Published online Dec 30, 2011. doi: 10.5318/wjo.v1.i1.4
Revised: December 17, 2011
Accepted: December 22, 2011
Published online: December 30, 2011
AIM: To assess changes in peripapillary retinal nerve fiber layer (RNFL) thickness and visual field (VF) in patients with glaucoma after reduction of intraocular pressure (IOP).
METHODS: Thirty-five consecutive patients with bilateral high tension glaucoma were included in the study. Thirty-five eyes underwent monocular deep sclerectomy (surgery group) and the medically treated fellow eyes served as controls (control group). Quantitative analyses of the peripapillary RNFL thickness by optical coherence tomography (OCT) and global VF indices by automated perimetry were performed before surgery and six months after surgery in both eyes. The changes in RNFL thickness overall and by quadrant were evaluated and studied with respect to age, best-corrected visual acuity (BCVA), preoperative global VF indices, postoperative IOP changes, and postoperative changes in global VF indices. Changes observed in RNFL thickness and VF indices were compared between eyes after surgery and fellow eyes.
RESULTS: Six months after surgery, the overall IOP decreased from a baseline mean of 24.5 ± 3.2 mmHg to 11.5 ± 2.7 mmHg (P < 0.001) at the time of OCT testing. A significant increase in the overall mean RNFL thickness was observed after surgery (P < 0.001). The preoperative VF mean deviation was significantly correlated with a postoperative increase in the RNFL thickness (P < 0.075). No correlation was found between RNFL thickness changes and age, BCVA, or changes in the global VF indices. There was no significant difference between eyes with an IOP reduction of more than 50% and those with a reduction in IOP less than 30% (P = 0.312).
CONCLUSION: A significant increase in the peripapillary RNFL thickness was associated with IOP reduction by glaucoma filtration surgery as measured by OCT.
- Citation: Ghanem AA, Mady SM, El-wady HES. Changes in peripapillary retinal nerve fiber layer thickness in patients with primary open-angle glaucoma after deep sclerectomy. World J Ophthalmol 2011; 1(1): 4-10
- URL: https://www.wjgnet.com/2218-6239/full/v1/i1/4.htm
- DOI: https://dx.doi.org/10.5318/wjo.v1.i1.4
Glaucoma is characterized by clinically detectable tissue loss in the nerve fiber head and retinal nerve fiber layer (RNFL). Defects in the peripapillary RNFL may even precede changes in optic nerve head appearance and visual field (VF) loss[1]. The optic disc sometimes is seen to be less excavated when the intraocular pressure (IOP) falls. This anatomic change has been documented previously after trabeculectomy by stereoscopic disc photography, computer-assisted planimetry, optic nerve head analysis, and confocal scanning laser ophthalmoscopy (CSLO)[1-4].
There is controversy about the effect of IOP reduction on the peripapillary RNFL, which also is considered a marker of structural optic nerve damage. However, the effect of IOP reduction on peripapillary RNFL is still unclear. Some studies have found no significant changes in the retinal cross-sectional area using CSLO and an optic nerve analyzer[4-5], whereas others have shown a significant increase in mean retinal height at the optic disc margin by CSLO[6,7].
Optical coherence tomography (OCT) evaluates and quantifies the peripapillary RNFL thickness in vivo. Aydin et al[8] reported a significant increase in the mean peripapillary RNFL thickness assessed by OCT scans performed with a noncommercial, prototype device in eyes undergoing filtering surgery, while Leung et al[9] reported structural and functional recovery in a patient with juvenile open-angle glaucoma, which was documented quantitatively by OCT after trabeculectomy.
Deep sclerectomy is a non-penetrating filtering procedure that facilitates IOP control with fewer complications than trabeculectomy[10,11]. The purpose of the present study was to assess changes in the peripapillary RNFL thickness and global VF indices in a prospective manner using a third generation OCT device in patients with primary open-angle glaucoma (POAG) after IOP lowering by surgical or medical treatment.
