INTRODUCTION
Pathological myopia is characterized by the excessive elongation of the globe and progressive degenerative changes and is a major cause of visual loss worldwide. The abnormal elongation of the eyeball in pathological myopia is associated with a spectrum of anatomical changes of the posterior pole, such as posterior staphyloma, atrophy of the retinal pigment epithelium (RPE), Bruch’s membrane cracks, subretinal hemorrhage, retinal detachment and choroidal neovascularization (CNV). Indeed, myopic CNV (mCNV) is one of the most vision threatening complications in pathological myopia[1]. It has been estimated that mCNV develops in 5%-11% of individuals with pathological myopia[2,3]. Affected individuals are often young and in their working life. The public health impact and socioeconomic cost associated with mCNV is therefore substantial.
There have been major advances in the treatment of mCNV in the past few years. Treatment modalities have evolved from thermal laser photocoagulation and photodynamic therapy, which aim to prevent vision loss, to the use of intravitreal anti-vascular endothelial growth factor (VEGF) agents, which have been shown to successfully restore vision in many patients. However, challenges to maintain long term vision remain, from the risk of recurrence and development of chorioretinal atrophy around the regressed CNV.
This article aims to provide a review of the literature of the epidemiology, progression, clinical course and treatment modalities as well as areas of future developments related to myopic CNV.
Definition of pathological myopia
There is currently no consensus on the definition of pathological myopia. Commonly used criteria for defining pathological myopic include refractive error and biometric criteria. In addition, clinical features commonly associated with pathological myopia, such as the presence of staphyloma and fundus changes such as lacquer cracks and chorioretinal atrophy, are often used[4,5]. In previously published population studies, a range of criteria have been used, such as refractive errors of -5 to -10 D and axial lengths of at least 25.0 mm to 26.5 mm. For example, the Blue Mountains Eye study, the Handan Study and the Hisayama study based their definition of high myopia on a refractive error of -5.0 D and worse. The Singapore Epidemiology of Eye disease program and the Shihpai study used a more stringent criterion of refractive error of -6.0 D and worse[6,7].
Epidemiology of myopia
The reported prevalence of pathological myopia based on population studies is estimated to be between 2% to 10% among adults aged 40 years and above[3,8-12]. There is a significant variation in the prevalence of high myopia between populations and East Asian countries have reported a significantly higher prevalence of high myopia compared to the rest of the world[13]. The overall prevalence of myopia also appears to be increasing, thus reflecting a complex interplay of genetic, environmental and epigenetic factors underlying the pathogenesis of this condition[2]. A predominantly Caucasian population in the United States, western Europe and Australia reported the prevalence of myopia (< -5 D) as 4.5%[14], compared to the Hisayama study in Japan which reported a higher prevalence of 5.7%[15]. In the Singapore Eye Study, the population prevalence was reported as 4.0%[6]. However, there was a significant difference between ethnic groups, with the prevalence among Chinese participants (6.1%)[9] 2-hold higher than that in Malay (3.0%)[8] and Indian participants (2.8%)[6]. Correspondingly, the impact of myopia is significantly higher in countries with a higher prevalence. In Japan, pathological myopia is the leading cause of blindness and in the Chinese population it has been reported to be the second commonest cause of blindness[7,16-18]. A recent systematic review reported the prevalence of pathological myopia to be 0.9%-3.1%. The prevalence of visual impairment attributable to pathological myopia was reported to range from 0.1%-0.5% in European studies and from 0.2%-1.4% in Asian studies[3].
Long term progression of myopic maculopathy
The myopic fundus has several clinical changes that may contribute to visual loss. However, it has been shown that these changes are not common in young myopes[19]. A study of myopia related changes in Singapore revealed that posterior staphyloma was the most common form of myopic macular change (23%), followed by chorioretinal atrophy (19.3%) in high myopes (< -6 D) over the age of 40[20]. These features increased in prevalence with increasing age, myopic refraction and axial length. Furthermore, long term follow-up of eyes with myopic maculopathy demonstrated that progression of lesions developed in a significant proportion[7,15,17,21,22]. Among individuals with retinal changes related to high myopia, it is estimated that 10% will develop CNV over 10 years[2,23]. However, a clear understanding of the relationship between the severity of myopia and the progression to sight threatening consequences remains elusive. One such theory suggests a linear relationship between the increased risks of pathology with each increase in spherical equivalent in diopters or axial length. Another theory suggests that there could be a threshold effect and the risk of pathology increases exponentially beyond a level of refractive error[24]. The thinning of the choroid and progressive stretching of the retina leading to choroidal ischemia and RPE atrophy may contribute towards the eventual formation of mCNV. Lacquer crack, which results from breaks in the Bruch’s membrane, is also considered a main predisposing factor for the formation of neovascularization.
