurrent oral cancer. Of these, 13 underwent surgery and 10 received chemotherapy or radiation therapy. The median overall survival in patients who have been undergoing surgical treatment significantly exceeded that in conservative treatment.
Zafereo et al[4] noted that the 3-year overall survival for patients who underwent salvage surgery, radiotherapy, palliative chemotherapy, or replacement therapy was 48.7%, 31.6%, 3.7%, and 5.1%, respectively[5-9].
According to Choe et al[9], 2-year survival rates in patients treated with chemoradiotherapy alone were significantly lower compared to those in patients undergoing salvage surgery (10.8% and 28.4%, respectively). The authors conclude that, due to the high risk of severe toxicity, repeated chemoradiotherapy should be performed only in a strictly select group of patients[10-14].
Kano et al[1], in an analysis of 11 patients who underwent salvage surgery and 24 who underwent conservative treatment, showed statistically significant differences in 5-year survival (49.1% and 16.3%, respectively).
Kropotov et al[5] points out that due to the emergence of new effective techniques for the surgical treatment of patients with oropharyngeal cancer, the chemoradiation approach is no longer considered as the method of choice in the treatment of such patients. However, further randomized trials are needed to individualize the treatment approach and choose the optimal treatment tactics for a particular patient[15-17]. The specific medical literature sources possess a considerable number of studies describing the effectiveness of surgical treatment of primary oropharyngeal cancers. Little attention is paid to exploring the process of therapy of relapsed tumors. In addition, as noted by Jayaram et al[6], the quality of many studies should not be considered good enough[18-22]: all studies were retrospective, some had an extremely small sample size (29 patients, 39 patients)[23-30]. The greatest hindrance to an adequate assessment is caused by the heterogeneity of the groups and the lack of adjustment of the result to possible predictors of effectiveness. Thus, the association of a tumor with the human papillomavirus was considered only in one clinical study[31]. Adams et al[7] reported that 74% of patients had a second relapse on average 9 months after salvage surgery[32-34], according to Zafereo et al[4] the recurrence rate was 66%, the onset time was 8 months on average[35,36].
Jayaram et al[6], based on meta-analysis of recurrent oropharyngeal malignancies, treatment showed the mean effect value for 3-year overall survival to be 26% with moderate heterogeneity (I2 = 40.7%), the mean effect value for 5-year overall survival was 23%, with high heterogeneity (I2 = 73.9%)[37,38]. However, it should be noted that this meta-analysis is based on studies conducted during the period 1976-2014, and the above indicators describe the effectiveness of rescue operations over the entire period.
At the same time, the authors indicate that there was a significant positive trend in 5-year survival over this period: 20% for studies conducted before 2000 and 35% after 2000 (P < 0.001). Such a phenomenon may be associated both with an improvement in the technique of surgical intervention, an increase in the quality of the algorithm for assessing the possibility of surgical treatment, and an increase in the relative frequency of tumors associated with the human papillomavirus.
This literature review considers the results of studies conducted in 2006-2016. Hamoir et al[7], considering the effectiveness of salvage operations in 29 patients with recurrent oropharyngeal cancer, determined 2-year survival rates of 64.5%, 5-year survival rates of 43.4%[39].
Fakhry et al[27] conducted a comparative analysis of the effectiveness of surgical treatment of recurrent oropharyngeal cancer in patients associated (49 patients) and non-associated (29 patients) with human papillomavirus. The overall 2-year survival rate for p16-positive patients was 72%, for p16-negative patients it was 45% (P = 0.004)[40-43].
In conclusion, we would like to point out that despite the impressive success of surgery as the best way of treatment for patients with oropharyngeal recurrent tumors, we strictly assume that this cohort of patients should be carefully stratified, managed and thoroughly discussed with multidisciplinary teams of specialists before surgery.