This prospective controlled study was performed in Mansoura Ophthalmic Center, Mansoura, Egypt, and was approved by Mansoura University Trust Ethics in accordance with the Declaration of Helsinki (1989) of the World Medical Association. Consecutive patients with bilateral high tension glaucoma scheduled for unilateral deep sclerectomy were enrolled. Informed consent was obtained from each patient. Eyes were scheduled for deep sclerectomy when the IOP exceeded the target pressure and/or VF defect and glaucomatous optic nerve damage showed progression despite maximum tolerated medical therapy.
Full ophthalmic examination was done, including assessing visual acuity, slit-lamp anterior and posterior segment biomicroscopy, IOP measurement by Goldmann applanation tonometry, gonioscopy using Goldmann three mirror contact lens, OCT, automated perimetry (Humphrey visual field analyzer, program 24-2), and cup/disc ratio estimation. A detailed medical history including age, gender, glaucoma medications, systemic hypertension, systemic medications, and previous ocular surgery was recorded
The IOP measurements were taken at least 5 times throughout the day from 8 am to 5 pm. Highest and lowest measured IOP values were used to determine IOP diurnal range. The VF categories were: (1) normal; (2) mild, an arcuate defect; (3) moderate, abnormal in one hemifield and not within 5 degrees of fixation; and (4) severe, abnormal in both hemifields or within 5 degrees of fixation. VF grading was done on the basis of the last reliable Humphrey VF test before surgery.
Changes in the RNFL thickness and VF indices in eyes undergoing deep sclerectomy (surgery group) were compared with those in the contralateral eyes in which the IOP was controlled medically (control group). The postoperative change in RNFL thickness was analyzed for several potential related factors (age, preoperative overall RNFL thickness, postoperative IOP change, and VF global indices).
Patients were selected based on their ability to perform reliable perimetry and on the clarity of the ocular media. Only patients with high tension glaucoma were included in this study. Patients were excluded if they had ocular pathologic features other than glaucoma such as diabetic retinopathy, age-related macular degeneration, parapapillary atrophy extending beyond 1.7 mm from the disc center, and inability to obtain adequate OCT images. Both Humphrey VF testing and OCT scanning were done preoperatively and six month postoperatively for all patients.
All patients underwent Humphrey VF testing using standard Humphrey 24-2 full threshold perimetry (Humphrey Instruments, Carl Zeiss Meditec, San Leandro, Dublin). A reliable VF test was defined as one with less than 30% fixation loss and false-positive or false-negative responses. The preoperative and postoperative mean deviation (MD) and pattern standard deviation (PSD) were used for the analysis.
Cross-sectional imaging of peripapillary RNL was performed with the Stratus OCT (model 3000, software version 4.4; Carl Zeiss Meditec, Dublin, CA) after pupillary dilation with 1% tropicamide to a minimum diameter of 5 mm. Circular 360° OCT scans were obtained using the fast RNFL thickness scan, with a diameter of 3.46 mm on the peripapillary RNFL. The scans include the single mean RNFL thickness, the average thickness within each of four quadrants (temporal, superior, nasal, and inferior), and average thickness within each of 12 sectors corresponding to clock hours.
Good scans were defined as focused images from the ocular fundus, with an adequate signal-to-noise ratio and a centered, circular ring around the optic disc. Images with less than 90% satisfactory A scan or a signal-to-noise ratio of less than 25 dB were excluded. If the amount of peripapillary atrophy exceeded the scan circle, which was visible and controlled by the operator, the patient was excluded. The average of the three qualified circular scans was used to calculate the mean and quadrantic RNFL thickness[12].
Local anesthesia was achieved using a peribulbar injection of 4 mL of a mixture of 4% xylocaine, and 0.75% marcaine. A fornix based conjunctival flap was made, the sclera was exposed, and hemostasis by wet-field cautery was performed. A one-third scleral thickness superficial flap (5.0 mm × 5.0 mm) was dissected at the 12-o’clock position at least 1.0 mm into the clear cornea. A second flap of deep sclera was dissected, Schlemm’s canal was deroofed, and a trabeculo-Descemet membrane window was created. The deep scleral flap was excised, and the juxta-canalicular trabeculum and Schlemm’s endothelium were removed using small blunt forceps. The superficial scleral flap was sutured with 2 to 4 interrupted nylon 10-0 buried sutures.
