Published online May 12, 2015. doi: 10.5318/wjo.v5.i2.45
Peer-review started: December 1, 2014
First decision: January 20, 2015
Revised: February 10, 2015
Accepted: April 1, 2015
Article in press: April 7, 2015
Published online: May 12, 2015
Processing time: 170 Days and 9 Hours
This work comprehensively reviews the latest treatment options for diabetic macular edema (DME) used in its management and presents further work on the topic. Diabetic retinopathy is an important and increasingly prevalent cause of preventable blindness worldwide. To meet this increasing burden there has recently been a proliferation of pharmacological therapies being used in clinical practice. A variety of medical treatment options now exist for DME. These include non-steroidal anti-inflammatory drugs such as nepafenac, as well as intravitreal steroids like triamcinolone (kenalog). Long-term results up to 7 years after commencing treatment are presented for triamcinolone. Studies are reviewed on the use of dexamethasone (ozurdex) and fluocinolone (Retisert and Iluvien implants) including the FAME studies. A variety of anti-vascular endothelial growth factor (anti-VEGF) agents used in DME are considered in detail including ranibizumab (lucentis) and the RESTORE, RIDE, RISE and Diabetic Retinopathy Clinical Research Network (DRCR.net) studies. Bevacizumab (avastin) and pegaptinib (macugen) are also considered. The use of aflibercept (eylea) is reviewed including the significance of the DA VINCI, VISTA-DME, VIVID-DME and the DRCR.net studies which have recently suggested potentially greater efficacy when treating DME for aflibercept in patients with more severely reduced visual acuity at baseline. Evidence for the anti-VEGF agent bevasiranib is also considered. Studies of anti-tumour necrosis factor agents like infliximab are reviewed. So are studies of other agents targeting inflammation including minocycline, rapamycin (sirolimus) and protein kinase C inhibitors such as midostaurin and ruboxistaurin. The protein kinase C β inhibitor Diabetic Macular Edema Study is considered. Other agents which have been suggested for DME are discussed including cyclo-oxygenase-2 inhibitors like celecoxib, phospholipase A2 inhibitors, recombinant erythropoietin, and monoclonal anti-interleukin antibodies such as canakinumab. The management of DME in a variety of clinical scenarios is also discussed - in newly diagnosed DME, refractory DME including after macular laser, and postoperatively after intraocular surgery. Results of long-term intravitreal triamcinolone for DME administered up to seven years after commencing treatment are considered in the context of the niche roles available for such agents in modern management of DME. This is alongside more widely used treatments available to the practitioner such as anti-VEGF agents like aflibercept (Eylea) and ranibizumab (Lucentis) which at present are the mainstay of pharmacological treatment of DME.
Core tip: Current evidence suggests the anti-vascular endothelial growth factor (anti-VEGF) agents aflibercept and ranibizumab are the most effective agents for most patients with diabetic macular edema. Aflibercept may be more effective when vision is very low. Other drugs retain niche roles including bevacizumab owing to lower costs, steroids like triamcinolone which can be effective many years later, dexamethasone and non-steroidal anti-inflammatory drugs like nepafenac. Also considered are anti-tumour necrosis factor agents like infliximab, anti-interleukins like canakinumab, anti-inflammatories including minocycline, rapamycin (sirolimus) and protein kinase C inhibitors midostaurin and ruboxistaurin. Fluocinolone implants, anti-VEGF agents bevasiranib and pegaptinib, cyclo-oxygenase-2 inhibitors like celecoxib, phospholipase A2 inhibitors and recombinant erythropoietin are discussed.
- Citation: Zaidi FH, Ansari E. New treatments for diabetic macular edema. World J Ophthalmol 2015; 5(2): 45-54
- URL: https://www.wjgnet.com/2218-6239/full/v5/i2/45.htm
- DOI: https://dx.doi.org/10.5318/wjo.v5.i2.45
Diabetic retinopathy is the principle cause of blindness in younger adults[1,2]. Almost 350 million people are affected by diabetes worldwide and this massive prevalence is expected to double by 2030[3]. The blinding complications of the disease make it a major cause of global visual morbidity in many countries[4-17]. While previously retinal laser had been the mainstay of treatment, a variety of non-laser treatment options have become available relatively recently for the treatment of diabetic macular edema (DME)[18-33]. These include anti-vascular endothelial growth factor (anti-VEGF) agents and a variety of steroid preparations as well as non-steroidal anti-inflammatory drugs (NSAIDs). These agents, alone and/or in combination with macular laser, are used to treat DME in varying treatment regimes in different parts of the world. Newer agents like infliximab are also being used to treat DME and interest is growing in monoclonal anti-interleukin antibodies such as canakinumab. The evidence for the use of these modalities of treatment will be considered as well as other targets for inflammation such as minocycline, rapamycin (sirolimus) and the protein kinase C Inhibitors midostaurin and ruboxistaurin. Other agents which have been suggested for DME are discussed including cyclo-oxygenase-2 (COX-2) inhibitors like celecoxib, phospholipase A2 inhibitors and recombinant erythropoietin.
Steroids are an older treatment for DME. Interest in these agents has recently been rekindled with the introduction of sustained release depot preparations. Despite new pharmacologic agents steroids still retain an important niche in modern clinical management - topical steroids are still used for the treatment of DME occurring after cataract surgery, as are NSAIDs.
Cataract surgery in patients with pre-existing DME may exacerbate the extent of edema[34-36]. It has been suggested by a number of studies that the incidence of DME increases after even uncomplicated cataract surgery in the absence of pre-operative DME[37-40]. Intensive postoperative topical steroids can help reduce macular thickness in postoperative DME, and may be given in combination with topical NSAIDs. A variety of NSAIDs have been used in this context. More recently a NSAID pro-drug, nepafenac 0.1%, administered topically to the eye, has been shown to have considerable efficacy with treatment usually taking 3-4 wk to make a significant benefit to visual acuity and macular thickness[41].
