Published online Aug 12, 2014. doi: 10.5318/wjo.v4.i3.52
Revised: May 16, 2014
Accepted: June 10, 2014
Published online: August 12, 2014
Processing time: 193 Days and 2 Hours
Ocular endoscopes enable ophthalmologists to observe any part of the retina without any limitations, including those caused by corneal opacities, the rim of the intraocular lens, cortical remnants, capsular opacities, a small pupil, and vitreous opacities. Moreover, ocular endoscopes enable the management of peripheral lesions without scleral indentation and are compatible with microincision vitrectomy surgery. The enlarged view under the endoscope, as obtained by drawing towards the lesion, appears to be another advantage. Rhegmatogenous retinal detachment with undetectable retinal breaks, trauma, endophthalmitis, scleral wounds with incarceration of the vitreous, and microcornea are indications for endoscopic vitrectomy. The combination of endoscopy and a wide-angle viewing system could compensate for the deficiencies of each technique and achieve more effective and safer surgical maneuvers. Endoscopy skills appear to be a great advantage for vitreoretinal surgeons; however, because endoscopies require a learning curve, becoming familiar with the handling of the endoscope through step-by-step learning is necessary.
Core tip: Ocular endoscopes enable ophthalmologists to observe inside the eye and perform surgical procedures independent of the status of the cornea, pupil size and media. Moreover, endoscopes enable the management of peripheral lesions without scleral indentation. The enlarged view under the endoscope, as obtained by drawing towards the lesion, appears to be another advantage. Having endoscopy skills appears to be an advantage for ophthalmologists; however, because endoscopies require a learning curve, becoming familiar with the handling of the endoscope is necessary.
- Citation: Kita M. Endoscope-assisted vitrectomy. World J Ophthalmol 2014; 4(3): 52-55
- URL: https://www.wjgnet.com/2218-6239/full/v4/i3/52.htm
- DOI: https://dx.doi.org/10.5318/wjo.v4.i3.52
Recently, endoscopic surgery has become popular in various fields of surgery. Overall, there has been a shift toward more non-invasive treatment, including in the field of vitreo-retinal surgery. In Japan, medical insurance has covered endoscopic vitrectomy since April 2012.
Many clinics are equipped with ocular endoscopes; however, many of these clinics lack skilled personnel to use the equipment. Performing endoscopic vitrectomy during surgery is difficult in many cases because of its learning curve. Becoming familiar with the handling of the endoscope is necessary in the clinical setting.
The advantages and indications of endoscope-assisted vitrectomy are presented here.
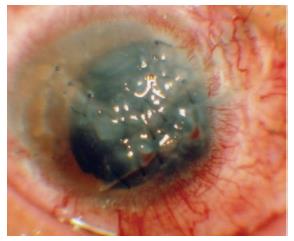
Because the endoscope combines illumination with image fibers, ophthalmologists can see areas where the endoscope illuminates[1,2]. Therefore, the ocular endoscope enables ophthalmologists to observe any part of the retina and manipulate surgical procedures independent of corneal opacities, the rim of intraocular lens, cortical remnants, capsular opacities, a small pupil, and vitreous opacities[3,4] (Figure 1).
Recently, a wide-angle viewing system has become popular, as it can easily provide a panoramic view of the surgical field. However, even in this system, an indentation of the sclera is inevitable when observing or manipulating the periphery, which could cause intraoperative pain and postoperative inflammatory reactions, such as fibrinous exudates.
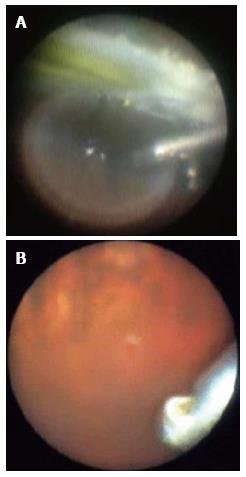
The endoscope enables ophthalmologists to observe the peripheral area of the fundus and the anterior part of the eye without scleral indentation (Figure 2A), which could contribute to a less invasive surgery and faster postoperative visual rehabilitation[1-4].
Moreover, when the perfluorocarbon liquid (PFCL) fills to the posterior surface of the iris, a stream of the infusion could cause the formation of PFCL droplets that appear as “fish-eggs”. Even if the PFCL is gently injected into the shape of a ball under the valved trocar system, scleral indentation might still cause the PFCL fish-eggs. Endoscopic maneuvers in the peripheral areas without scleral indentation can prevent the formation of PFCL fish-eggs.
A wide-angle viewing system is unsuitable for the observation or detection of subtle changes in the fundus because the image in the system is small. On the other hand, because endoscopes can magnify the view by closing in on the retina, the images obtained are clearer and larger than those obtained with a wide-angle viewing system or a microscope. Therefore, endoscopes can facilitate the detection and management of tiny lesions[5] (Figure 2B).