Postoperative treatment included a combination of dexamethasone and tobramycin 4 times daily for 2 wk. The dosage was tapered by one drop weekly until discontinuation after 8 wk. When the vessel density increased or flattening occurred, we intensified the postoperative anti-inflammatory treatment (prednisolone acetate every 1 h to 2 h during waking hours). When filtration through the trabeculo-Descemet membrane was insufficient because of an elevated IOP, a goniopuncture was performed with the neodymium:yttrium-aluminum-garnet (ND:YAG) laser in the thin driest anterior portion of the trabeculo-descemet membrane.
A statistics program (SPSS version 15.0 for Windows; SPSS Inc., Chicago, IL) was used for all analyses. The distribution of data was determined using the Kolmogorov-Smirnov test. A paired t test was used to analyze RNFL thickness differences in individual eyes basis and to compare parameters before and after surgery. A comparison between the two study groups was carried out, and Pearson’s correlation was used to analyze the association between parameters. The number of glaucoma medications and the visual acuity were compared using the Wilcoxon signed rank test. Since multiple correlations were investigated, the P value was adjusted using the Bonferroni correction. A P value of 0.05 or less was considered to be statistically significant.
Thirty-five eyes of 35 patients were qualified for this study; thirty-five eyes underwent deep sclerectomy and the fellow eyes served as the control eyes. The patient demographics and baseline characteristics for both groups are presented in Table 1.
| Parameters | Surgery group | Control group | P value1 |
| No. of patients | 35 | ||
| Age (yr) | 62.2 ± 2.5 | ||
| Gender | |||
| Male | 18 (51%) | ||
| Female | 17 (49%) | ||
| Diabetes mellitus | 8 | ||
| Hypertension | 25 | ||
| Cup/Disc ratio | 0.76 ± 2.4 | 0.57 ± 2.1 | F = 3.52 |
| P = 0.0121 | |||
| IOP diurnal range2 | 12.17 ± 2.21 | 8.26 ± 3.31 | F = 4.64 |
| P = 0.0151 | |||
| Preoperative IOP (mmHg) | 23.4 ± 6.1 | 18.5 ± 2.3 | F = 3.54 |
| P = 0.0151 | |||
| Preoperative medication | 3.1 ± 0.5 | 1.7 ± 0.4 | F = 2.56 |
| P = 0.0211 | |||
| Preoperative VF MD (dB) | -9.2 ± 5.3 | -4.5 ± 4.1 | F = 4.21 |
| P = 0.0171 | |||
| Preoperative VF PSD (dB) | 5.7 ± 3.1 | 4.8 ± 2.9 | F = 4.56 |
| P = 0.25 | |||
| Preoperative VA (logMAR) | 0.73 ± 0.4 | 0.86 ± 0.2 | F = 3.61 |
| P = 0.36 | |||
The mean IOP, the mean number of medications used, and VF indices before surgery were significantly lower in the control group than in the surgery group (P = 0.001). The mean preoperative IOP in the surgery group was 23.4 ± 6.1 mmHg; 6 mo after surgery, the mean IOP decreased to 10.6 ± 25 mmHg (P < 0.001). The mean percent change in IOP was 45.4% ± 16.5% (range, 16% to 65%). In 24 eyes (68.6%), the IOP reduction exceeded 30%.
At six months, complete success, defined as IOP of 21 mmHg or less and IOP reduction of greater than or equal to 20% without anti-glaucoma medication, was achieved in 91.4% (32/35) of the patients, qualified success, defined as having an IOP of 21 mm Hg or less and an IOP reduction of greater than or equal to 20% with anti-glaucoma medication, was achieved in 5.7% (2/35) of the patients, and failed surgery, defined as having an additional surgery, occurred in one patient (2.8%). ND:YAG laser goniopuncture was performed in 6 eyes (17.1%).