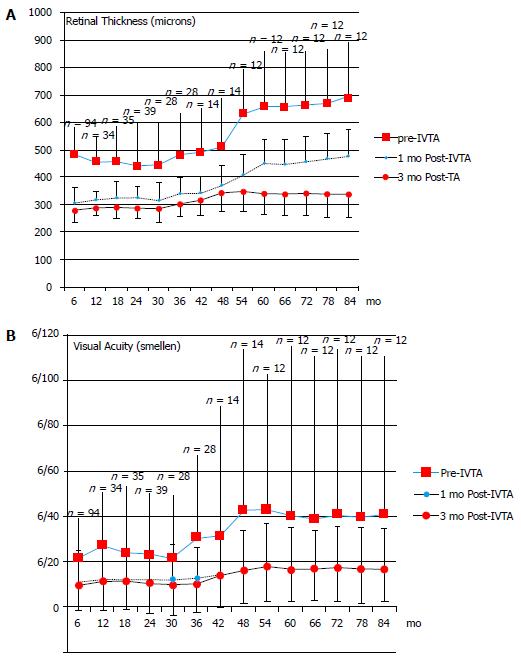
Triamcinolone (kenalog), a short-acting intravitreal steroid, is better-established in clinical practice and has been shown to improve visual acuity and central macular thickness in DME even several years after starting injections in selected patients[42]. Triamcinolone still retains a niche in the management of DME[42-61]. For example some patients do not want to undergo three intravitreal loading doses required in most anti-VEGF treatment protocols for DME. Further, evidence exists for long-term retinal complications including atrophy with anti-VEGF use in age-related macular degeneration, and the drugs are not freely available in a sterile form in all parts of the world[62]. A further practical utility is that triamcinolone permits the effect of intravitreal steroids, including on intraocular pressure, to be evaluated in patients before administering a longer-term depot steroid for DME. Identification of steroid-responders prior to administering a longer term depot steroid can be of significant benefit to selected patients where such a tendency is suspected[43]. Patients from initial work by the authors of 92 eyes administered intravitreal triamcinolone (IVTA) over 5 years have been followed up for a total of 7 years[42]. Inclusion criteria comprised all eyes with diabetic macular oedema injected with 4 mg/mL IVTA till treatment failed or was discontinued, often owing to the emergence of anti-VEGF treatment (frequently after 7 years). Exclusion criteria were subjects with non-diabetic oedema (uveitis, vascular, post-operative) and baseline foveal ischaemia. Visual acuity, central retinal thickness from optical coherence tomography prior to, 1 mo after (± 1 wk) and 3 mo post-IVTA (± 2 wk), the presence of complications, and fundus fluorescein angiographic data were recorded. Repeat IVTA injections continued to be effective in improving visual acuity and reducing DME in 76% of subjects (P < 0.02), including after multiple injections (mean 10 IVTA injections/patient by seven years) (Figure 1). In 24% of subjects foveal ischaemia limited outcome, usually 36-54 mo post-initial treatment. In 8% (n = 7) of subjects one repeat injection of IVTA was sufficient to stop leakage or cause a persistent reduction in macular thickness on OCT in excess of 100 microns for 2 to 3 years. IVTA could offer significant sustained visual benefit and reduction in macular thickness up to 7 years after initiation of therapy in some select patients, including after multiple injections. In certain subjects not selected for anti-VEGF treatment therapeutic potential was limited by the development of foveal ischaemia 2 to 7 years after treatment was commenced.
However it is worth remembering that treatment with IVTA is associated with cataract and also glaucoma which is significant in over 50% of patients[43]. Triamcinolone has also been associated with a reduction in progression of diabetic retinopathy but only in eyes with proliferative diabetic retinopathy, which is relevant since this can co-exist with DME[63]. However in this context the newer anti-VEGF agent ranibizumab remains more effective than triamcinolone, and also reduces progression of diabetic retinopathy in the absence of proliferative disease, a situation where triamcinolone is of limited value[63].
Dexamethasone sustained-release intravitreal implant (Ozurdex, Allergan, Inc.) is a relatively new drug that is injected as a depot into the eye at a dose of 0.7 mg. It is not used in aphakes as the depot may migrate to the corneal endothelium and cause corneal decompensation. It has been combined with laser photocoagulation and compared with laser treatment alone in diffuse DME in a 12-mo multicentre randomised controlled trial conducted by Callanan et al[64]. Patients with diffuse DME on fluorescein angiography had a greater mean improvement in best corrected visual acuity (BCVA) with Ozurdex combined with laser treatment in comparison to laser therapy alone (7.9. to 2.3 letters). There was also an additional reduction in vascular leakage with the additional Ozurdex implant beyond the use of laser therapy alone. Predictably there was an increase in intraocular pressure with Ozurdex. By month 12 of the study there was no significant difference between the two groups, though during the study consistent improvements in visual acuity were found in patients treated with combined Ozurdex and laser. Sustained release depot steroids are relatively contraindicated in patients with glaucoma and in non-pseudophakes but they do offer utility in patients who are unwilling to undergo the higher injection frequency necessitated with intravitreal ranibizumab. The initial implantation method could cause serious technical complications till the recent past, however the current injection technique and injectors are much safer and experience and confidence in their use has grown recently.