CNV secondary to pathological myopia
Myopia is the most common cause of CNV in individuals below the age of 50[25]. mCNV is also the second most common cause of CNV following age-related macular degeneration, constituting 5%-10% of all CNV[12]. Sequelae of mCNV[3] include macular atrophy and scarring, which are major causes of visual loss in the long term[22,26-28]. The overall prevalence of mCNV is estimated to be 0.04% to 0.05% in the general population[2]. In pathological myopes, however, the incidence and prevalence have been reported to be as high as 10% and 0.5% respectively[29] and bilateral in 15%[3]. The risk of developing mCNV has been reported to increase with increasing severity of myopia[29,30] and macular changes, such as tessellated fundi, lacquer cracks, diffuse atrophy and patchy atrophy[23,31,32].
Clinical characteristics
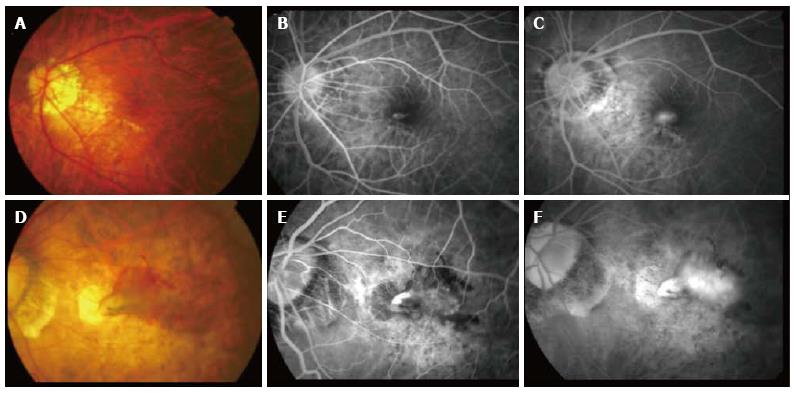
CNV secondary to pathological myopia exhibits significant differences in clinical characteristics when compared to CNV secondary to age-related macular degeneration (AMD). Patients with mCNV often present earlier with better visual acuity. They may report subtle visual symptoms such as metamorphopsia and central scotoma[28]. On clinical examination, the CNV typically appears as a grayish membrane with or without retinal hemorrhages. The tessellated fundus often makes determination of retinal swelling challenging. In addition, the amount of intraretinal and subretinal fluid that accompanies the mCNV is often less than that seen in CNVs secondary to AMD. A more effective pump mechanism in the retinal pigment epithelium in these eyes, especially in younger patients, has been postulated as a potential explanation of this difference. The minimal exudation is postulated to be due to the attenuated nature of the choroidal blood circulation in the pathological myopic fundus[33]. In an older patient, however, the clinical features tend to show more overlapping features with CNVs secondary to AMD (Figure 1). These eyes often have larger lesions and more exudative changes and may eventually lead to the formation of disciform scars[27]. If the underlying etiology of the CNV is not clear, the presence of myopic changes in the fundus, such as the presence of staphyloma, lacquer crack and peripapillary atrophy, may help distinguish a mCNV from AMD. In addition, type I CNVs, which are the most common type in AMD, are relatively uncommon in pathological myopia[34,35].
Figure 1 Clinical features of a typical choroidal neovascularization secondary to pathological myopia.
(A) Color fundus photography and corresponding fluorescein angiography (FA) (B and C) showing a younger patient with a small choroidal neovascularization lesion adjacent to the lacquer crack compared to (D) color fundus photograph and corresponding FA (E and F) showing an older patient with a much larger lesion with significant intraretinal fluid and extensive background atrophic changes.