The mean number of medications used decreased significantly from 3.1 ± 0.5 before surgery to 0.9 ± 0.2 after deep sclerectomy (P < 0.001). No significant change was found between the visual acuity before and 6 months after surgery (P = 0.365).
The mean preoperative and postoperative MD was -9.2 ± 5.3 and -8.6 ± 4.6, respectively (P = 0.361). The mean preoperative and postoperative PSDs were 5.7 ± 3.1 and 5.3 ± 3.1, respectively (P = 0.325). These results revealed no difference between preoperative and postoperative MD and PSD of the VF results in the study group.
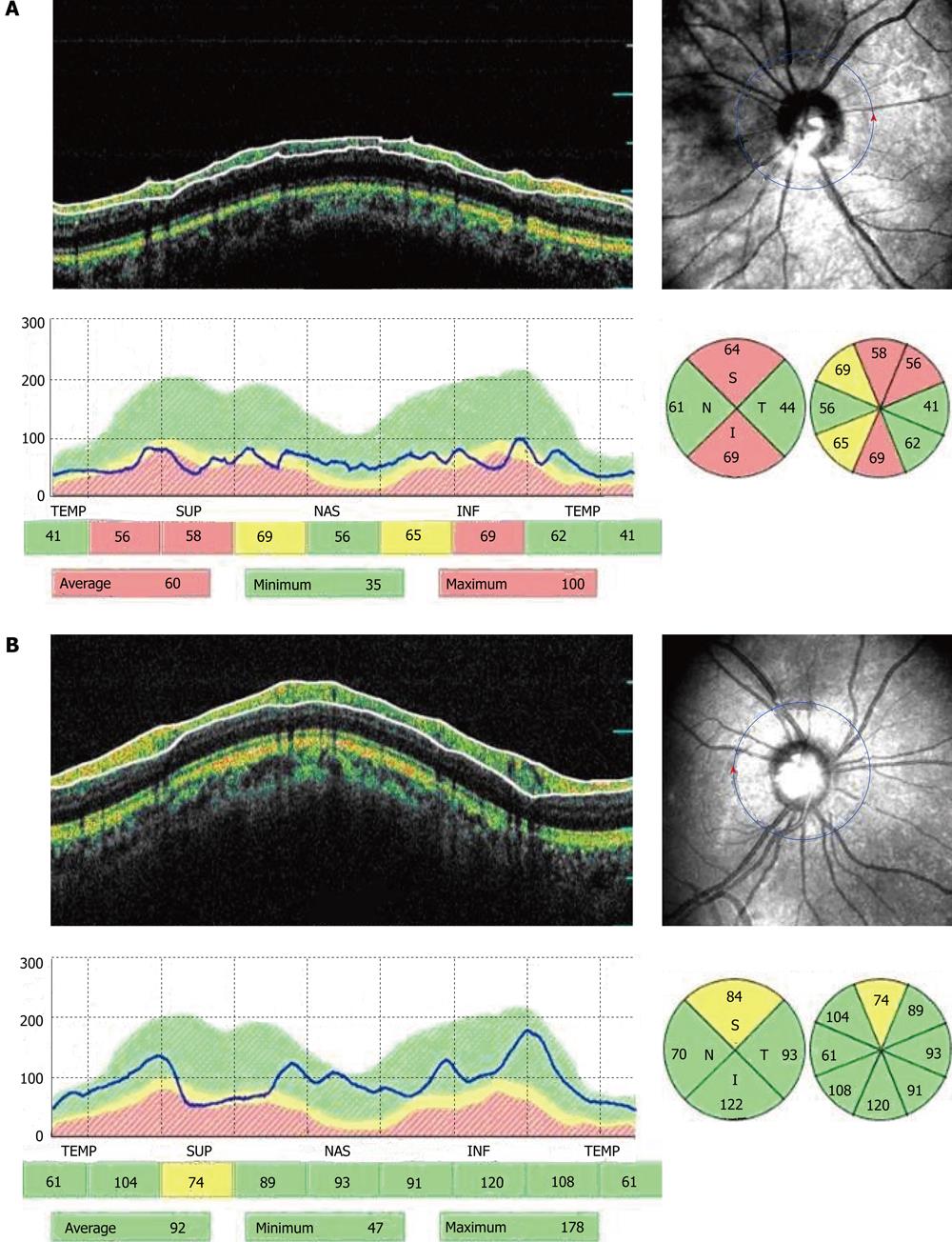
Table 2 summarizes the mean peripapillary RNFL thickness measured by OCT for the entire study. A significant increase was found in the mean overall and quadrantic RNFL thickness in the surgery group. The mean overall RNFL thickness change in the surgery group was 7.5 ± 9.6 μm (range, -28.3 μmto 15.2 μm; median, -1.35 μm, P = 0.001). In the surgery group, the RNFL thickness increased in 32 eyes (91.4%) after surgery. In the control group, the mean overall RNFL thickness change was -4.3 ± 5.4 μm (range, -17.8 μm to 10.5 μm, P = 0.145).
| Parameter | Pre-operative (μm) | Post-operative (μm) | P value1 |
| Overall | |||
| Surgery group | 71.6 ± 18.6 | 89.5 ± 21.2 | 0.0151 |
| Control group | 72.5 ± 17.8 | 71.7 ± 20.3 | 0.165 |
| Superior quadrant | |||
| Surgery group | 85.8 ± 25.1 | 96.7 ± 23.2 | 0.0211 |
| Control group | 85.7 ± 24.1 | 86.5 ± 21.5 | 0.114 |
| Nasal quadrant | |||