In rhegmatogenous retinal detachment, one of the poor prognostic factors for surgical success is the inability to detect retinal breaks preoperatively. Undiagnosed retinal breaks, which are typically characterized as tiny breaks located near the ora serrata, appear to be the main cause for the lower success rate of initial surgery in pseudophakic and aphakic retinal detachment compared to phakic cases. Furthermore, capsular opacity, lens remnants, and/or a small pupil could prevent the identification of these retinal breaks. Therefore, the visualization of retinal breaks can simplify surgery and improve the reattachment rate.
There are several advantages for using the endoscope[5]. Endoscopes are suitable for the observation of the periphery, independent of small pupil and media opacity, without causing scleral depression, and help detect tiny lesions in the retina by enlarging the images (Figure 2B). Dynamic scleral depressions sometimes make tiny retinal breaks unclear to close the retinal flaps.
The endoscopic identification of retinal breaks enables ophthalmologists to perform retinopexy only around the breaks. In contrast, when using a standard 360-degree peripheral laser or cryoretinopexy for vitrectomy, retinal breaks remain unidentified. Excessive retinopexy may cause intraoperative pain and complications, such as vitreous hemorrhage or iatrogenic breaks, postoperative inflammation and proliferative change.
In severe penetrating corneal injuries, using a temporary keratoprosthesis during vitrectomy followed by keratoplasty is thought to be beneficial because of the difficulty of observation through the cornea. However, complications may arise, including suprachoroidal hemorrhage and graft failure.
With a floating contact on the cornea or a wide-angle viewing system, endoscopes can overcome poor corneal conditions, which impair observations into the eye (Figure 1). Furthermore, the endoscope allows ophthalmologists to observe the peripheral part of the retina, vitreous base, pars plana, and pars plicata without manipulating the anterior chamber and causing scleral depression, which could cause fluid leakage or hemorrhage from the penetrating wounds in open eye injuries[6].
The enlarged, clear image with an endoscope can facilitate the detection of retinal breaks not preoperatively identified.
In inflammation, observations via the pupil are sometimes difficult due to the poor media conditions, such as corneal opacity, keratoprecipitate, posterior synechiae of the iris, small pupil, and cell adherence to IOL (intraocular lens). Managing vitrectomies via the pupil when severe inflammatory cells invade the cornea is impossible because of the dense corneal opacity. These cases are an absolute indication of endoscopic use[3,4].
Endoscopic vitrectomy without any manipulation of the anterior chamber and scleral depression could reduce the risks of intraoperative perforations of the eye wall and severe postoperative inflammation.
When retinal breaks are generated due to the incarceration of the vitreous or/and retina into the scleral wound or trocar, depressing the sclera to observe the lesion through a microscope is dangerous because of the risk of enlarging the retinal break.
In these situations, releasing the incarceration under the endoscope, without indenting the sclera, appears to be inevitably safer[3,4].
Microcornea is a rare congenital eye malformation. In most reports of microcornea, microphthalmia has also been observed. However, microcornea can also be associated with normal size globes or even macrophthalmia. In these cases, especially with a small pupil, the morphological features prevent the observation of the peripheral retina, even with a scleral depression and/or wide-angle viewing system.
Endoscope-assisted vitrectomy is advantageous for the management of these lesions, such as retinal detachments[7].
Recently, microincision vitrectomy surgery (MIVS) has become popular and 20G, 23G, and 25G endoscope fibers for small-gage surgery are commercially available in Japan.
The size of the field of view depends on the focusing lens attached to the fiber; therefore, there is no difference in the size of view between the 20G and small-gage systems. However, the size of the image depends on the pixels of the fiber; therefore, a smaller gage system tends to have a smaller image. However, the size of the image can be enlarged with a special instrument called the iS Board (Fiber Tec, Tokyo, Japan), which can modify the size, contrast, brightness and color tone of the image in real-time. The iS Board allows ophthalmologists to more easily perform endoscopic vitrectomies in MIVS[1-4].
In MIVS, scleral depression is sometimes difficult because of the transconjunctival approach. Furthermore, compared with 20G vitrectomy, a longer period of time is required to restore the intra-ocular pressure after releasing the scleral depression because less fluid is supplied from the smaller gage infusion. Therefore, endoscopic vitrectomy is advantageous for the peripheral management in MIVS where scleral indentation is likely[3,4].
The wide-angle viewing system enables ophthalmologists to instantaneously observe a panoramic fundus; however, the image is small. In contrast, endoscopes enable ophthalmologists to enlarge the image by closing in on the retina. However, this field of observation is narrow, and the view is non-stereoscopic. It is more efficient to choose the best tool of visualization at each step of the surgery. For example, in retinal detachment cases, core vitrectomy, creating PVD and F/A exchange should be visualized with a wide- angle view, contact lens are suitable for membrane peeling, and endoscopes are suitable for peripheral maneuvers.