DIAGNOSTIC CHARACTERISTICS
Optical coherence tomography
Optical coherence tomography (OCT) is a non-invasive imaging modality, which provides an in vivo cross section tomograph of the retina. It provides valuable information regarding the localization, character and activity of CNV.
Myopic CNV typically appears as a hyperreflective lesion above the reflective band corresponding to the retinal pigment epithelium (RPE). Intraretinal fluid and disruption of the retinal layers often accompany the hyperreflective lesion. However, the amount of intraretinal or subretinal fluid may vary. In younger patients with small mCNV there may be relatively limited surrounding intraretinal fluid and the contour of the internal limiting membrane may be minimally altered in some of these cases. As the lesion becomes less active, OCT can be used to monitor the decrease in the size of the lesion. The outline of the lesion also becomes increasingly distinct and exhibits high reflectivity. Correspondingly, the amount of intraretinal fluid surrounding the lesion can be seen to decrease progressively as activity decreases (Figure 2)[34].
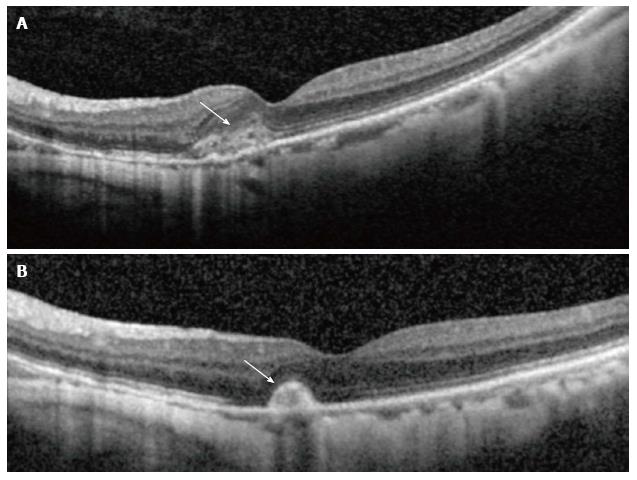
Figure 2 Optical coherence tomography showing corresponding changes in activity.
(A) Shows hyperreflective lesion corresponding to a small juxtafoveal myopic choroidal neovascularization (arrow) located above the retinal pigment epithelial cell layer with minimal exudation. After 3 mo (B), the lesion had scarred up, represented by a highly reflective lesion with sharp outline, and no intraretinal fluid is seen, which suggests an inactive lesion (arrow).
In addition to demonstrating the mCNV lesion, other myopia-related morphological changes are often seen on OCT. These include the presence of staphyloma and a relatively thin choroid. In addition, other co-existing pathologies, such as epiretinal membrane and retinoschisis can also be documented.
There are, however, some limitations in the use of OCT, especially in highly myopic eyes. The image resolution may be affected by optical factors in the presence of very long axial length and posterior staphyloma. The accuracy of quantitative data, such as central macular thickness measurement, in highly myopic eyes can be variable due to distortion in highly elongated eyes and the lack of normative data. Recently, Keane et al[36] developed software that attempts to improve the accuracy of retinal thickness measurement and may be helpful in the outcomes of treatment.
The ability of using enhanced depth imaging (EDI)-OCT techniques to image choroidal thickness has led to considerable interest in studying the role of the choroid in the pathogenesis of mCNV[37-39]. It has been shown that choroidal thickness decreases with age and myopia. Several studies have suggested that reduced choroidal thickness along with the presence of lacquer cracks and posterior staphylomas are significant risk factors for developing myopic CNV[40-43]. In addition, regional variations in choroidal thickness have also been shown. This thinning of the retina and choroid coupled with age related retinal-choroidal attenuation leads to a loss in choroidal vasculature, which is unable to meet the oxygen demands of the retina, and predisposes these eyes to developing myopic related retinal dysfunction[44]. Using 3-dimensional reconstruction, other authors have demonstrated that the edge of staphyloma may contribute towards a dome-shaped macula appearance described in some eyes[45].