| Surgery group | 54.6 ± 21.5 | 72.0 ± 25.1 | 0.0141 |
| Control group | 56.5 ± 18.9 | 58.2 ± 22.1 | 0.356 |
| Inferior quadrant | |||
| Surgery group | 81.3 ± 25.1 | 69.2 ± 22.5 | 0.0231 |
| Control group | 83.1 ± 18.2 | 83.3 ± 20.1 | 0.453 |
| Temporal quadrant | |||
| Surgery group | 56.2 ± 19.2 | 75.2 ± 20.1 | 0.0161 |
| Control group | 57.0 ± 18.5 | 58.1 ± 21.5 | 0.451 |
The correlations between the RNFL changes after surgery and age, IOP change (mmHg), percent change in IOP, preoperative MD, PSD, BCVA, and the change in the VF MD and PSD are shown in Table 3. A significant correlation was found between the RNFL thickness changes after surgery and the preoperative MD (P = 0.374 and P = 0.075, respectively). There were no significant changes in the control group in the MD (P = 0.132), PSD (P = 0.145), or IOP (P = 0.127) at the 6-mo follow up.
| r | P value1 | |
| Age | -0.164 | 0.276 |
| IOP change (mmHg) | -0.235 | 0.023 |
| IOP change (%) | -0.453 | 0.036 |
| BCVA | -0.153 | 0.216 |
| Preoperative VF MD (dB) | 0.374 | 0.075 |
| Preoperative VF PSD (dB) | -0.182 | 0.383 |
| Change in VF MD | 0.395 | 0.064 |
| Change in VF PSD | 0.186 | 0.625 |
In the present study we did not found a significant correlation between BCVA and overall or segmental RNFL thickness in the study group. There was a significant correlation between BCVA and overall RNFL thickness, and also between BCVA and the RNFL thickness by quadrants (temporal, inferior, and superior) in the control group (Figure 1).