Fluocinolone has been used in two delivery systems to treat DME. First a non-bio-erodable extended-release implant was sutured onto the sclera (Retisert, Bausch and Lomb, Rochester, New York). Two phase-II studies showed benefit to macular thickness in DME[65]. Later an extended-release injectable device (iluvien, alimera, alpharetta, georgia) was studied, including in the FAME studies[66]. These were two Phase III randomised control trials of 956 patients with persistent DME who had previously undergone macular laser. Patients received either intravitreal fluocinolone acetonide or sham injection. By the end of the study 28% of patients receiving fluocinolone acetonide found an improvement in BCVA of 15 letters at 24 mo as opposed to 16% of sham-treated patients[66]. Both modes of fluocinolone acetonide administration have been associated with cataract formation and a rise in intraocular pressure.
VEGF is elevated in the aqueous and vitreous humour in proportion to the extent of DME[67]. Monoclonal antibodies (anti-VEGF agents) have been used to target VEGF. Ranibizumab (Lucentis) has rapidly become the default treatment for DME in many countries in view of significant prolonged improvements in visual acuity[68,69]. Muether et al[70] studied VEGF-A levels in aqueous humour samples from 17 eyes in patients with DME before injection of intravitreal ranibizumab. They found total suppression of VEGF-A in all patients after ranibizumab injections for, on average, 33.7 d (median 34 d) with considerable variation between individuals (range: 27-42 d). RESTORE was a 12-mo phase III randomised controlled trial with 345 subjects. It found ranibizumab either on its own or when combined with laser therapy was better than laser in terms of improving mean BCVA for the entire duration of the study[68]. These improvements have been found to continue into 36 mo after commencing treatment in a phase III 3-year randomised controlled trial conducted by Brown et al[71].
RIDE and RISE are also phase III randomised clinical trials and aim to evaluate the safety and efficacy of intravitreal ranibizumab in DME[69]. The proportions of patients gaining 15 letters or more from baseline in month 36 were as follows in the sham, 0.3 mg, and 0.5 mg ranibizumab groups (patients receiving sham injections were able to cross over to 0.5 mg in the third year of the study): in RIDE 19.2%, 36.8%, and 40.2%, respectively, and in RISE 22.0%, 51.2%, and 41.6%, respectively. The incidence of serious adverse events which might possibly be related to anti-VEGF suppression were 19.7% in the 0.5 mg ranibizumab group compared with 16.8% in the 0.3 mg group.
Unlike ranibizumab there is considerably less data on outcomes for bevacizumab (avastin), which worldwide is another widely-used anti-VEGF agent[72]. There is evidence that in patients with a central macular thickness of 400 μm the retina is less responsive to bevacizumab in comparison with ranibizumab[73]. In a randomised study of 60 eyes out of 45 patients who completed the study Nepomuceno et al[67] compared intravitreal bevacizumab with intravitreal ranibizumab in DME. While there was a significant rise in mean BCVA in both groups, as well as at all stages of the study (P < 0.05), this benefit was significantly greater in the group of eyes receiving intravitreal ranibizumab compared with the intravitreal bevacizumab group throughout weeks 8 (P = 0.032) and 32 (P = 0.042). Mean central subfield thickness improvement was noted in both groups at all study visits but with no difference between the groups. Intravitreal injections can be very painful for some patients (occasionally excruciatingly so) and it is hence worth noting that the mean number of injections administered was significantly higher (P = 0.005) in the group receiving intravitreal bevacizumab (9.84) over the intravitreal ranibizumab group (7.67). The conclusions of the authors of this study are important. Through one whole year of follow-up, while intravitreal bevacizumab and intravitreal ranibizumab appear to be associated with a similar reduction in central macular thickness, intravitreal ranibizumab is associated with greater improvement in BCVA at some visits. Further, intravitreal bevacizumab is associated with a greater number of intravitreal injections.
The evidence suggests that ranibizumab certainly appears more effective than bevacizumab for the management of DME. However in developing countries cost is an important factor to bear in mind, as ranibizumab (lucentis) is vastly more expensive than bevacizumab (avastin). The Diabetic Retinopathy Clinical Research Network have reported that ranibizumab can cause transient regression of proliferative diabetic retinopathy[49]. Other workers have shown it may decrease the cumulative probability of deterioration of diabetic retinopathy[74]. These factors are relevant to appraising the drug in DME especially where proliferative disease is co-existing.
An interesting concept with relevance to the clinician is whether VEGF suppression may prevent postoperative diabetic macular oedema in patients undergoing cataract surgery. It has been shown that VEGF levels in aqueous humour peak one day after cataract surgery and normalize one month after cataract surgery[75]. In a randomised controlled trial Chae et al[76] evaluated whether intravitreal ranibizumab administered at the time of cataract surgery prevents macular edema in patients without DME but with otherwise stable diabetic retinopathy. The sham group compared with the ranibizumab group had significantly greater increases in central macula thickness and macula volume, and worse BCVA from baseline to six months postoperatively. This suggests that ranibizumab is an effective prophylactic agent in reducing the severity and risk of DME at the time of phacoemulsification cataract surgery. However, in this regard, bevacizumab has also been shown to be effective when used in this capacity in two randomised controlled trials, one of 30 eyes by Salehi et al[36] and one of 68 eyes undergoing cataract surgery by Cheema et al[77].
Intraocular pressure rises acutely after intravitreal injection. However evidence is accumulating that anti-VEGF agents may increase the risk of long-term sustained rises in intra-ocular pressure. Very recently a major randomised control trial of 582 eyes from 486 patients has been published by Bressler and colleagues to address this issue. Patients were randomised to intravitreal ranibizumab with deferred macula laser or to sham injection with early laser. The researchers found evidence for sustained long-term pressure rises necessitating topical pressure-lowering treatment in patients receiving ranibizumab. The cumulative probability of a sustained elevation of intraocular pressure or commencing of pressure-lowering treatment at 3 years was 9.5% for patients in the ranibizumab arm vs 3.4% for patients in the sham injection arm[78].
Aflibercept (eylea) is a recombinant fusion protein which binds to VEGF serving as a “VEGF Trap” thereby inhibiting the action of VEGF-A, VEGF-B and placental growth factor[79,80]. The DA VINCI study enrolled 221 patients with centre-involving DME and a BCVA of between 20/40 and 20/320 who were randomised into four groups each receiving various dosing regimes of intravitreal VEGF-Trap and one other group receiving macular laser in place of VEGF-Trap[80]. Improvements in BCVA were found in eyes injected with VEGF-Trap of 8.5 to 11.4 letters vs 2.5 letters in eyes receiving laser. By week 52 eyes receiving VEGF-Trap displayed a mean change in BCVA of 9.7 to 13.1 letters vs a loss of 1.3 letters in eyes receiving laser. As there was no significant difference between groups receiving VEGF-Trap this supported the lower dosing frequency regime of 8-weekly rather than 4-weekly injections with VEGF-Trap. The VISTA-DME and VIVID-DME studies were large studies of aflibercept which aimed to have sufficient power to study the safety profile of VEGF-Trap[81]. They were both similarly designed phase 3 randomised control trials enrolling in total 872 patients with DME who were randomised to various dosing regimes of intravitreal aflibercept or macular laser. The study groups joined their findings to increase the power of the study. Eyes receiving aflibercept performed significantly better by week 52 after starting treatment and in terms of safety profile aflibercept was well-tolerated.
Most recently the Diabetic Retinopathy Clinical Network has published a randomised control trial of 660 patients comparing aflibercept, ranibizumab and bevacizumab[82]. The principle outcome studied was the effect of intravitreal injections of these agents on visual acuity at one year. At low levels of initial visual acuity aflibercept was more effective in improving visual acuity at one year, while at higher initial levels of visual acuity the three agents were very similar in their effect of visual acuity at one year.
Pegaptinib (macugen) is a smaller molecule - a pegylated anti-VEGF agent aptamer which binds anti-VEGF. It has been studied in 260 subjects with DME and BCVA of 20/50 to 20/200. Subjects were randomised to receive either intravitreal pegaptinib or sham injection every 6 wk for 102 wk. Subjects received macular laser at 18 wk. By the end of the study subjects treated with pegaptinib gained on average 6.1 letters of vision compared with 1.3 letters in the sham group (P < 0.01). There was a similar incidence of side effects in the two groups, suggesting an acceptable systemic safety profile[83].
Bevasiranib is small interfering RNA molecule (siRNA) which inhibits intracellular transcription of VEGF messenger-RNA[84]. The RACE trial studied different doses of bevasiranib given for 3 mo[85]. Macular thickness was reduced from weeks 8 to 12 with improvements in visual acuity.
Tumour necrosis factor (TNF) is an important cytokine which has a fundamental role in the activity of the immune system as well as the human cell cycle. Infliximab is a monoclonal antibody that targets human TNF. It is typically administered systemically every 4-8 wk. The drug is currently at an early stage of evaluation in the context of reducing severity of diabetic retinopathy and studies are only of small numbers of patients. However the results offer some promise. A clinical improvement in vision from DME has been noted after two infusions of infliximab in 4 of 6 studied eyes with DME by Sfikakis et al[86]. A subsequent small Phase III study by the same group found an improvement of almost 25% in visual acuity in infliximab-treated eyes over eyes treated with placebo[87]. Systemic side effects were minimal. These side effects can sometimes be serious and are theoretically reduced by intravitreal formulation, which also enables the drug to be targeted to the retina. The drug has been formulated for intraocular use recently and intravitreal infliximab has recently been tried in Behcet’s Syndrome, and is likely to be trialled in DME in the near future[88].
It is well-recognised that inflammation has a role in DME[89]. Recently it has been suggested that up-regulation of the immune system in diabetes may in part be due to neuropathy of the bone marrow causing increased synthesis of inflammatory white cells and reduced production of endothelial progenitor cells affecting the permeability of the blood-retina barrier[89,90]. The increased inflammation may affect the hypothalamus to induce insulin resistance. Suppressing inflammation has been a target in DME. Recently minocycline, administered systemically, has been found to reduce central macular thickness in DME together with improvement in vision and vascular leakage[90]. It has been postulated that this is by inhibiting retinal microglial function, which otherwise shows a pattern of activation and aggregation in regions of DME[89].
Rapamycin (sirolimus) is a macrolide antibiotic which also suppresses the immune system[91,92]. It forms an intracellular complex which inhibits the mammalian target of rapamycin (mTOR), which is a protein kinase integrating growth factor-activated signals. These include those promoting VEGF-mediated angiogenesis. A “double” effect of rapamycin is that by inhibiting mTOR it may also down-regulate VEGF transcription. A small pilot study of five adult participants with DME has suggested a reasonable safety profile for rapamycin administered via this route and some potential benefit to vision and macular thickness, however the relatively small numbers preclude any conclusive statement on its efficacy in DME[93].
Hyperglycemic states induce de novo synthesis of diacylglycerol which activates protein kinase C (PKC)[94]. The oral PKC inhibitor midostaurin is both a protein kinase C inhibitor and anti-VEGF inhibitor, making it an attractive drug for use in DME. Further, the oral selective PKC β inhibitor ruboxistaurin may also have potential for improving or maintaining visual acuity in DME. A randomised study of 141 patients with DME receiving a variety of oral doses of PKC412 (which is midostaurin) vs placebo showed a significant reduction in macular thickness and a small improvement in visual acuity of 4.36 letters (P = 0.007) in patients receiving 100 mg per day of PKC412 by 3 mo[95]. However, gastrointestinal side effects were common owing to the lack of specificity of this group of drugs, and dose-related effects on glycaemic control and hepatotoxicity were also noted. In view of this the authors suggested targeting the drug for local ocular delivery. In the PKC-DRS2 study oral ruboxistaurin reduced the extent of sustained moderate visual loss, delayed progression of DME, reduced the need for laser treatment and improved visual outcomes in patients with nonproliferative diabetic retinopathy[96,97]. The protein kinase C β inhibitor Diabetic Macular Edema Study specifically studied outcomes in DME and showed that patients administered oral ruboxistaurin had less progression of DME compared with a placebo group during a 30-mo period[98].