Fundus fluorescein angiography
Although OCT provides valuable information for follow-up, fundus fluorescein angiography (FA) remains the gold standard for the confirmation of any CNV lesion at baseline. FA is also more sensitive in detecting mild activity from leakage in cases where OCT shows questionable presence of intraretinal fluid. The lesions typically display a classic pattern of leakage, in keeping with a type 2 CNV[46]. It is important to distinguish a subfoveal mCNV from a juxtafoveal or extrafoveal mCNV as the location of the lesion has been found to be an important prognostic factor. In addition, the amount of leakage on FFA is a good indicator of the level of activity of the lesion, which is an important factor in determining treatment options for the disease.
Indocyanine green angiography
As most mCVN lesions are type 2 CNV, FA and OCT provide adequate information in most cases. However, ICGA may provide additional information in selected cases, particularly where masking from blood might obscure visualization of the CNV on FA. A simple bleed associated with lacquer crack without CNV, or Fuchs’ hemorrhage, can be distinguished from mCNV on ICGA findings. Abnormal vasculature or a hyperfluorescence on ICGA may indicate the presence of CNV when information from FA is limited due to masking by blood. In Fuchs’ hemorrhage, however, no abnormalities of the choroidal vasculature will be seen on ICGA[47]. ICGA can also be used to detect and characterize lacquer cracks, which appear as linear hypofluorescent streaks in late phase ICGA[48].
Treatment options for myopic CNV
Over the last decade, various treatment options for mCNV have been proposed, including thermal laser photocoagulation, photodynamic therapy and submacular surgery. The success rate of these treatment modalities was variable. With the success of anti-vascular endothelial growth factors (VEGF) described in the treatment of CNV secondary to AMD, there have been many cases series published advocating their use for mCNV as an off label option. Recently, the results of two large clinical trials using anti-VEGF therapy in mCNV have been released, both of which reported very favorable results.
Thermal laser photocoagulation
Thermal laser photocoagulation and surgery no longer constitute the mainstay of treatment for myopic CNV. This is due to the irreversible scarring, central scotoma and high rates of recurrence.
For many years, laser thermal photocoagulation was the only modality for treating mCNV. Due to the immediate severe reduction in vision and central scotoma, laser thermal photocoagulation was limited to extrafoveal lesions. However, even in these cases, the long term efficacy is limited by atrophic scar creep and the high rate of recurrence[49].
Surgery
Myopic CNV, which is predominately a type 2 CNV, theoretically can be excised surgically as it is located anterior to the RPE and hence can be removed with relative preservation of the RPE layer. However, surgical excision of subfoveal myopic CNV has had disappointing results due to post-operative atrophic scar formation, central scotoma and a high rate of recurrences[50-52]. With less invasive therapies available, surgical excision is no longer a viable option.
Other surgical techniques such as macular translocation (MT) have been proposed. The benefits stem from the displacement of the neurosensory retina at the fovea to an area that has a presumed healthier RPE-Bruch’s complex together with the conversion of a subfoveal to an extrafoveal lesion which also allows for treatment modalities that might otherwise harm the fovea[53]. The clinical efficacy of such procedures is variable and limited to reports from case series[54-56].
Photodynamic therapy
The efficacy and safety of PDT in mCNV lesions was studied in the verteporfin randomized, double masked, placebo-controlled clinical trial[57]. The VIP study demonstrated a stabilization of visual acuity in 72% of eyes with subfoveal CNV following PDT over a period of 12 mo. However, there was still a mean loss of visual acuity of 2.8 letters at month 12. At month 24, the initial stabilization was not maintained. These findings are complemented by several smaller case series also showing benefit in visual stabilization during the early phase[57-60]. Based on the VIP study, PDT was approved for treating subfoveal mCNV.
Anti-VEGF treatment
The efficacy of anti-vascular endothelial growth factor (anti-VEGF) therapy has already been demonstrated in CNV secondary to AMD, diabetic macula edema and macular edema secondary to retinal vein occlusion. Favorable results of anti-VEGF therapy in mCNV have been reported in a series of mostly non-randomized, uncontrolled studies. Rapid gain of vision of 10-15 letters within an average of 1 to 3 injections over a 12 mo period has previously been reported[61-66].
The REPAIR study is a phase 2, open-label, single arm study which investigated the efficacy of intravitreal ranibizumab in mCNV. After a single ranibizumab injection at baseline, patients were retreated on a pro re nata (PRN) basis. At month 5, the mean best corrected visual acuity (BCVA) improved by 12.2 letters compared to baseline. The mean number of retreatments was 1.9 injections up to month 6[67].
The strongest evidence for the beneficial effects of anti-VEGF therapy in the treatment of mCNV comes from two recently completed phase III clinical trials; the Ranibizumab and PDT (verteporfin) evaluation in myopic choroidal neovascularization (RADIANCE) trial and the VEGF Trap-Eye in Choroidal Neovascularization Secondary to Pathological Myopia (MYRROR) Study.
The RADIANCE trial was a phase III, 12 mo, randomized, double-masked, multi-center, active-controlled study which compared the efficacy and safety of ranibizumab 0.5 mg against verteporfin photodynamic therapy (vPDT) in 277 patients with myopic CNV. This study demonstrated that ranibizumab treatment provided superior best-corrected visual acuity (BCVA) gains (10 ETDRS letters) vs vPDT (2.2 ETDRS letters) at 3 mo. The secondary outcome showed that ranibizumab treatment guided by disease activity criteria (2.5 injections) was non-inferior to VA stabilization criteria (3.5 injections) up to month 6. Over 12 mo, individualized ranibizumab treatment was effective in improving and sustaining BCVA and was generally well tolerated in patients with myopic CNV. The anatomical outcomes of this study were consistent with the gains in BCVA. There was a reduction in the proportion of patients with subretinal fluid, intraretinal edema and/or intraretinal cysts from baseline to 1 year, with a median of 3.5 injections in the disease activity criteria group and 4.6 injections in the VA stabilization group[68].
The MYRROR study was a multicenter, randomized, double-masked, sham-controlled trial which assessed the efficacy and safety of intravitreal administration of aflibercept (VEGF Trap-Eye; Eylea)[69]. The study was conducted in 20 sites across 5 Asian countries between 2010 and 2013. Patients were randomized in a 3:1 (aflibercept: sham injections) and followed up for 24 wk. Patients in the active treatment arm received one initial 2 mg dose of aflibercept. Patients were subsequently evaluated every 4 wk and received additional aflibercept injections determined by visual and anatomical criteria, through 20 wk. Patients on the sham arm received monthly sham injections through week 20. Starting at week 24, patients in both arms were eligible to receive aflibercept injections on an as needed basis through week 48. At 24 wk, the aflibercept group gained 12.1 ETDRS letters, which was significantly better than the sham injection group, which experienced a 2 letters loss. The efficacy gains at week 24 in the treatment arm extended further until week 48. Patients in the treatment group received a median of 2 injections in the first quarter of the study (baseline to week 12). In each of the following three quarters, the median of injections was 0.
These results support the efficacy of anti-VEGF therapy in mCNV (Figure 3). However, there are currently no randomized controlled trials on the use of other anti-VEGF agents, such as bevacizumab, in mCNV. Neither are there high quality head-to-head comparison studies to examine whether there may be difference in the efficacy and safety between different anti-VEGF agents. A retrospective study by Lai et al[70], however, shows promising results in the long term efficacy of both bevacizumab or ranibizumab as primary treatment for subfoveal mCNV where visual gains and number of retreatments appeared to be similar between bevacizumab and ranibizumab.