Several studies reported less cupping of the optic disc after IOP reduction in some patients after glaucoma surgery. This observation is more likely to be due to a simple shift in anatomic structures rather than recovery or reversal of damage. This anatomic change has been documented by stereoscopic disc photography, computer-assisted planimetry, optic nerve head analysis, and conofocal scanning laser ophthalmoscopy[1-4]. When IOP is lowered, there is less stretch on the lamina cribrosa, and the disc is able to return to its normal position. However, there is no consensus regarding whether the changes associated with IOP reduction occur only in the optic nerve head or also in the peripapillary RNFL[4].
Until recently, the assessment of the RNFL has been largely subjective. OCT, developed to assess tissue thickness in vivo, is a non-invasive imaging technique that allows high-resolution cross-sectional ocular imaging and evaluates and quantifies the peripapillary RNFL thickness. OCT provides real-time, immediate, objective, and reproducible quantitative measurements of the RNFL within a short time during first visit and offers a reproducible technique with a standard deviation of measurements of 10 μm to 20 μm for the mean overall RNFL thickness[13,14].
In the present study, we prospectively assessed the functional changes in peripapillary RNFL thickness by OCT and global VF indices by automated perimetry in 35 patients who underwent monocular deep sclerectomy. In addition, our purpose was to evaluate the correlation of global indices with the structural glaucomatous damage. We used OCT with fast RNFL thickness scan, which reduces the examination time and improves the accuracy and centration of the scans[13].
We found a significant change in the peripapillary RNFL thickness in the surgery group. The RNFL thickening was significant for the overall measurement and in all quadrants. These results are consistent with those reported by Aydin et al[8], who found a significant increase in the overall peripapillary RNFL from 72.8 μm to 81.7 μm after filtration surgery measured by OCT in 18 eyes that underwent trabeculectomy and in 20 eyes that underwent combined trabeculectomy and cataract extraction.
Several studies reported no significant changes in the peripapillary RNFL thickness. Using a Rodenstock optic nerve head analyzer, Sogano et al[5] found that although the cup volume decreased and the rim area increased significantly after trabeculectomy, the RNFL height did not change 2 mo to 6 mo after surgery. Moreover, Irak et al[4] used confocal scanning laser ophthalmoscopy to evaluate 49 eyes 3 mo after filtration surgery and did not find a significant change in the RNFL cross-sectional area.
The differences between our findings and those of Aydin et al[8] could be attributed to several factors. First, the absence of a control group in their study precluded achieving definite conclusions. Moreover, their study was retrospective and the results obtained should be interpreted cautiously. In addition, those authors assumed that the RNFL thickness does not change after cataract surgery, and they combined the data obtained from trabeculectomy or combined cataract extraction and trabeculectomy.
The mean preoperative RNFL thickness was 6.2 μm lower in our study than in the study of Aydin et al[8]. Curiously, despite a higher mean thickness before surgery than in our patients, the mean preoperative MD was worse. This finding can be explained partially by an increase in the diffuse VF defects as the result of cataract artifacts. In fact, to avoid the effect of cataract removal on the VF test, they analyzed the data in only 35 eyes that had undergone only deep sclerectomy.
It has been reported that glaucomatous progression is more likely to be detected using OCT compared to HVF. This may reflect OCT hypersensitivity or true damage identified by OCT before detection by conventional methods[15]. In addition, the differences may be the result of different degrees of preexisting glaucomatous damage. Some experimental and clinical studies have shown that restoration of anatomic position is more likely to occur in the early stages of glaucoma[6,16,17].
The physiological basis of the improvement in optic nerve head appearance and RNFL thickness with IOP reduction is not clear. It has been suggested that IOP reduction results in less posterior bowing of the lamina criborsa[18,19].
Our results revealed that overall and segmental RNFL thickness seems to be more reliable index. Deep structural alterations revealed by OCT constitute an important indication of early functional changes. The VF MD seems to be more sensitive for the patients with POAG.
Several studies have shown a high correlation between the degree of improvement in the morphologic features of the optic nerve head and the percent of IOP reduction[2-4]. Aydin et al[8] reported that after filtration surgery, a 0.5-μm increase in the mean RNFL could be expected for each 1-mmHg decrease in IOP. Although a difference in IOP reduction could explain different results, the mean IOP change after surgery obtained in our study was 1.5 mmHg more than that obtained by Aydin et al[8]. Moreover, in the present study, the mean percent change in IOP was 45.3% ± 16.4%. Twenty-seven eyes (77.1%) had an IOP reduction of more than 30%, which is similar to data (73.7%) reported by Aydin et al[8].
In the present study we did not find a significant correlation between BCVA and overall or segmental RNFL thickness in the study group. There was a significant correlation between BCVA and overall RNFL thickness, and also between BCVA and the RNFL thickness in all quadrants (temporal, inferior and superior) in the control group. The highest correlation between BCVA and the RNFL thickness in the temporal sector (r = 0.432, P < 0.001) was most likely due to the location of the maculopapillary bundle in this region of the optic disc.
Mechanisms that explain an improvement in RNFL thickness with IOP reduction are unclear. After the retinal nerve fiber is damaged, it cannot regenerate. One plausible explanation is recovery of compressed RNFL. Moreover, it is possible that some axons are able to function marginally while the IOP is high and can recover some physiologic functions when the IOP is lowered, but this is a biomechanical or physiologic restoration, not an anatomic one.
In the present study, we found a significant increase in peripapillary RNFL thickness after successful deep sclerectomy. The only factor significantly correlating with changes in the RNFL thickness was the preoperative VF MD.
In conclusion, our results showed an increase in RNFL thickness after deep sclerectomy that correlates with IOP reduction. OCT measurements are affected by IOP reduction after deep sclerectomy as these changes may have some clinical significance in long-term follow-up. Thus, we recommend obtaining OCT images after deep sclerectomy procedure as a baseline for follow-up of POAG patients.
The authors thank Taha Baker for his care and diligence during writing the paper.
There is controversy about the effect of intraocular pressure (IOP) reduction on the peripapillary retinal nerve fiber layer (RNFL), which also is considered a marker of structural optic nerve damage. However, the effect of IOP reduction on peripapillary RNFL is still unclear. Some studies have found no significant changes in the retinal cross-sectional area using confocal scanning laser ophthalmoscopy (CSLO) and an optic nerve analyzer, whereas others have shown a significant increase in mean retinal height at the optic disc margin by CSLO.
The study found a significant change in the peripapillary RNFL thickness in the surgery group. The RNFL thickening was significant for the overall measurement and in all quadrants.
The mean preoperative RNFL thickness was 6.2 μm lower in our study than in the study of Aydin et al. Also, the authors found a significant change in the peripapillary RNFL thickness in the surgery group. The RNFL thickening was significant for the overall measurement and in all quadrants. These results are consistent with those reported by Aydin et al, who found a significant increase in the overall peripapillary RNFL from 72.8 μm to 81.7 μm after filtration surgery.
The results showed an increase in RNF thickness after deep sclerectomy that correlates with IOP reduction. optical coherence tomography (OCT) measurements are affected by IOP reduction after deep sclerectomy as these changes may have some clinical significance in long-term follow-up. Thus, the authors recommend obtaining OCT images after deep sclerectomy procedure as a baseline for follow-up of primary open-angle glaucoma patients.
This manuscript describes a study in which retinal nerve fiber layer thickness was measured before and after deep sclerectomy using optical coherence tomography. This study is generally well designed.
Peer reviewers: Colin Ian Clement, BSc, MBBS, PhD, FRANZC, Glaucoma Unit, Sydney Eye Hospital, 8 Macquarie Street, Sydney, NSW 2000, Australia; Necip Kara, MD, Department of Ophthalmology, Kanuni Sultan Suleyman Education and Research Hospital, 34290 Istanbul, Turkey
S- Editor Wu X L- Editor Wang TQ E- Editor Li JY
| 1. | Katz LJ, Spaeth GL, Cantor LB, Poryzees EM, Steinmann WC. Reversible optic disk cupping and visual field improvement in adults with glaucoma. Am J Ophthalmol. 1989;107:485-492. [PubMed] |
| 2. | Lesk MR, Spaeth GL, Azuara-Blanco A, Araujo SV, Katz LJ, Terebuh AK, Wilson RP, Moster MR, Schmidt CM. Reversal of optic disc cupping after glaucoma surgery analyzed with a scanning laser tomograph. Ophthalmology. 1999;106:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Kotecha A, Siriwardena D, Fitzke FW, Hitchings RA, Khaw PT. Optic disc changes following trabeculectomy: longitudinal and localisation of change. Br J Ophthalmol. 2001;85:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Irak I, Zangwill L, Garden V, Shakiba S, Weinreb RN. Change in optic disk topography after trabeculectomy. Am J Ophthalmol. 1996;122:690-695. [PubMed] |
| 5. | Sogano S, Tomita G, Kitazawa Y. Changes in retinal nerve fiber layer thickness after reduction of intraocular pressure in chronic open-angle glaucoma. Ophthalmology. 1993;100:1253-1258. [PubMed] |
| 6. | Shirakashi M, Nanba K, Iwata K. Reversal of cupping in experimental glaucoma. Ophthalmologica. 1991;202:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Raitta C, Tomita G, Vesti E, Harju M, Nakao H. Optic disc topography before and after trabeculectomy in advanced glaucoma. Ophthalmic Surg Lasers. 1996;27:349-354. [PubMed] |
| 8. | Aydin A, Wollstein G, Price LL, Fujimoto JG, Schuman JS. Optical coherence tomography assessment of retinal nerve fiber layer thickness changes after glaucoma surgery. Ophthalmology. 2003;110:1506-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Leung CK, Woo J, Tsang MK, Tse KK. Structural and functional recovery in juvenile open angle glaucoma after trabeculectomy. Eye (Lond). 2006;20:132-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Shaarawy T, Mansouri K, Schnyder C, Ravinet E, Achache F, Mermoud A. Long-term results of deep sclerectomy with collagen implant. J Cataract Refract Surg. 2004;30:1225-1231. [RCA] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | El Sayyad F, Helal M, El-Kholify H, Khalil M, El-Maghraby A. Nonpenetrating deep sclerectomy versus trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology. 2000;107:1671-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 183] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Stratus OCT. Model 3000 User Manual. Dublin, CA: Carl Zeiss Meditec 2003; 4-6. |
| 13. | Paunescu LA, Schuman JS, Price LL, Stark PC, Beaton S, Ishikawa H, Wollstein G, Fujimoto JG. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using Stratus OCT. Invest Ophthalmol Vis Sci. 2004;45:1716-1724. [RCA] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 366] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 14. | Schuman JS, Pedut-Kloizman T, Hertzmark E, Hee MR, Wilkins JR, Coker JG, Puliafito CA, Fujimoto JG, Swanson EA. Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology. 1996;103:1889-1898. [PubMed] |
| 15. | Wollstein G, Schuman JS, Price LL, Aydin A, Stark PC, Hertzmark E, Lai E, Ishikawa H, Mattox C, Fujimoto JG. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 261] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 16. | Topouzis F, Peng F, Kotas-Neumann R, Garcia R, Sanguinet J, Yu F, Coleman AL. Longitudinal changes in optic disc topography of adult patients after trabeculectomy. Ophthalmology. 1999;106:1147-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Coleman AL, Quigley HA, Vitale S, Dunkelberger G. Displacement of the optic nerve head by acute changes in intraocular pressure in monkey eyes. Ophthalmology. 1991;98:35-40. [PubMed] |
| 18. | Quigley HA. The pathogenesis of reversible cupping in congenital glaucoma. Am J Ophthalmol. 1977;84:358-370. [PubMed] |
| 19. | Albon J, Purslow PP, Karwatowski WS, Easty DL. Age related compliance of the lamina cribrosa in human eyes. Br J Ophthalmol. 2000;84:318-323. [PubMed] |