Not all pharmacological agents have proven to be of benefit in treating DME. On the basis of the efficacy of NSAIDs it was thought that COX-2 inhibitors may be of benefit in diabetic retinopathy. However studies of the COX-2 inhibitor celecoxib have not shown any significant benefit in improving vision in DME, though did find some reduction in leakage on angiography[99]. Other drugs targeting the immune system are currently being studied in trials including phospholipase A2 inhibitors, recombinant erythropoietin, and anti-interleukin antibodies[89,100]. In fact a large number of potential agents have been suggested for use in diabetic retinopathy to target various components of the inflammatory pathway, many of which have not found clinical use. The most promising at present seem agents such as canakinumab which are monoclonal antibodies targeting interleukin. Animal studies have shown breakdown of the blood retina barrier and neurotoxicity to ganglion cells in the inner retina occurs in diabetes under the effect of oxidative stress and pro-inflammatory cytokines such as interleukin[100]. Studies in humans of antibodies blocking these pathways are still at an early stage but are being conducted to assess the effect of canakinumab in DME[89].
Evidence from a number of human studies and trials show several pharmacological agents have benefit in DME, to varying degrees. Till very recently the efficacy of ranibizumab seemed greatest, and remains accompanied by a large body of evidence, and a good ocular safety profile. Very recently evidence has emerged from a large RCT that aflibercept may be more efficacious in patients with poor vision at baseline[82]. However a variety of other drugs also carry benefits. These different drugs are relevant and important to consider as practical alternatives to ranibizumab and grid/focal macular laser, both of which may be perceived to be costly in some healthcare systems across the world. Further, DME is often a refractory and recurrent disease and diabetics undergo cataract and vitreoretinal surgery more frequently than most patients - clinical scenarios where the plurality of therapeutic options is highly useful for managing this common sight-threatening disease.
Most new pharmacological therapies are being investigated as multiple inflammatory pathways are involved in the development of DME[100]. In the longer term adjunctive treatments which block these pathways will likely be used alongside suppressors of vascular leakage[19,100]. For example, while ranibizumab reduces retinal oedema in DME, in future agents which protect ganglion cells may be used adjunctively alongside suppressors of capillary leakage to provide a multi-faceted approach to the management of DME.
P- Reviewer: Iacono P, Koleva-Georgieva DN S- Editor: Ma YJ L- Editor: A E- Editor: Liu SQ
| 1. | Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Ophthalmology. 1995;102:647-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 291] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54:1-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 356] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 3. | Park YG, Kim EY, Roh YJ. Laser-based strategies to treat diabetic macular edema: history and new promising therapies. J Ophthalmol. 2014;2014:769213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Jonas JB, Bourne RR, White RA, Flaxman SR, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Price H, Wong TY. Visual impairment and blindness due to macular diseases globally: a systematic review and meta-analysis. Am J Ophthalmol. 2014;158:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Katulanda P, Ranasinghe P, Jayawardena R. Prevalence of retinopathy among adults with self-reported diabetes mellitus: the Sri Lanka diabetes and Cardiovascular Study. BMC Ophthalmol. 2014;14:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Lamparter J, Raum P, Pfeiffer N, Peto T, Höhn R, Elflein H, Wild P, Schulz A, Schneider A, Mirshahi A. Prevalence and associations of diabetic retinopathy in a large cohort of prediabetic subjects: the Gutenberg Health Study. J Diabetes Complications. 2014;28:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Looker HC, Nyangoma SO, Cromie DT, Olson JA, Leese GP, Black MW, Doig J, Lee N, Lindsay RS, McKnight JA. Rates of referable eye disease in the Scottish National Diabetic Retinopathy Screening Programme. Br J Ophthalmol. 2014;98:790-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Jingi AM, Noubiap JJ, Ellong A, Bigna JJ, Mvogo CE. Epidemiology and treatment outcomes of diabetic retinopathy in a diabetic population from Cameroon. BMC Ophthalmol. 2014;14:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Keenan TD, Johnston RL, Donachie PH, Sparrow JM, Stratton IM, Scanlon P. United Kingdom National Ophthalmology Database Study: Diabetic Retinopathy; Report 1: prevalence of centre-involving diabetic macular oedema and other grades of maculopathy and retinopathy in hospital eye services. Eye (Lond). 2013;27:1397-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Looker HC, Nyangoma SO, Cromie DT, Olson JA, Leese GP, Philip S, Black MW, Doig J, Lee N, Briggs A. Predicted impact of extending the screening interval for diabetic retinopathy: the Scottish Diabetic Retinopathy Screening programme. Diabetologia. 2013;56:1716-1725. [PubMed] |
| 11. | Mackenzie S, Schmermer C, Charnley A, Sim D, Vikas Tah M, Nussey S, Egan C. SDOCT imaging to identify macular pathology in patients diagnosed with diabetic maculopathy by a digital photographic retinal screening programme. PLoS One. 2011;6:e14811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Mohamed QA, Ross A, Chu CJ. Diabetic retinopathy (treatment). BMJ Clin Evid. 2011;2011. [PubMed] |
| 13. | Rauf A, Malik R, Bunce C, Wormald R. The British Asian community eye study: outline of results on the prevalence of eye disease in British Asians with origins from the Indian subcontinent. Indian J Ophthalmol. 2013;61:53-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Jammal H, Khader Y, Alkhatib S, Abujbara M, Alomari M, Ajlouni K. Diabetic retinopathy in patients with newly diagnosed type 2 diabetes mellitus in Jordan: prevalence and associated factors. J Diabetes. 2013;5:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Burgess PI, MacCormick IJ, Harding SP, Bastawrous A, Beare NA, Garner P. Epidemiology of diabetic retinopathy and maculopathy in Africa: a systematic review. Diabet Med. 2013;30:399-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Al-Akily SA, Bamashmus MA, Gunaid AA. Causes of visual impairment and blindness among Yemenis with diabetes: a hospital-based study. East Mediterr Health J. 2011;17:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Al-Shakarchi FI. Blindness in iraq: leading causes, target patients, and barriers to treatment. Middle East Afr J Ophthalmol. 2011;18:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Martin DF, Maguire MG. Treatment Choice for Diabetic Macular Edema. N Engl J Med. 2015;372:1260-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retin Eye Res. 2008;27:622-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 295] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 20. | Azad R, Sain S, Sharma YR, Mahajan D. Comparison of intravitreal bevacizumab, intravitreal triamcinolone acetonide, and macular grid augmentation in refractory diffuse diabetic macular edema: A prospective, randomized study. Oman J Ophthalmol. 2012;5:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Soheilian M, Garfami KH, Ramezani A, Yaseri M, Peyman GA. Two-year results of a randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus laser in diabetic macular edema. Retina. 2012;32:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Soheilian M, Ramezani A, Yaseri M, Mirdehghan SA, Obudi A, Bijanzadeh B. Initial macular thickness and response to treatment in diabetic macular edema. Retina. 2011;31:1564-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. |
Shah AM, Bressler NM, Jampol LM; Does laser still have a role in the management of retinal vascular and neovascular diseases? |
| 24. | Ockrim Z, Yorston D. Managing diabetic retinopathy. BMJ. 2010;341:c5400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Mirshahi A, Shenazandi H, Lashay A, Faghihi H, Alimahmoudi A, Dianat S. Intravitreal triamcinolone as an adjunct to standard laser therapy in coexisting high-risk proliferative diabetic retinopathy and clinically significant macular edema. Retina. 2010;30:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Gillies MC, Simpson JM, Gaston C, Hunt G, Ali H, Zhu M, Sutter F. Five-year results of a randomized trial with open-label extension of triamcinolone acetonide for refractory diabetic macular edema. Ophthalmology. 2009;116:2182-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Rudnisky CJ, Lavergne V, Katz D. Visual acuity after intravitreal triamcinolone for diabetic macular edema refractory to laser treatment: a meta-analysis. Can J Ophthalmol. 2009;44:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M, Ahmadieh H, Dehghan MH, Azarmina M, Moradian S. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology. 2009;116:1142-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Faghihi H, Roohipoor R, Mohammadi SF, Hojat-Jalali K, Mirshahi A, Lashay A, Piri N, Faghihi Sh. Intravitreal bevacizumab versus combined bevacizumab-triamcinolone versus macular laser photocoagulation in diabetic macular edema. Eur J Ophthalmol. 2008;18:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Manasseh GS, Shao EH, Taylor SR. Treatment of diabetic maculopathy. Br J Hosp Med (Lond). 2015;76:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Farahvash MS, Mahmoudi AH, Farahvash MM, Tabatabaee A, Riazi M, Mohammadzadeh S, Faghihi H, Nilli-Ahmadabadi M, Mirshahi A, Karkhaneh R. The impact of macular laser photocoagulation on contrast sensitivity function in patients with clinically significant macular edema. Arch Iran Med. 2008;11:143-147. [PubMed] |
| 32. | Soheilian M, Ramezani A, Bijanzadeh B, Yaseri M, Ahmadieh H, Dehghan MH, Azarmina M, Moradian S, Tabatabaei H, Peyman GA. Intravitreal bevacizumab (avastin) injection alone or combined with triamcinolone versus macular photocoagulation as primary treatment of diabetic macular edema. Retina. 2007;27:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Dehghan MH, Ahmadieh H, Ramezani A, Entezari M, Anisian A. A randomized, placebo-controlled clinical trial of intravitreal triamcinolone for refractory diabetic macular edema. Int Ophthalmol. 2008;28:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Tsilimbaris MK, Tsika C, Diakonis V, Karavitaki A, Pallikaris I. Macular Edema and Cataract Surgery. Zaidi FH, Editor: US: InTech 2013; 323-336. |
| 35. | Javadi MA, Zarei-Ghanavati S. Cataracts in diabetic patients: a review article. J Ophthalmic Vis Res. 2008;3:52-65. [PubMed] |
| 36. | Salehi A, Beni AN, Razmjoo H, Beni ZN. Phacoemulcification with intravitreal bevacizumab injection in patients with cataract and coexisting diabetic retinopathy: prospective randomized study. J Ocul Pharmacol Ther. 2012;28:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Dowler JG, Hykin PG, Lightman SL, Hamilton AM. Visual acuity following extracapsular cataract extraction in diabetes: a meta-analysis. Eye (Lond). 1995;9:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Dowler JG, Hykin PG, Hamilton AM. Phacoemulsification versus extracapsular cataract extraction in patients with diabetes. Ophthalmology. 2000;107:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Dowler J, Hykin PG. Cataract surgery in diabetes. Curr Opin Ophthalmol. 2001;12:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Schatz H, Atienza D, McDonald HR, Johnson RN. Severe diabetic retinopathy after cataract surgery. Am J Ophthalmol. 1994;117:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Hariprasad SM, Callanan D, Gainey S, He YG, Warren K. Cystoid and diabetic macular edema treated with nepafenac 0.1%. J Ocul Pharmacol Ther. 2007;23:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Zaidi F, Ansari E. Long-term result of intravitreal steroid for macular oedema: 2 to 5 year follow-up of 92 eyes. Acta Ophthalmologica. 2009;87:s244. |
| 43. | Ansari EA, Ali N. Intraocular pressure following intravitreal injection of triamcinolone acetonide. Open Ophthalmol J. 2008;2:119-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Grover D, Li TJ, Chong CC. Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev. 2008;CD005656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Yilmaz T, Weaver CD, Gallagher MJ, Cordero-Coma M, Cervantes-Castaneda RA, Klisovic D, Lavaque AJ, Larson RJ. Intravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: a systematic review. Ophthalmology. 2009;116:902-911; quiz 912-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Gibran SK, Khan K, Jungkim S, Cleary PE. Optical coherence tomographic pattern may predict visual outcome after intravitreal triamcinolone for diabetic macular edema. Ophthalmology. 2007;114:890-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Gómez-Ulla F, Marticorena J, Alfaro DV, Fernández M, Méndez ER, Rothen M. Intravitreal triamcinolone for the treatment of diabetic macular edema. Curr Diabetes Rev. 2006;2:99-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Schwartz SG, Flynn HW, Scott IU. Pharmacotherapy for diabetic retinopathy. Expert Opin Pharmacother. 2009;10:1123-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064-1077.e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1061] [Cited by in RCA: 1029] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 50. | Jonas JB, Söfker A. Intraocular injection of crystalline cortisone as adjunctive treatment of diabetic macular edema. Am J Ophthalmol. 2001;132:425-427. [PubMed] |
| 51. | Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 270] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 52. | Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL, Friedman SM, Glassman AR, Scott IU, Stockdale CR, Sun JK. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 413] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 53. | Diabetic Retinopathy Clinical Research Network (DRCR. net), Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, Hartnett E, Ip MS, Kim JE, Kollman C. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 54. | Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R, Caulin C, Gaudric A. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218-224; discussion 224-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 55. | Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trial. Ophthalmology. 2004;111:2044-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 56. | Moshfeghi DM, Kaiser PK, Bakri SJ, Kaiser RS, Maturi RK, Sears JE, Scott IU, Belmont J, Beer PM, Quiroz-Mercado H. Presumed sterile endophthalmitis following intravitreal triamcinolone acetonide injection. Ophthalmic Surg Lasers Imaging. 2005;36:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 299] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 57. | Ockrim ZK, Sivaprasad S, Falk S, Roghani S, Bunce C, Gregor Z, Hykin P. Intravitreal triamcinolone versus laser photocoagulation for persistent diabetic macular oedema. Br J Ophthalmol. 2008;92:795-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Jonas JB, Degenring R, Kreissig I, Akkoyun I. Safety of intravitreal high-dose reinjections of triamcinolone acetonide. Am J Ophthalmol. 2004;138:1054-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Jonas JB, Harder B, Kamppeter BA. Inter-eye difference in diabetic macular edema after unilateral intravitreal injection of triamcinolone acetonide. Am J Ophthalmol. 2004;138:970-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Gillies MC, Islam FM, Zhu M, Larsson J, Wong TY. Efficacy and safety of multiple intravitreal triamcinolone injections for refractory diabetic macular oedema. Br J Ophthalmol. 2007;91:1323-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Shimura M, Nakazawa T, Yasuda K, Shiono T, Iida T, Sakamoto T, Nishida K. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008;145:854-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 62. | Tanaka E, Chaikitmongkol V, Bressler SB, Bressler NM. Vision-threatening lesions developing with longer-term follow-up after treatment of neovascular age-related macular degeneration. Ophthalmology. 2015;122:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Bressler SB, Qin H, Melia M, Bressler NM, Beck RW, Chan CK, Grover S, Miller DG. Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol. 2013;131:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 64. | Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, Liu CC, Li XY, Hollander DA, Schiffman RM, Whitcup SM; Ozurdex PLACID Study Group. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013;120:1843-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 65. | Schwartz SG, Flynn HW Jr, Scott IU. Intravitreal Corticosteroids in the Management of Diabetic Macular Edema. Curr Ophthalmol Rep. 2013;1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, Tolentino M, Gupta A, Duarte L, Madreperla S, Gonder J, Kapik B, Billman K, Kane FE; FAME Study Group. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626-635.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 67. | Nepomuceno AB, Takaki E, Paes de Almeida FP, Peroni R, Cardillo JA, Siqueira RC, Scott IU, Messias A, Jorge R. A prospective randomized trial of intravitreal bevacizumab versus ranibizumab for the management of diabetic macular edema. Am J Ophthalmol. 2013;156:502-10.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 68. | Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A; RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 991] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 69. | Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS; RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1229] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 70. | Muether PS, Droege KM, Fauser S. Vascular endothelial growth factor suppression times in patients with diabetic macular oedema treated with ranibizumab. Br J Ophthalmol. 2014;98:179-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG, Adamis AP, Ehrlich JS, Hopkins JJ; RIDE and RISE Research Group. RIDE and RISE Research Group. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 636] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 72. | Arevalo JF. Diabetic macular edema: changing treatment paradigms. Curr Opin Ophthalmol. 2014;25:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Sivaprasad S, Crosby-Nwaobi R, Esposti SD, Peto T, Rajendram R, Michaelides M, Hykin P. Structural and functional measures of efficacy in response to bevacizumab monotherapy in diabetic macular oedema: exploratory analyses of the BOLT study (report 4). PLoS One. 2013;8:e72755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130:1145-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 75. | Patel JI, Hykin PG, Cree IA. Diabetic cataract removal: postoperative progression of maculopathy--growth factor and clinical analysis. Br J Ophthalmol. 