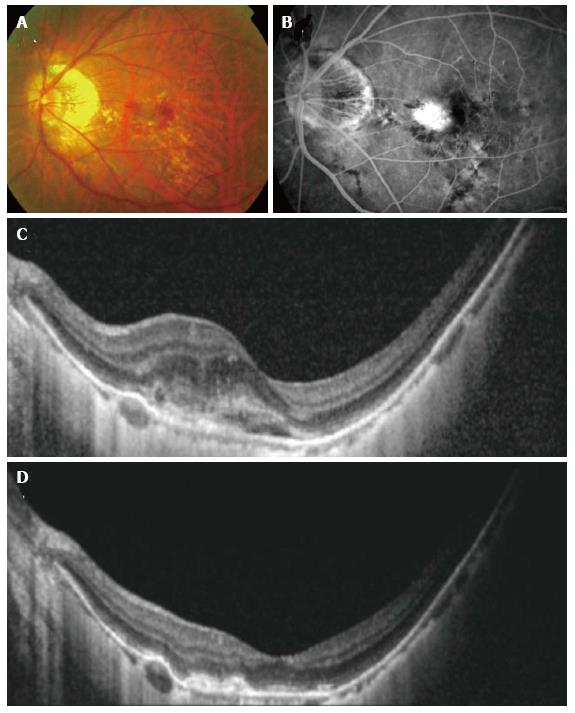
Figure 3 Resolution seen with anti-vascular endothelial growth factor treatment in myopic.
Choroidal neovascularization (CNV) color fundus photograph (A), fluorescein angiography (FA) (B) and optical coherence tomography (OCT) (C) showing a larger juxtafoveal myopic CNV with significant amount of intraretinal fluid. Note the lacquer cracks which are clearly visible on the FA. After a course of intravitreal bevacizumab, OCT demonstrated resolution of intraretinal fluid and consolidation of the CNV (D).
TREATMENT REGIMENS
Loading regimen
A 3 monthly injection (loading phase) is often practiced in anti-VEGF therapy in AMD. However, current evidence suggests this may not be necessary in mCNV. Indeed, the VEGF load in mCNV has been suggested to be lower than that in AMD[71]. A series of uncontrolled studies have reported favorable results using a pro re nata (PRN) regimen. Iacono et al[72] demonstrated favorable outcomes with stabilization of vision and > 90% closure of CNV with the use of bevacizumab on an as-needed basis with an average of 4.74 injections in the first year. Wakabayashi et al[73] compared a PRN regimen with a 3 monthly followed by PRN regimen and concluded that there was similar visual outcome over 12 mo with fewer injections in the group without initial loading[73]. Neither the RADIANCE nor MYRROR studies mandated an initial loading phase and reported low (< 3) mean and median number of injections up to month 6 and week 24 respectively.
Follow-up regimen
Both the RADIANCE and MYRROR studies followed-up participants on a monthly basis. However, this may be challenging in the clinical setting. In the case of AMD, various modifications to allow longer follow-up have been studied in trials such as the efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration (EXCITE study), Prospective OCT Study With Lucentis for Neovascular AMD (PrONTO study) and treatment-and-extend regimen[74-77]. In mCNV, however, there are currently no published data in this respect.
Retreatment
The assessment of treatment response and decision of when to stop treatment is based on multiple factors, including visual acuity, symptomatology, clinical assessment and imaging results. Common regimens include “treat to dry” based on OCT assessment and “treat to stable vision” based on visual acuity. In the RADIANCE study, two dosing regimens were compared. In the visual acuity stabilization group, dosing was stopped if there was no change in BCVA compared to 2 preceding monthly visits. In the disease activity group, dosing was stopped if there was no disease activity based on vision impairment attributable to intra or subretinal fluid or active leakage. The study reported that ranibizumab treatment driven by disease activity showed non-inferiority to treatment driven by stabilization criteria, with respect to mean BCVA from baseline to month 6, gaining 11.7 and 11.9 letters respectively. The corresponding number of injections was 2.5 in the disease activity group and 3.5 in the stabilization group.
Safety of anti-VEGF therapy
The systemic safety of anti-VEGF has been questioned and remains a possible concern[78-80]. The pivotal clinical trials of ranibizumab and aflibercept in AMD did not show a significant increase in stroke risk or thrombotic events[81-83]. However, a pooled analysis of five randomized controlled trials using ranibizumab suggested that patients with a history of previous stroke may be at increased risk of developing stoke after anti-VEGF therapy[84]. It is also unclear whether the anti-VEGF agents differ significantly with respect to safety. Carneiro et al[85] reported that VEGF plasma levels decreased 42% in patients treated by intraocular injection of bevacizumab but not in ranibizumab-treated patients, potentially highlighting the safety profile between the two drugs[85]. In a US Medicare study, higher risks of stroke and all-cause mortality were observed with intravitreal bevacizumab compared with ranibizumab[86]. Furthermore, there are potentially additional ocular specific complications in highly myopic eyes. Worsening of retinoschisis, macular hole and macular detachment have been described after intravitreal anti-VEGF for mCNV[87]. As seen in other treatment modalities, progression of chorioretinal atrophy around the mCNV has also been described following anti-VEGF therapy[26,88] (Figure 4). However, the proof of any causal relationship remains difficult with no clear evidence from clinical trials.
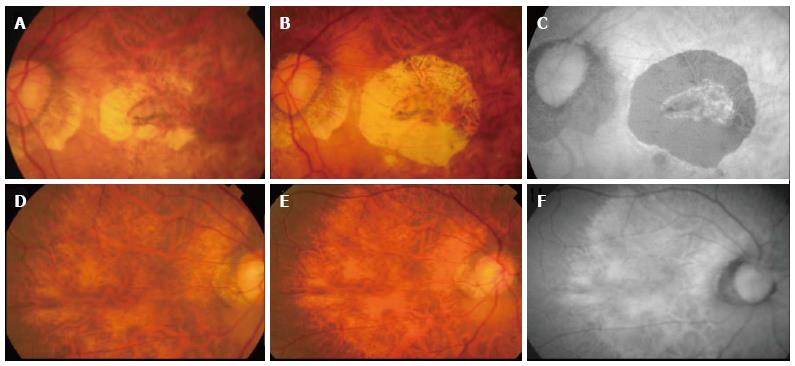
Figure 4 Progression of atrophy around treated choroidal neovascularization.
Color fundus photograph (A) showing inactive choroidal neovascularization (CNV) after 1 year therapy with ranibizumab, color fundus photography (B) showing increase in CNV related chorioretinal atrophy two years later. No further therapy was given during the intervening period. Autofluorescence imaging (C) demonstrating clearly the area of retinal pigment epithelial atrophy as hypoautofluorescent area. The fellow eye showed diffuse atrophy but no significant progression was seen during the same follow-up period (D-F).
Monitoring of disease activity
FA remains the standard for diagnosis and disease activity monitoring of mCNV[89,90]. With the advent of anti-VEGF treatment, there is growing importance in the use of OCT as a simple non-invasive alternative of disease monitoring. There are, however, shortfalls of the use of OCT in disease monitoring of mCNV. In myopic eyes, the retina and choroids are thin and mCNV typically have minimal leakage with minimal intra/subretinal fluid; hence, OCT findings may not be as informative compared to its use in AMD CNV. Introni et al[91] suggest that there is no evidence for central retinal thickness or sub/intraretinal fluid in mCNV. Instead, they suggest the use of outer retinal characteristics on SD OCT: identification of a hyperreflective lesion with fuzzy borders and a more highly reflective core above the RPE, and “absent or altered” IS/OS junction as signs of activity, and they found a regression of these findings and RPE thickening after treatment[91].
Prognostic factors
Many studies have studied the prognostic factors for mCNV. Prognostic factors for visual outcome after anti-VEGF therapy include age, CNV size and location, baseline VA, presence of chorioretinal atrophy, choroidal thickness and recurrence of mCNV[92]. Other factors such as refractive error, axial length and lens status have been variably described. Furthermore, prior PDT has also been suggested to limit visual prognosis. In addition, age, CNV size, baseline choroidal thickness and the presence of lacquer cracks have been described as prognostic factors for needing a larger number of injections[93].
Age of onset of CNV: Younger patients generally obtained more favorable results than elderly subjects. In various studies, a significant improvement in vision was generally seen in younger patients (mean age 48-53) than studies enrolling older patients (mean age 60). This may be attributed to the age related deterioration of the RPE which decreases the inhibition of CNV growth. Elderly patients require more anti-VEGF injections compared to younger subjects. In addition, the younger subjects have a smaller area of pre-existing chorioretinal degeneration, resulting in the significant improvement in vision following anti-VEGF treatment[72,94-96].
Location of CNV: Subjects with subfoveal CNV generally had a worse final VA compared to subjects with lesions that were non subfoveal. Hayashi et al[97] also showed that the incidence of chorioretinal atrophy in subfoveal CNV was 80% compared to 6% in non subfoveal CNV, with a significant difference in the size of chorioretinal atrophy[97].
Size of CNV: Nakanishi et al[95] showed that pre-treatment CNV size was significantly associated with both the BCVA and the change in the BCVA at 24 mo after the initial anti-VEGF therapy. Eyes with smaller mCNV had both better BCVA and better improvement of BCVA at 24 mo after the initial treatment than those with larger myopic CNV. Similar findings were reported for age-related macular degeneration (AMD) where the size of the CNV before PDT or anti-VEGF therapy was a predictive factor for the post-treatment BCVA. However, the mechanism of how the CNV lesion size influences the visual outcome after these treatments has not been determined[95].
Prior PDT: Several studies have performed sub group analysis of eye outcomes with anti-VEGF treatment with and without prior PDT treatment. Ruiz-Moreno et al[94] specifically studied the influence of PDT on visual outcomes in eyes with myopic CNV treated with intravitreal bevacizumab. These studies all show similar conclusions that prior treatment with PDT seems to adversely affect the BCVA outcome with additional anti-VEGF treatment. Poorer visual outcome in this group with prior PDT may due to several factors, including choroidal ischemia, damage to RPE and photoreceptors and choriocapillary atrophy[94,98].
Baseline vision: In a multivariate analysis of a retrospective, observational case series of 103 eyes of 89 consecutive patients with subfoveal myopic CNV by Yang et al[92], baseline BCVA, along with other factors such as choroidal thickness and CNV size, was associated significantly with poor final BCVA. This poor functional outcome in eyes with poorer baseline VA may just reflect the more aggressive CNV which would have a poor prognosis with any treatment modality[92].
Recurrence
Recurrence of mCNV is a well-recognized challenge. In the RADIANCE study, 19.0% to 29.1% of eyes continued to have CNV leakage on FA at month 12. In the disease activity group, 37.1% of eyes required additional injections according to retreatment criteria between month 6 and month 11[68]. In a retrospective observational case series of 103 eyes with mCNV, recurrence was reported in 23.3%. Most of the first recurrences (72.7%) occurred during the first year of follow-up. Baseline CNV size and the presence of lacquer cracks have been described as prognostic factors for recurrence[92].
CONCLUSION
Myopic CNV is one of the most common vision threatening complications of pathological myopia, with a significant socioeconomic impact as it affects a younger, working age group of patients compared to other common blinding diseases. The natural progression of mCNV shows an early stabilization of vision followed by gradual decrease in VA over time due to the development of chorioretinal atrophy. The final visual outcome relates closely with the distance of CNV from the fovea and inversely with the size of CNV. Subfoveal location of CNV is associated with worse visual outcome when compared with a juxtafoveal and extrafoveal location; however, there is a high likelihood of conversion of these CNVs to subfoveal type or extension of the CNV within the fovea.
Currently, anti-VEGF treatment appears to be the most promising treatment modality for myopic CNV. Compared to previous treatment options like PDT which have been shown to only stabilize vision in the short term, there is now level 1 evidence to support the efficacy of specific anti-VEGF agents in mCNV with visual outcome superior to that achieved with PDT.
While these studies affirm anti-VEGF treatment for short term gains in vision, further research is still needed regarding the optimal follow-up interval, rate and risk of recurrence and late atrophy on treated eyes. In the long term, the development and enlargement of chorioretinal atrophy around regressed CNV remain the determining factors on final visual outcome. Hence, further research is necessary to investigate the underlying mechanisms of chorioretinal atrophy and to establish the best treatment modalities to prevent these late complications.
Other future directions of study would be to determine the risk factors associated with the development of myopic CNV in pathological myopic eyes. With newer imaging technologies available, such as swept source OCT, the state and health of the choroid in myopes can be better assessed. This can help in the understanding of the changes in retinal metabolic support in highly myopic patients and the role of choroidal abnormalities in the pathogenesis of myopic degenerative diseases.
In summary, myopic CNV remains a common cause of vision loss. With better understanding of the pathophysiology, risk factors and natural history, better therapies can be developed to both prevent and treat the disease.