Combining the maneuvers of an endoscope and a wide-angle viewing system, called “hybrid vitrectomy”, could compensate for the deficits of each system and allow a more effective and safer surgical management[3,4].
Because some training is required to obtain endoscopy skills, a step-by-step process is recommended to shorten the learning curve[8-10]. First, the endoscope should be used in the left hand to illuminate. Next, observations of the peripheral fundus should be attempted while the right hand stays out of the eye. The endoscope probe should be more horizontal than would be expected to observe the ora serrata. Endoscopic observation should be attempted while the vitrectomy cutter in the right hand is inside the eye. Subsequently, cutting the vitreous hemorrhage or applying laser photocoagulation under the endoscope should be attempted. Until an ophthalmologist feels comfortable using the endoscope, endoscopes are more suitable for use in usual cases, where the endoscopes are not necessary to perform vitrectomies. Beginning to use an endoscope in cases where the endoscope is necessary is difficult. Ophthalmologists should become familiar with manipulating the endoscope by frequently using it.
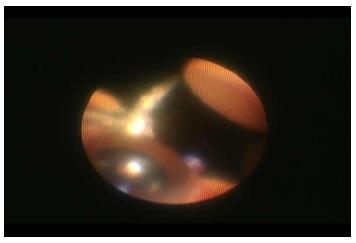
Orientation is the most important point when using the endoscope. First, the direction should be properly arranged by projecting the fingers of the right hand outside of the eye (Figure 3). Then, the endoscopic probe is inserted into the eye, followed by the re-arrangement of the direction by projecting the vitrectomy cutter at the 4:00 position on the screen[8-10].
To observe the peripheral area at approximately 2:00, the endoscope probe should be held with the right hand instead of the usual left hand and inserted from the port at approximately 10:00. A 360-degree periphery can be observed under the endoscope by the manipulation with both hands.
The monitor for the endoscopic view is another key point, and it should be located at a comfortable position for the surgeon to turn from the microscope to its monitor.
The maintenance of the fiber is also important for the proper visualization of the fundus through the endoscope during surgery.
In Japan, not only disposable fibers but also re-usable fibers for MIVS have been available recently. According to the smaller-gage vitrectomy system, such as 27G or 29G, smaller endoscope fibers could be developed in the near future. A multifunctional endoscope with an attachment laser or angiographic filter might be useful. A system that can provide 3-dimensional images might make manipulations easier during surgeries.
In endoscopy, ophthalmologists have a narrow field of mono-vision. Because microscopes have a wider stereovision, they are advantageous in most situations in vitrectomy. Therefore, it is unnecessary to use endoscopic maneuvers from the beginning to end of the surgery. Choosing the best tool for visualization at each step of the surgery is important.
The efficacy of the endoscope in vitrectomy surgery is clear; therefore, obtaining understanding of and competency with the endoscope is an advantage.
Increasing the frequency and proper use of the endoscope by more surgeons can ensure the improvement of the endoscope as a more convenient tool in vitrectomy surgeries.
P- Reviewer: Arevalo JF, Jhanji V, Peng SM S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Kita M. Progress in ocular endoscopy. Rinsho Gannka. 2008;62:171-175. |
| 2. | Kita M. System for ocular endoscopy. Textbook for Ophthalmic Surgery. Tokyo: Bunkodo 2012; 116-117. |
| 3. | Kita M. Endoscope assisted vitrectomy. Rinsho Gannka. 2011;65:156-161. |
| 4. | Kita M. Endoscopic vitrectomy. Gannka Ophthalmology. 2011;53:1269-1274. |
| 5. | Kita M, Yoshimura N. Endoscope-assisted vitrectomy in the management of pseudophakic and aphakic retinal detachments with undetected retinal breaks. Retina. 2011;31:1347-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Morishita S, Kita M, Yoshitake S, Hirose M, Oh H. 23-gauge vitrectomy assisted by combined endoscopy and a wide-angle viewing system for retinal detachment with severe penetrating corneal injury: a case report. Clin Ophthalmol. 2011;5:1767-1770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Yoshitake S, Oh H, Kita M. Endoscope-assisted vitrectomy for retinal detachment in an eye with microcornea. Jpn J Ophthalmol. 2012;56:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Kita M. Tricks of endoscopy in vitrectomy surgery. Jpn J of Ophthalmic Surgery. 2008;21:72-74. |
| 9. | Kita M. Endoscope assisted vitrectomy. Trics and pitfalls 2. Tokyo: Nakayama Shoten 2008; 118-119. |
| 10. | Kita M. Surgical maneuvers in endoscopic vitrectomy. Textbook for Ophthalmic Surgery. Tokyo: Bunkodo 2012; 261-267. |