| 1. | Kano S, Homma A, Hayashi R, Kawabata K, Yoshino K, Iwae S, Hasegawa Y, Nibu K, Kato T, Shiga K, Matsuura K, Monden N, Fujii M. Salvage surgery for recurrent oropharyngeal cancer after chemoradiotherapy. Int J Clin Oncol. 2013;18:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | White H, Ford S, Bush B, Holsinger FC, Moore E, Ghanem T, Carroll W, Rosenthal E, Sweeny L, Magnuson JS. Salvage surgery for recurrent cancers of the oropharynx: comparing TORS with standard open surgical approaches. JAMA Otolaryngol Head Neck Surg. 2013;139:773-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Koo BS, Lim YC, Lee JS, Choi EC. Recurrence and salvage treatment of squamous cell carcinoma of the oral cavity. Oral Oncol. 2006;42:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Zafereo ME, Hanasono MM, Rosenthal DI, Sturgis EM, Lewin JS, Roberts DB, Weber RS. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009;115:5723-5733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Yakovleva LP, Kropotov MA, Khodos AV, Tigrov MS, Vyalov AS, Gavrishchuk PA, Bochkar VA, Guseinov II, Alizade GR. P-165 Opportunities for surgical treatment of elderly patients with head and neck tumors. Oral Oncology. 2021;118. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Jayaram SC, Muzaffar SJ, Ahmed I, Dhanda J, Paleri V, Mehanna H. Efficacy, outcomes, and complication rates of different surgical and nonsurgical treatment modalities for recurrent/residual oropharyngeal carcinoma: A systematic review and meta-analysis. Head Neck. 2016;38:1855-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Adams ES, Deivasigamani S, Mottaghi M, Huang J, Gupta RT, Polascik TJ. Evaluation of Recurrent Disease after Radiation Therapy for Patients Considering Local Salvage Therapy: Past vs. Contemporary Management. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Hamoir M, Schmitz S, Suarez C, Strojan P, Hutcheson KA, Rodrigo JP, Mendenhall WM, Simo R, Saba NF, D'Cruz AK, Haigentz M Jr, Bradford CR, Genden EM, Rinaldo A, Ferlito A. The Current Role of Salvage Surgery in Recurrent Head and Neck Squamous Cell Carcinoma. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Choe KS, Haraf DJ, Solanki A, Cohen EE, Seiwert TY, Stenson KM, Blair EA, Portugal L, Villaflor VM, Witt ME, Vokes EE, Salama JK. Prior chemoradiotherapy adversely impacts outcomes of recurrent and second primary head and neck cancer treated with concurrent chemotherapy and reirradiation. Cancer. 2011;117:4671-4678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Perrone F, Mariani L, Pastore E, Orsenigo M, Suardi S, Marcomini B, DaRiva L, Licitra L, Carbone A, Pierotti MA, Pilotti S. p53 codon 72 polymorphisms in human papillomavirus-negative and human papillomavirus-positive squamous cell carcinomas of the oropharynx. Cancer. 2007;109:2461-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Rivera F, García-Castaño A, Vega N, Vega-Villegas ME, Gutiérrez-Sanz L. Cetuximab in metastatic or recurrent head and neck cancer: the EXTREME trial. Expert Rev Anticancer Ther. 2009;9:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Seiwert TY, Salama JK, Vokes EE. The concurrent chemoradiation paradigm--general principles. Nat Clin Pract Oncol. 2007;4:86-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 303] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 13. | Kurul S, Dinçer M, Kizir A, Uzunismail A, Darendeliler E. Plastic surgery in irradiated areas: analysis of 200 consecutive cases. Eur J Surg Oncol. 1997;23:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Specenier PM, Vermorken JB. Targeted therapies in head and neck cancer. Targ Oncol. 2007;2: 73-88. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Stuckensen T, Kovács AF, Adams S, Baum RP. Staging of the neck in patients with oral cavity squamous cell carcinomas: a prospective comparison of PET, ultrasound, CT and MRI. J Craniomaxillofac Surg. 2000;28:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 143] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Reynolds LF, Rigby MH, Trites J, Hart R, Taylor SM. Outcomes of transoral laser microsurgery for recurrent head and neck cancer. J Laryngol Otol. 2013;127:982-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Sinha P, Hackman T, Nussenbaum B, Wu N, Lewis JS Jr, Haughey BH. Transoral laser microsurgery for oral squamous cell carcinoma: oncologic outcomes and prognostic factors. Head Neck. 2014;36:340-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Roh J, Muelleman T, Tawfik O, Thomas SM. Perineural growth in head and neck squamous cell carcinoma: a review. Oral Oncol. 2015;51:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Tan HK, Giger R, Auperin A, Bourhis J, Janot F, Temam S. Salvage surgery after concomitant chemoradiation in head and neck squamous cell carcinomas - stratification for postsalvage survival. Head Neck. 2010;32:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Tanvetyanon T, Padhya T, McCaffrey J, Zhu W, Boulware D, Deconti R, Trotti A. Prognostic factors for survival after salvage reirradiation of head and neck cancer. J Clin Oncol. 2009;27:1983-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Vandersteen C, Dassonville O, Chamorey E, Poissonnet G, Nao EE, Pierre CS, Leyssale A, Peyrade F, Falewee MN, Sudaka A, Haudebourg J, Demard F, Santini J, Bozec A. Impact of patient comorbidities on head and neck microvascular reconstruction. A report on 423 cases. Eur Arch Otorhinolaryngol. 2013;270:1741-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21 Suppl 7:vii252-vii261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 23. | Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2546] [Cited by in RCA: 2546] [Article Influence: 149.8] [Reference Citation Analysis (0)] |
| 24. | Vermorken JB, Stöhlmacher-Williams J, Davidenko I, Licitra L, Winquist E, Villanueva C, Foa P, Rottey S, Skladowski K, Tahara M, Pai VR, Faivre S, Blajman CR, Forastiere AA, Stein BN, Oliner KS, Pan Z, Bach BA; SPECTRUM investigators. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 326] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 25. | Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5387] [Cited by in RCA: 4979] [Article Influence: 331.9] [Reference Citation Analysis (0)] |
| 26. | Bachar GY, Goh C, Goldstein DP, O'Sullivan B, Irish JC. Long-term outcome analysis after surgical salvage for recurrent tonsil carcinoma following radical radiotherapy. Eur Arch Otorhinolaryngol. 2010;267:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Benatar MJ, Dassonville O, Chamorey E, Poissonnet G, Ettaiche M, Pierre CS, Benezery K, Hechema R, Demard F, Santini J, Bozec A. Impact of preoperative radiotherapy on head and neck free flap reconstruction: a report on 429 cases. J Plast Reconstr Aesthet Surg. 2013;66:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Broglie MA, Soltermann A, Haile SR, Röösli C, Huber GF, Schmid S, Stoeckli SJ. Quality of life of oropharyngeal cancer patients with respect to treatment strategy and p16-positivity. Laryngoscope. 2013;123:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1169] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 30. | Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2814] [Cited by in RCA: 2766] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 31. | Fakhry C, Zhang Q, Nguyen-Tan PF, Rosenthal D, El-Naggar A, Garden AS, Soulieres D, Trotti A, Avizonis V, Ridge JA, Harris J, Le QT, Gillison M. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32:3365-3373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 422] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 32. | Chen AM, Daly ME, Luu Q, Donald PJ, Farwell DG. Comparison of functional outcomes and quality of life between transoral surgery and definitive chemoradiotherapy for oropharyngeal cancer. Head Neck. 2015;37:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma--an update. CA Cancer J Clin. 2015;65:401-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 750] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 34. | Patel RS, Clark JR, Dirven R, Wyten R, Gao K, O'Brien CJ. Prognostic factors in the surgical treatment of patients with oral carcinoma. ANZ J Surg. 2009;79:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Cohan DM, Popat S, Kaplan SE, Rigual N, Loree T, Hicks WL Jr. Oropharyngeal cancer: current understanding and management. Curr Opin Otolaryngol Head Neck Surg. 2009;17:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2644-2652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 37. | Culié D, Benezery K, Chamorey E, Ettaiche M, Fernandez J, Poissonnet G, Riss JC, Hannoun-Lévi JM, Chand ME, Leysalle A, Saada E, Sudaka A, Haudebourg J, Demard F, Santini J, Peyrade F, Dassonville O, Bozec A. Salvage surgery for recurrent oropharyngeal cancer: post-operative oncologic and functional outcomes. Acta Otolaryngol. 2015;135:1323-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Datema FR, Poldermans D, Baatenburg de Jong RJ. Incidence and prediction of major cardiovascular complications in head and neck surgery. Head Neck. 2010;32:1485-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Dean NR, Rosenthal EL, Carroll WR, Kostrzewa JP, Jones VL, Desmond RA, Clemons L, Magnuson JS. Robotic-assisted surgery for primary or recurrent oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1930] [Cited by in RCA: 1834] [Article Influence: 101.9] [Reference Citation Analysis (0)] |
| 41. | Ansarin M, Pietrobon G, Tagliabue M, Mossinelli C, Ruju F, Maffini F, Rocca MC, Alterio D, Simon C, Zorzi SF. Salvage transoral robotic surgery in recurrent oropharyngeal carcinoma: a single-center retrospective study. Eur Arch Otorhinolaryngol. 2024;281:3167-3177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Sievert M, Goncalves M, Zbidat A, Traxdorf M, Mueller SK, Iro H, Gostian AO. Outcomes of transoral laser microsurgery and transoral robotic surgery in oropharyngeal squamous cell carcinoma. Auris Nasus Larynx. 2021;48:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Rujan SA, Bertesteanu SVG, Grigore R, Popescu B, Condeescu-Cojocarita M, Alexandru N, Bertesteanu GS, Schipor-Diaconu TE, Cirstea AI, Tudosie MD, Popescu ID, Taher BP. A Review and Comparative Analysis of Transoral Surgical Treatment versus Conservative Management in Early-Stage Oropharyngeal Cancer. J Pers Med. 2024;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |