INTRODUCTION
The primary goal of diabetic vitrectomy is the restoration of vision. To achieve this goal, we remove the vitreous blood, reattach the macula and retina where traction is present, and remove any cataracts that are present. The second and extremely important aim of surgery is to control the diabetic neovascularization process to promote long-term anatomic and visual success. Diabetic patients undergo surgery consisting of core vitrectomy; the removal of the posterior hyaloid as far as the retinal detachment area, which is then dissected using delamination, segmentation, bimanual or en bloc techniques or combined procedure; the endophotocoagulation of the ischemic retina; and, in the majority of cases, the use of a tamponade, which could be gas (sulfur hexafluoride or perfluoropropane) or silicone oil (SO)[1-3]. When these surgical objectives are achieved and the 6 mo outcome is good, the eyes tend to remain stable for many years[1,4,5]. Tractional retinal detachment (TRD) involving the macula and nonclearing vitreous hemorrhage are the most common indications for diabetic vitrectomy[1]. Intraocular hemorrhage is a serious event during diabetic vitrectomy. Extensive hemorrhage may prevent the successful conclusion of surgery and may increase intraoperative complications. The removal of clotted blood may not only extend a preexisting retinal break but may also create new retinal breaks[6-8].
The most common indication for reoperation after diabetic surgery is recurrent vitreous hemorrhage. Approximately 60% of eyes develop recurrent vitreous hemorrhage sometime in the postoperative period[9]. Hemorrhage usually occurs within the first few days after surgery but may occur months or years later. Two-thirds of all hemorrhages occur during the first 6 mo[10]. Various surgical maneuvers have been utilized to prevent intraoperative vitreous hemorrhaging, such as increasing the intraocular pressure (IOP) by increasing the infusion of balanced salt solution (BSS), using the vented gas forced infusion (VGFI) setting on the vitrectomy machine, and using endodiathermy or perfluorocarbon as a surgical tool to stop the bleeding[11]. Bevacizumab (Avastin, Genentech Inc., San Francisco, CA), a full-length humanized monoclonal antibody against vascular endothelial growth factor (VEGF), was approved by the US Food and Drug Administration for the treatment of metastatic colorectal cancer[12-14]. Recent reports have indicated that intravitreal bevacizumab (IVB) injections show promise for targeting the VEGF-implicated intraocular neovascularization associated with age-related macular degeneration[15] and proliferative diabetic retinopathy (PDR)[16]. IVB has recently been shown to enhance the clearance of vitreous hemorrhage and to induce the involution of both retinal neovascularization[16,17] and anterior segment neovascularization[16,18,19]. Recently, numerous studies have reported the clinical outcomes of IVB applied as an adjunct to vitrectomy in the management of diabetic retinopathy. Bevacizumab can induce the regression of retinal neovascularization in patients with diabetes; therefore, it has been suggested that the presurgical administration of IVB might reduce intraoperative bleeding during vitrectomy for PDR[20]. However, the presurgical administration of IVB remains controversial[21]. Some studies have reported that bevacizumab pretreatment for diabetic vitrectomy did not influence the rates of postoperative vitreous hemorrhage or final visual acuity (VA). Although many surgeons perform IVB before vitrectomy in patients with diabetes, there are limited systematic studies or studies with large sample sizes demonstrating the benefits of IVB in facilitating surgery and improving clinical outcomes[21]. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without IVB pretreatment for severe diabetic retinopathy revealed that IVB injection before vitrectomy for PDR could reduce intraoperative bleeding, the frequency of endodiathermy, the mean surgical time, the reabsorption time of blood after vitrectomy and the incidence of recurrent vitreous hemorrhage (VH), as well as improving the best-corrected visual acuity (BCVA)[21] .
Despite the success of pars plana vitrectomy (PPV) in managing the severe complications of diabetic retinopathy, significant operative and postoperative complications still occur and may lead to anatomical failure and blindness. The recurrence of retinal detachment secondary to fibrovascular proliferation, progression of neovascularization with neovascular glaucoma, hypotony with subsequent phthisis bulbi, and fibrinoid syndrome are some of the many reported postoperative complications of diabetic vitrectomy. SO facilitates retinal reattachment by providing extended intraocular tamponade[22]. Oil may compartmentalize the eye and may play a role in inhibiting progressive neovascularization in the anterior segment by preventing the diffusion of angiogenic substances. In addition, SO can prevent hypotony and subsequent phthisis bulbi[23]. Castellarin et al[3] demonstrated the effectiveness of SO tamponade in cases of severe diabetic retinopathy.
MATERIALS AND METHODS
A retrospective interventional serial case study was performed on 84 eyes of 64 patients who underwent vitrectomy for severe diabetic retinal detachment at Holhos Uberlandia Eye Hospital between March 2007 and August 2013. The same surgeon (MAF) performed all of the surgeries. The possible risks and benefits of the treatment were explained to the patients before surgery, as was the off-label use of IVB. Informed consent was obtained before the procedures, in accordance with the Helsinki Declaration. Approval to review the patient data was obtained from the institutional review board. All patients underwent a complete ophthalmological examination with refraction, slit lamp examination, IOP measurement, fundus photography and fluorescein angiography, if possible. Additionally, ultrasound was performed in cases where the fundus could not be examined because of a cataract, vitreous hemorrhage or any other condition that obscured the fundus. Preoperative data were obtained from the final examination before surgery and intraoperative data were collected from the surgical description. Postoperative data were collected one day, one week and monthly after the surgery and data were also collected after SO removal, except for VA measurements which were performed before the surgery and at least one month after the SO removal. After surgery, the patients were instructed to return in the event of complications such as pain, decreased vision or any changes; otherwise, they returned on the following appointment date. The BCVA was measured using the Snellen chart and VA was converted to a logMAR score for analysis. All patients received a preoperative 1.25-mg IVB injection (Avastin, Genentech Inc.) and underwent 23-gauge transconjunctival sutureless vitrectomy using the Accurus vitrectomy machine (until January 2012) and the CONSTELLATION machine (after that period) (Alcon, Fort Worth, TX) and 1300-centistokes SO tamponade (Bausch and Lomb, Rochester, NY). Intraoperative complications, postoperative complications and postoperative outcomes were analyzed. The primary outcome was intraoperative and postoperative bleeding occurring during and after vitrectomy and SO removal. The secondary outcomes were any other complications that occurred during and after the two surgeries, the surgical time, the status of the lens and a comparison of the preoperative and postoperative BCVA values in logMAR. The statistical analysis was performed with GraphPad Prism 5 (GraphPad Software, La Jolla, CA) using a column analysis for the noncomparative analysis and Student’s paired t-test for the comparative study; P < 0.05 was considered statistically significant.
RESULTS
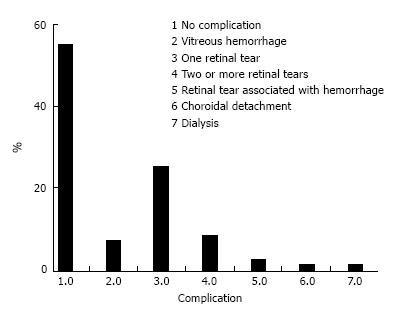
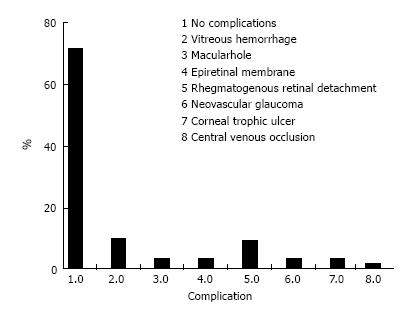
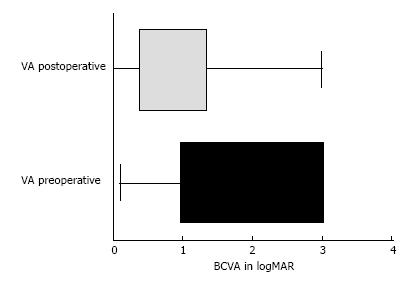
At the beginning of the chart review, 88 eyes of 68 patients met the inclusion criteria; however, 4 eyes (0.45%) developed phthisis bulbi during the follow-up period and were excluded from the statistical analysis. Thus, 84 eyes of 64 patients were included in the study. Forty-two patients were male (65.6%), and 22 (34.4%) were female. All the patients had severe or complicated diabetic retinal detachment. Three of 84 eyes (3.6%) had severe TRD; six patients (7.1%) had combined tractional and rhegmatogenous retinal detachment; and 75 (89.3%) had tractional retinal detachment with some degree of vitreous hemorrhage (Figures 1 and 2). The patients’ ages ranged from 30 to 86 years, with a mean ± SD of 61.25 ± 12.29 years. Bevacizumab was injected between 1 and 10 d before surgery, with a mean ± SD of 3.7 ± 2.2 d. Forty-six eyes (54.8%) had no complications during the surgery; 6 (7.1%) had vitreous hemorrhage; 21 (25%) had one retinal tear; 7 (8.3%) had two or more retinal tears, one of which was in the posterior pole, temporal to the fovea; 2 (2.4%) had a retinal tear associated with hemorrhage; 1 (1.2%) had choroidal detachment; and 1 (1.2%) had dialysis in the temporal entrance of the trocar (Figure 3). For the six patients (7.1%) who had hemorrhage during the vitrectomy, the hemorrhage was confined to the area between the SO and retina and the blood was reabsorbed in 30-90 d. In two other patients (2.4%), the hemorrhage occurred in the tear location and was also confined to the area between the SO and retina; the blood was reabsorbed in 90 and 120 d. The time until the removal of the SO ranged from 1 to 8 mo, with a mean ± SD of 4.16 ± 1.59. All patients had attached retinas and none had a vitreous cavity or preretinal hemorrhage before SO removal. During the postoperative period after SO removal, 60 eyes (71.4%) had no complications, 8 (9.5%) had vitreous hemorrhage, 2 (2.4%) had a macular hole (MH), 2 (2.4%) had an epiretinal membrane (ERM), 7 (8.3%) had rhegmatogenous retinal detachment, 2 (2.4%) had neovascular glaucoma, 2 (2.4%) had corneal trophic ulcers, and 1 (1.2%) had central venous occlusion (Figure 4). In the group that presented with vitreous hemorrhage after SO removal, 8 (9.5%) patients were followed for at least one month and were reoperated if the hemorrhage did not improve during this period. Five patients improved over periods varying from 30 to 60 d, and three patients were reoperated after 30 d of no improvement. In the patients who were reoperated, a new IVB injection was performed after the vitrectomy was completed. These patients did not present with rebleeding. Two patients (2.4%) had an MH that developed subsequently, after SO removal (1 year and 11 mo and 2 years and 5 mo). These patients were reoperated with the closure of the MH, using SF6 gas as a tamponade. Seven patients (8.3%) who had rhegmatogenous retinal detachment were reoperated to attach the retina; the retina remained attached until the final follow-up. The 2 (2.4%) patients who had ERM were reoperated with a good anatomic aspect of the macula. The two patients who presented with neovascular glaucoma were treated with the injection of 1.25 mg of bevacizumab into the anterior chamber and vitreous cavity as well as with the topical administration of brimonidine tartrate, timolol maleate, dorzolamide hydrochloride, atropine and prednisolone acetate. The IOP of one patient was controlled; the other patient underwent glaucoma surgery. The surgical time ranged from 40 to 120 min, with a mean of 77.8 ± 20.7 min. The follow-up ranged from 6 to 84 mo, with a mean of 33.90 ± 22.97 mo. The final status of the lens was 34 phakic eyes (40.5%) and 24 pseudophakic eyes (28.5%); in 26 eyes (31%), the lens was removed during vitrectomy or SO removal. The preoperative BCVA in logMAR ranged from 0.1 to 3.0, with a mean of 1.6 ± 0.9; the postoperative BCVA in logMAR ranged from 0.0 to 3.0, with a mean of 0.9 ± 0.7; the preoperative and postoperative BCVA values were significantly different (P < 0.0001) (Figure 5).
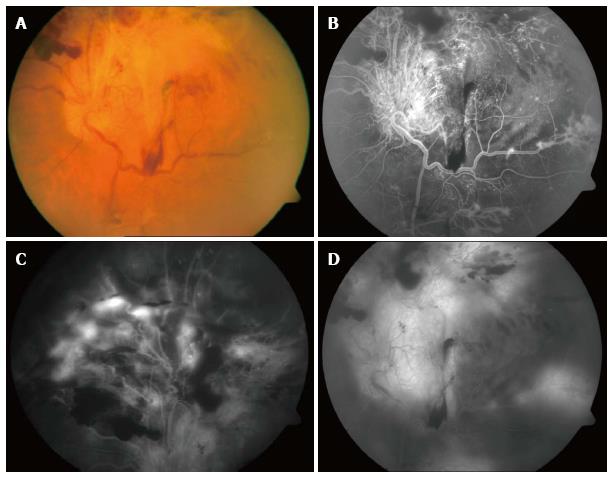
Figure 1 Preoperative images of the retina showing diabetic retinal detachment involving the posterior pole (A), the superior retina (B), and the region near the arcade (C), as well as subhyaloid and preretinal hemorrhage in the equator (D).
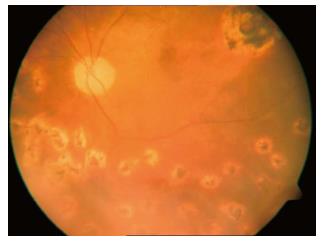
Figure 2 Postoperative image of the retina after silicone oil removal showing the retina attached and the posterior pole retinal tear treated with a laser.
Figure 3 The percentage of intraoperative complications.
Figure 4 Histogram showing postoperative complications: Frequency distribution.
Figure 5 Comparison of pre- and postoperative visual acuity: Horizontal box-and - whiskers plot.
VA: Visual acuity; BCVA: Best-corrected visual acuity.
DISCUSSION
Bevacizumab is a recombinant humanized monoclonal anti-VEGF antibody that is used to induce the regression of neovascularization and to reduce permeability of the vessels[24]. Bevacizumab is increasingly used to treat choroidal neovascularization and diabetic macular edema[25,26] and it has proven to be effective for the treatment of PDR complicated by vitreous hemorrhage[16,27,28]. In one study, the results of fluorescein angiography revealed a reduction in the leakage from the foci of neovascularization and the regression of the neovascular component of the fibrovascular tissue in eyes with PDR within 1 wk after IVB. Based on these observations, it was suggested that IVB may reduce the incidence of intraoperative hemorrhage during diabetic vitrectomy[29]. Chen first reported that IVB was helpful in facilitating vitrectomy for severe PDR[17]. Many clinical trials have shown that IVB before vitrectomy improves the condition of the fundus. In a study by Ahmadieh, preoperative IVB injection led to a significant resolution of VH and improvement of vision in nine eyes (25.7%) initially scheduled for vitrectomy to the degree that surgical intervention was no longer required[30].
Based on surgeons’ experience, the regression of the vascular component of the fibrovascular complexes after IVB facilitates the segmentation and delamination of membranes[31]. This result is due to the membranes being less adhesive to the underlying retina and being readily separated from the retina. The hemodynamic changes in retinal circulation that occur after IVB, such as constriction and decreased flow in new vessels, greatly reduce the likelihood of intraoperative bleeding. The analysis of our study’s primary outcome revealed that only 6 of 84 eyes (7.1%) had hemorrhage during the primary vitrectomy and 2 eyes (2.4%) had a retinal tear associated with hemorrhage at the location of the tear. Our study included 84 eyes; the majority of previously published studies had smaller numbers of patients[11,31-33]. Rizzo et al[11] observed mild intraoperative bleeding in 3 of 11 cases of PPV with IVB (PPV + IVB) and in 7 of 11 cases of PPV without IVB (PPV alone); they also reported severe intraoperative bleeding in 2 of 11 PPV+ IVB cases and 9 of 11 PPV alone cases. El-Batarny observed 6.8 ± 1.5 bleeding attacks/patient (range: 4-9) in 15 cases of PPV alone and 1.9 ± 1.1 bleeding attacks/patient (range: 0-4) in 15 cases of PPV + IVB[33]. In a systematic review and meta-analysis of the clinical outcomes of vitrectomy with or without IVB pretreatment for severe diabetic retinopathy, Zhao et al[21] found that IVB injection before vitrectomy for PDR reduced intraoperative bleeding, the frequency of endodiathermy and the mean surgical time. The above studies demonstrate that IVB is an important tool for reducing intraoperative bleeding and facilitating intraoperative surgical maneuvers, which is consistent with our findings. In the present work, postoperative bleeding after vitrectomy was confined to the area between the retina and the SO, which is in agreement with the findings of Castellarin et al[3] and Yeh et al[31].
Our surgical time ranged from 40 to 120 min, with a mean of 77.8 ± 20.7 min. This time was longer than that reported by El-Batarny[33] and Rizzo et al[11] for PPV associated with IVB. However, our study was retrospective and our surgical time was calculated from the anesthesiologist’s records and was most likely overestimated. The surgical time reported by El-Batarny was 61.6 ± 14.5 min (range: 40-90 min) in the PPV + IVB group[33]. Rizzo et al[11] reported a mean surgical time of 57 ± 9 min in the PPV+ IVB group. The diabetic retinal detachment in our study had a simple classification compared with the “Elliott” grading system used by Yeh et al[31] but most papers do not classify diabetic retinal detachment using this classification; as a result, comparing the cases would be impossible. Our study was limited to patients with TRD that encompassed a broad area; that threatened or involved the macula; that was or was not associated with vitreous hemorrhage; that varied in degree from mild, only in the inferior retina, to massive, involving the vitreous and subhyaloid spaces; and that sometimes covered the posterior pole or extended to close to the arcades. We also had several patients with combined retinal detachment. Our study included 3 eyes (3.6%) with severe TRD, six eyes (7.1%) with combined tractional and rhegmatogenous retinal detachment and 75 eyes (89.3%) with TRD with some degree of vitreous hemorrhage. After SO removal, 8 eyes (9.5%) had rebleeding; these were followed for at least one month and were reoperated if the hemorrhage did not improve in this period. Five patients improved over periods varying from 30 to 60 d, and three eyes (3.6%) were reoperated after 30 d of no improvement. In these patients, a new IVB injection was performed after the completion of the vitrectomy and rebleeding did not occur. Our rate of rebleeding after SO removal was low at 9.5% and only three patients (3.6%) had to undergo reoperation. El-Batarny reported a postoperative bleeding rate of 26.6% in the PPV group and none in the PPV + IVB group; however, there were only 15 patients in each group. We followed our patients for an average of 33.90 ± 22.97 mo (range: 6-84 mo); our follow-up period was long because we performed a single retrospective study with data collection beginning in March of 2007. This follow-up period was much longer than those of other studies which had follow-ups of approximately 6 mo[21]. We had 21 eyes (25%) with one retinal tear, 7 eyes (8.3%) with two or more retinal tears, 2 (2.4%) with retinal tears associated with hemorrhage and 1 case (1.2%) with dialysis in the temporal entrance of the trocar. We had 31 eyes with iatrogenic retinal tears (36.9%), a higher number than in other reports. Rizzo et al[11] reported 4/11 iatrogenic retinal tears in the PPV alone group and none in the PPV + IVB group. El-Batarny[33] reported iatrogenic breaks in 6 cases (40%) in the PPV alone group and in 3 cases (20%) in the PPV + IVB group. In the present work, the preoperative BCVA in logMAR ranged from 0.1 to 3.0, with a mean of 1.6 ± 0.9, and the postoperative BCVA in logMAR ranged from 0.0 to 3.0, with a mean of 0.9 ± 0.7; the preoperative and postoperative values were significantly different (P < 0.0001). Similar findings were reported by Zhao et al[21] in a meta-analysis of preoperative IVB for diabetic vitrectomy.
In conclusion, IVB may diminish intraoperative and postoperative bleeding, thus possibly facilitating intraoperative maneuvers, diminishing the complications in these very complex cases and playing a role in the final outcomes of these eyes. The postoperative bleeding in the SO-filled eyes was confined to the area between the retina and SO; therefore, SO may act as a secondary tool to prevent postoperative bleeding. Additional prospective studies with control groups and larger numbers of eyes will be necessary to confirm these hypotheses.
COMMENTS
Background
Retinal detachment is an important cause of visual impairment and blindness in diabetic patients; to treat these cases, the authors performed vitrectomy. In such cases, bleeding during vitrectomy is very common; for the vitrectomy to be successful, the surgeon must stop the bleeding. The intravitreal injection of bevacizumab (Avastin), a monoclonal antibody against vascular endothelial growth factor (VEGF), is used to prevent or decrease bleeding during the surgery, thus facilitating surgical maneuvers.
Research frontiers
Intravitreal bevacizumab (IVB) has been used for many conditions, including metastatic colon cancer and ocular conditions associated with VEGF, such as aged-related macular degeneration (ARMD), diabetic macular edema, retinal vein occlusion and proliferative diabetic retinopathy (PDR). Based on these findings, the use of Avastin to stop or decrease bleeding during vitrectomy in diabetic patients has become popular among retinal specialists.
Innovations and breakthroughs
This study in which a large number of patients were followed for long time after vitrectomy demonstrated that the use of Avastin is very promising in these cases because it decreases bleeding during and after surgery, which in turn facilitates the procedure and the surgical maneuvers by allowing the physician to operate in a clear medium, resulting in better postoperative outcomes.
Applications
These results suggest that Avastin should be prospectively compared to a sham or placebo in a large group, such as that in our study, to provide an evidence-based demonstration of the efficacy of this antibody.
Terminology
VEGF, or vascular endothelial growth factor, is directly involved in the neovascularization that occurs during pathological processes in the eye, such as ARMD and PDR. Bevacizumab is an antibody against VEGF, or an anti-VEGF antibody, which inhibits or blocks the growth of new vessels.
Peer review
The manuscript is a retrospective review. The only novel point is the long-term follow up.
P- Reviewer: Jhanji V, Malik A S- Editor: Song XX L- Editor: Roemmele A E- Editor: Lu YJ