2006;90:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Chae JB, Joe SG, Yang SJ, Lee JY, Sung KR, Kim JY, Kim JG, Yoon YH. Effect of combined cataract surgery and ranibizumab injection in postoperative macular edema in nonproliferative diabetic retinopathy. Retina. 2014;34:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Cheema RA, Al-Mubarak MM, Amin YM, Cheema MA. Role of combined cataract surgery and intravitreal bevacizumab injection in preventing progression of diabetic retinopathy: prospective randomized study. J Cataract Refract Surg. 2009;35:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Bressler SB, Almukhtar T, Bhorade A, Bressler NM, Glassman AR, Huang SS, Jampol LM, Kim JE, Melia M; for the Diabetic Retinopathy Clinical Research Network Investigators. Repeated Intravitreous Ranibizumab Injections for Diabetic Macular Edema and the Risk of Sustained Elevation of Intraocular Pressure or the Need for Ocular Hypotensive Treatment. JAMA Ophthalmol. 2015;Feb 26; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 79. | Stefanini FR, Badaró E, Falabella P, Koss M, Farah ME, Maia M. Anti-VEGF for the management of diabetic macular edema. J Immunol Res. 2014;2014:632307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | Do DV, Schmidt-Erfurth U, Gonzalez VH, Gordon CM, Tolentino M, Berliner AJ, Vitti R, Rückert R, Sandbrink R, Stein D. The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011;118:1819-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 81. | Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H, Marcus DM. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 595] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 82. | The Diabetic Retinopathy Clinical Research Network. Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, Melia M, Pieramici DJ, Sun JK, Beck RW. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. N Engl J Med. 2015;372:1193-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1174] [Cited by in RCA: 1137] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 83. | Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS; Macugen 1013 Study Group. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology. 2011;118:1107-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 84. | Mousa SA, Mousa SS. Current status of vascular endothelial growth factor inhibition in age-related macular degeneration. BioDrugs. 2010;24:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 85. | Bandello F, De Benedetto U, Knutsson KA, Parodi MB, Cascavilla ML, Iacono P. Evidence for Anti-vascular Endothelial Growth Factor Treatment of Diabetic Macular Oedema. European Endocrinol. 2012;8:36-41. |
| 86. | Sfikakis PP, Markomichelakis N, Theodossiadis GP, Grigoropoulos V, Katsilambros N, Theodossiadis PG. Regression of sight-threatening macular edema in type 2 diabetes following treatment with the anti-tumor necrosis factor monoclonal antibody infliximab. Diabetes Care. 2005;28:445-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Sfikakis PP, Grigoropoulos V, Emfietzoglou I, Theodossiadis G, Tentolouris N, Delicha E, Katsiari C, Alexiadou K, Hatziagelaki E, Theodossiadis PG. Infliximab for diabetic macular edema refractory to laser photocoagulation: a randomized, double-blind, placebo-controlled, crossover, 32-week study. Diabetes Care. 2010;33:1523-1528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 88. | Markomichelakis N, Delicha E, Masselos S, Sfikakis PP. Intravitreal infliximab for sight-threatening relapsing uveitis in Behçet disease: a pilot study in 15 patients. Am J Ophthalmol. 2012;154:534-541.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 89. | Lingam G, Wong TY. Systemic medical management of diabetic retinopathy. Middle East Afr J Ophthalmol. 2013;20:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 90. | Cukras CA, Petrou P, Chew EY, Meyerle CB, Wong WT. Oral minocycline for the treatment of diabetic macular edema (DME): results of a phase I/II clinical study. Invest Ophthalmol Vis Sci. 2012;53:3865-3874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 91. | Sausville EA, Elsayed Y, Monga M, Kim G. Signal transduction--directed cancer treatments. Annu Rev Pharmacol Toxicol. 2003;43:199-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S-14S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 532] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 93. | Krishnadev N, Forooghian F, Cukras C, Wong W, Saligan L, Chew EY, Nussenblatt R, Ferris F, Meyerle C. Subconjunctival sirolimus in the treatment of diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2011;249:1627-1633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Abu El-Asrar AM. Evolving strategies in the management of diabetic retinopathy. Middle East Afr J Ophthalmol. 2013;20:273-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Campochiaro PA; C99-PKC412-003 Study Group. Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Invest Ophthalmol Vis Sci. 2004;45:922-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 96. | PKC-DRS2 Group, Aiello LP, Davis MD, Girach A, Kles KA, Milton RC, Sheetz MJ, Vignati L, Zhi XE. Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113:2221-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Aiello LP, Vignati L, Sheetz MJ, Zhi X, Girach A, Davis MD, Wolka AM, Shahri N, Milton RC; PKC-DRS and PKC-DRS2 Study Groups. Oral protein kinase c β inhibition using ruboxistaurin: efficacy, safety, and causes of vision loss among 813 patients (1,392 eyes) with diabetic retinopathy in the Protein Kinase C β Inhibitor-Diabetic Retinopathy Study and the Protein Kinase C β Inhibitor-Diabetic Retinopathy Study 2. Retina. 2011;31:2084-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 98. | PKC-DMES Study Group. Effect of ruboxistaurin in patients with diabetic macular edema: thirty-month results of the randomized PKC-DMES clinical trial. Arch Ophthalmol. 2007;125:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 99. | Chew EY, Kim J, Coleman HR, Aiello LP, Fish G, Ip M, Haller JA, Figueroa M, Martin D, Callanan D. Preliminary assessment of celecoxib and microdiode pulse laser treatment of diabetic macular edema. Retina. 2010;30:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 100. | Safi SZ, Qvist R, Kumar S, Batumalaie K, Ismail IS. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed Res Int. 2014;2014:801269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |