Published online Dec 30, 2011. doi: 10.5318/wjo.v1.i1.11
Revised: December 23, 2011
Accepted: December 23, 2011
Published online: December 30, 2011
AIM: To evaluate the efficacy and safety of laser-assisted subepithelial keratectomy (LASIK) for myopic correction done under thin flaps (120 μm) and compare with results obtained under thick flaps (150 μm).
METHODS: The study included 150 myopic eyes of 75 patients without previous refractive surgery who underwent LASIK prospectively. Two microkeratome heads (90 and 130) were used to create a flap with thickness of 120 μm and 150 μm, respectively. Thin flap group (120 μm) included 75 eyes while thick flap group included 75 eyes. Follow-up period was 12 mo. Efficacy, safety, and stability were evaluated and compared between the two groups.
RESULTS: In 150 eyes, the mean preoperative spherical equivalent refraction was -8.65 ± 2.6 D, mean sphere was -4.4 ± 3.5 D, and mean cylinder was -1.0 ± 1.3 D. The amount of ablation was significantly larger in the thin flap (88.5 ± 32.21 μm) group than in the thick flap group (64 ± 28.13 μm). Percentage of safety was higher in the thin flap group (94.8%) than in the thick flap group (91.7%). There were no intraoperative complications, especially flap-related problems. Subjective symptoms of dry eye occurred in 20.7% and 33.3% of eyes in the thin and thick flap groups, respectively.
CONCLUSION: Thin-flap LASIK is effective and safe in correcting myopic defects. It achieves better visual results, rapid visual recovery, and stable postoperative refraction than LASIK with thick flaps.
-
Citation: Ghanem AA, Nematallah EH. Evaluation of laser
in situ keratomileusis for myopic correction performed under thin flaps. World J Ophthalmol 2011; 1(1): 11-16 - URL: https://www.wjgnet.com/2218-6239/full/v1/i1/11.htm
- DOI: https://dx.doi.org/10.5318/wjo.v1.i1.11
Recent reports of post-laser-assisted subepithelial keratectomy (LASEK) ectasia have produced a renewed interest in surface ablation techniques, such as photorefractive keratectomy, LASEK and Epi-LASIK, to eliminate the need to perform a corneal flap and to preserve a thicker stromal bed less prone to mechanical destabilization[1]. Despite good refractive results, surface techniques are associated with greater pain, discomfort, and slower visual recovery in the immediate postoperative period.
Currently, LASIK is still the main refractive procedure performed because of its rapid and comfortable visual rehabilitation, good refractive stability, and lower potential for haze formation. Recent controversies concerning the advantages of surface versus lamellar refractive procedures have led to the development of thin-flap LASIK (in which LASIK is performed after creating intended regular thin flaps), which blends the advantages of both techniques by providing a faster and more comfortable visual recovery. Thin-flap LASIK also serves to preserve more stromal tissue, inducing less compromise of the corneal architecture and thereby theoretically reducing the possibility of ectasia secondary to thin stromal beds[2-5]. Additionally, thin-flap LASIK permits higher myopic and astigmatic corrections and allows wider optical zones that minimize unwanted scotopic visual symptoms.
The purpose of the present study was to evaluate the efficacy, safety, and stability of LASIK for myopic correction done under thin flaps and compare the results with those achieved with thick flaps using the Moria M2 microkeratome and the Allegretto wave eye Q excimer laser.
All procedures in this prospective, randomized, double-masked, controlled clinical study were performed in Mansoura Ophthalmic Center, Mansoura, Egypt. Patients included in the study were given a detailed explanation of the procedure and the risk/benefits of laser refractive surgery. Visual, verbal, or written informed consent was obtained from each patient. This study was approved by the human subjects committee and was performed in accordance with the Declaration of Helsinki (1989) of the World Medical Association.
The study included 150 eyes of 75 patients having spherical myopia, simple or compound myopic astigmatism without a history of previous refractive surgery. The mean age of the patients was 31 ± 9.2 years (range, 18-44 years). Forty-five patients were females and thirty patients were males.
Inclusion criteria were: (1) stable refraction for at least one year; (2) absence of any ocular pathology or surgical complications that might compromise the final visual outcome; (3) normal tear film with absence of dry eye; and (4) best-corrected visual acuity of 6/6 or 6/9 preoperatively.
Exclusion criteria were: (1) patients with previous intraocular surgery, corneal scarring, or active inflammation; (2) pachymetry value of less than 500 μm; (3) keratoconus; (4) schirmer test of less than 5.0 mm; and (5) associated posterior segment pathology.
All patients had a complete preoperative evaluation, including uncorrected and best-corrected visual acuity (UCVA, BCVA) using Landolt's broken ring chart, manifest and cycloplegic refraction, slit lamp biomicroscopy, Goldmann applanation tonometry, direct and indirect ophthalmoscopy, ultrasonic pachymetry (Nidek Up 1000), and corneal topography (Shin-Nippon CT-1000). The evaluation included dry eye symptoms (soreness, scratchiness, dryness and burning), tear film stability (break-up time), ocular surface staining and tear excursion (Schirmer I test). Contact lenses were discontinued two weeks before surgery.
The selected eyes were randomly divided into two groups according to the intraoperative flap thickness created: (1) thin flap (120 μm) group included 75 eyes; (2) thick flap (150 μm) group included 75 eyes. The measurements of actual flap thickness were done by intraoperative subtraction pachymetry.
Data of the operated eye were introduced into the computer of the excimer Laser machine. The calculated treatment data included optic zone, ablation zone, ablation depth and residual stromal depth. They were selected on the basis of the degree of refractive error, central corneal thickness, and microkeratome head used so as to achieve postoperative emmetropia with a minimum residual stromal bed depth of 275 μm[6].
The surgical procedure was performed with benoxinate hydrochloride 0.4% eye drops. The Moria M2 automated microkeratome (Moria, Antony, France) was used in all cases. Two heads (90 and 130) were used to create a flap thickness of 120 μm and 150 μm in the thin and thick flap groups, respectively. In all cases the standard speed (speed 2) was used. Suction rings and stop were used according to the M2 microkeratome nomogram on the basis of the keratometric readings. Superior hinged flap was created. After the cut, suction was released and the corneal flap was lifted with a spatula. The ablation was performed in the stromal bed using the Allegretto wave eye Q excimer laser (Wave-light, Amswolfsmantel, Erlanger, Germany). The corneal flap and hinge were irrigated with balanced salt solution and the flap was gently repositioned and stretched onto the eye. A therapeutic contact lens (Acuvue, base curve 9.1 m) was applied for corneal flap protection.
Postoperative treatment involved topical moxifloxacin 0.5% and prednisolone acetate 1% eye drops four times daily for 1 wk together with artificial tears eye drops three times per day for 6 wk. Gradual withdrawal of topical steroids was done over 2 wk. Patients were followed up postoperatively at first week, and then every month until 12 mo.
Patients’ data were recorded at each postoperative examination. To minimize bias all measurements were taken by the same examiner, who was masked with regard of two groups.
The follow-up examinations included: uncorrected visual acuity, slit-lamp biomicroscopy, keratometry, pachymetry, and corneal topography. A questionnaire was used at each visit to record the patient’s subjective symptoms of dry eye: dryness, fluctuations of vision, blurring of vision, and foreign body sensation. Postoperative results were assessed as follows:
Visual outcome: (1) Efficacy was represented as the percentage of eyes having no difference between postoperative UCVA and preoperative BCVA at one-week follow-up[3]; and (2) safety was assessed as the ratio postoperative BCVA /preoperative BCVA at the end of follow-up period[6].
Refractive outcome: Stability of the surgical procedure was measured as the percentage of eyes with postoperative manifest refractive spherical equivalent within ± 1.0 diopter at the end of follow-up period[7].
Data were collected and analyzed using the Statistical Program for the Social Sciences (SPSS) software (version 15; SPSS, Chigao, IL, United States). The quantitative variables were expressed as range or mean ± SD . Student t test was used to compare the quantitative data of the two groups while chi-square test was used to compare qualitative frequencies. A P value of less than 0.05 was considered to be statistically significant.
In all 150 eyes, the mean preoperative spherical equivalent refraction was -8.65 ± 2.6 D (range, -1.5 to -15 D), mean sphere was -4.4 ± 3.5 D (range, -1.25 to -14.0 D), and mean cylinder was -1.0 ± 1.3 D (range, 0 to 5.0 DC). The mean central corneal thickness was 546 ± 36.18 μm (range 500 μm to 645 μm).
Table 1 shows the preoperative data by group. There were significant differences between the thin (120 μm) and thick (150 μm) flap groups regarding pachymetry (thicker corneas had thicker flaps) and keratometric value (steeper cornea had thicker flaps). Although thin flaps were associated with higher degree of sphere and spherical equivalents to be corrected, the differences were insignificant.
| Thin flap group(120 mm)n = 75 | Thick flap group (150 mm)n = 75 | P value1 | |
| Sphere (D) | |||
| Mean ± SD | -5.0 ± 1.48 | -3.0 ± 2.18 | 0.0701 |
| Range | -2.0 to -14.0 | -1.25 to -12.0 | |
| Cylinder (D) | |||
| Mean ± SD | -1.0 ± 1.21 | -1.0 ± 1.85 | 0.0604 |
| Range | 0.0 to -5.0 | 0.0 to -3.0 | |
| Spherical equivalent (D) | |||
| Mean ± SD | -8.0 ± 4.06 | -7.75 ± 2.37 | 0.8063 |
| Range | -2.0 to -15.0 | -1.5 to -13.0 | |
| Pachymetry (mm) | |||
| Mean ± SD | 530 ± 35.88 | 545 ± 32.7 | 0.00062 |
| Range | 450 to 625 | 495 to 634 | |
| Keratometric value | |||
| Mean ± SD | 43.62 ± 1.3 | 44.72 ± 1.6 | 0.01222 |
| Range | 40.0 to 47.1 | 40.80 to 47.50 | |
LASIK was performed on 75 eyes with thin flaps (120 μm) using the 90 microkeratome head. The mean ablation depth was 88.6 ± 31.21 μm (range, 28-158 μm), and mean optic zone was 6.5 mm. Sixty-five eyes (43.3%) were operated after 150 μm flap creation using the 130 microkeratome head. The mean ablation depth was 66 ± 28.16 μm (range, 28-134 μm), and mean optic zone was 6.0 mm. The amount of ablation was significantly larger in the thin flap group than in the thick flap group (P = 0.004). The difference in the optic zone was insignificant.
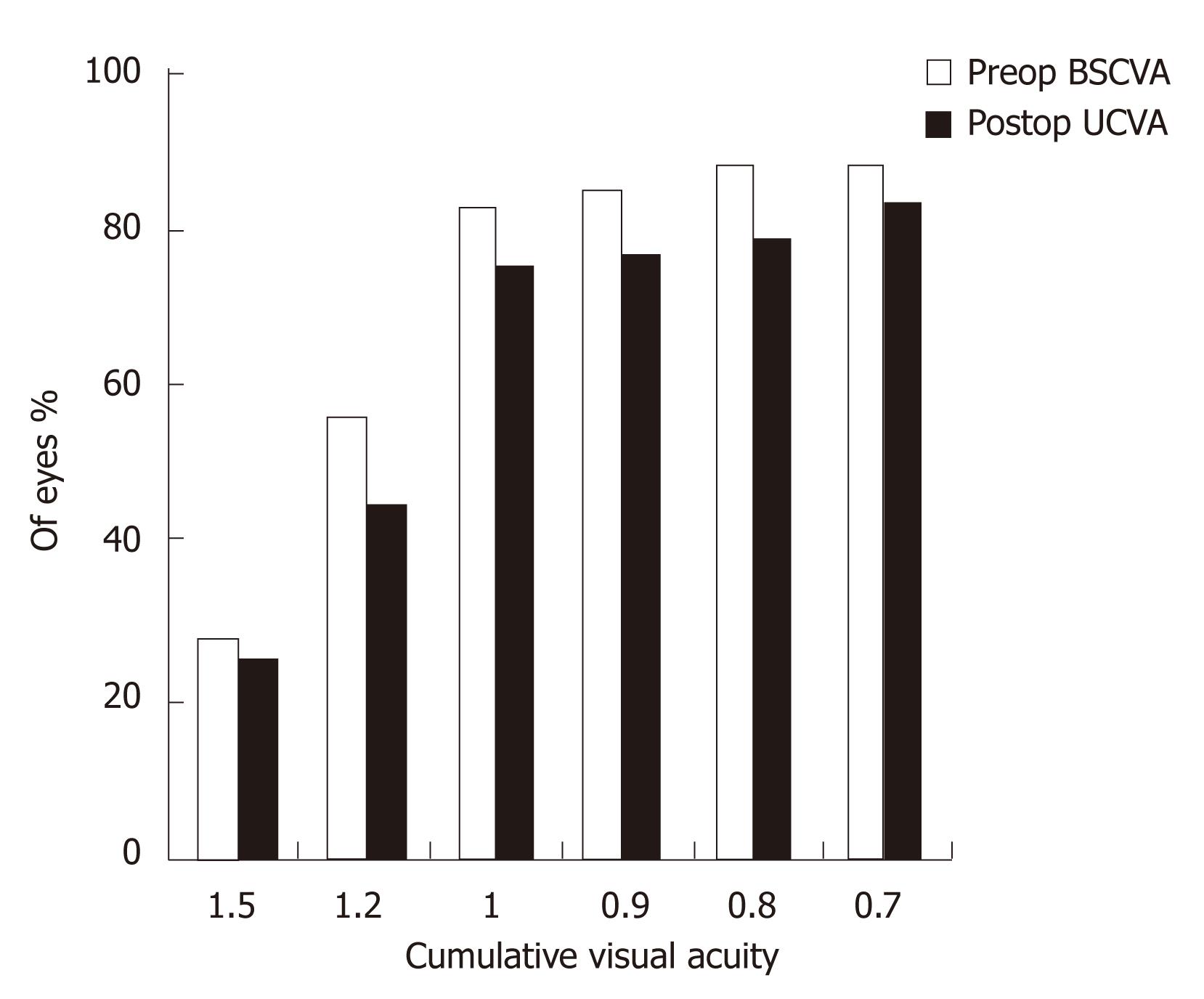
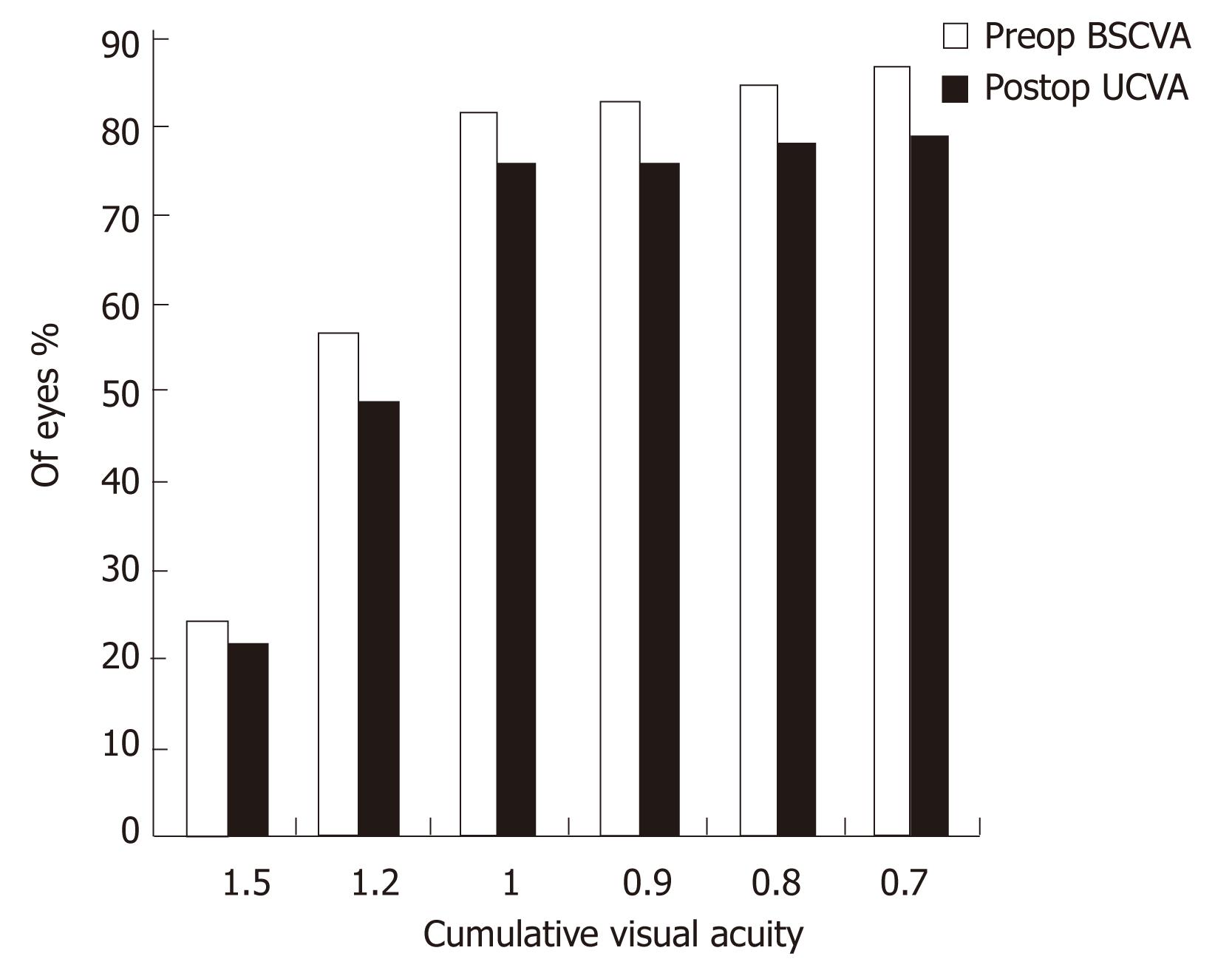
Figures 1 and 2 show the cumulative visual acuity in the thin and thick flap groups, respectively. No significant differences were found between the thin and thick flap groups.
Seventy-one eyes in the thin flap group achieved the preoperative BCVA with efficacy percentage of 94.8% as compared to 90.8% in the thick flap group (67 eyes). This difference was statistically significant (P < 0.05). Three month postoperatively, all the operated eyes had UCVA equal to the preoperative BCVA in the two groups with efficacy percentage of 100% (Table 2).
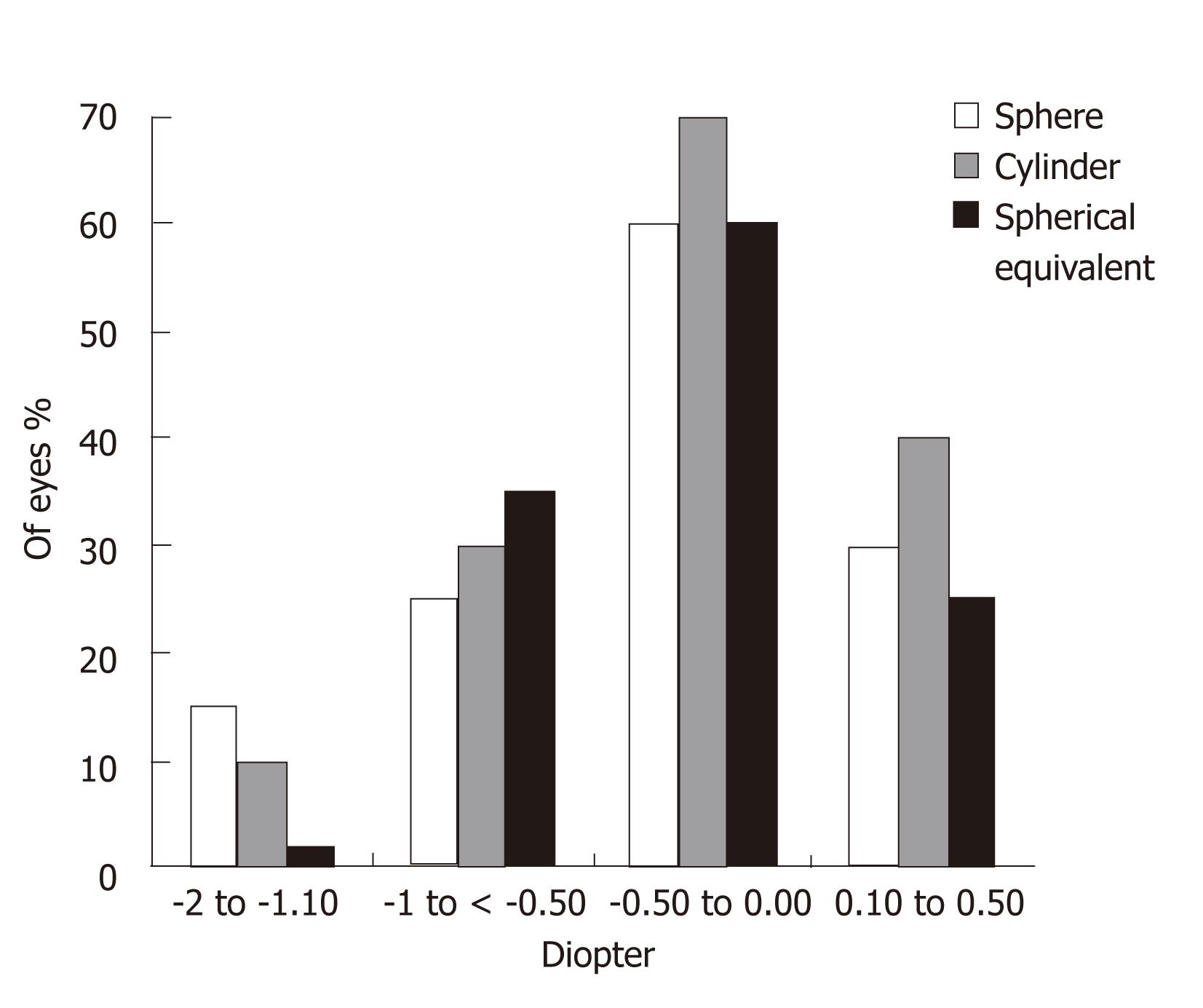
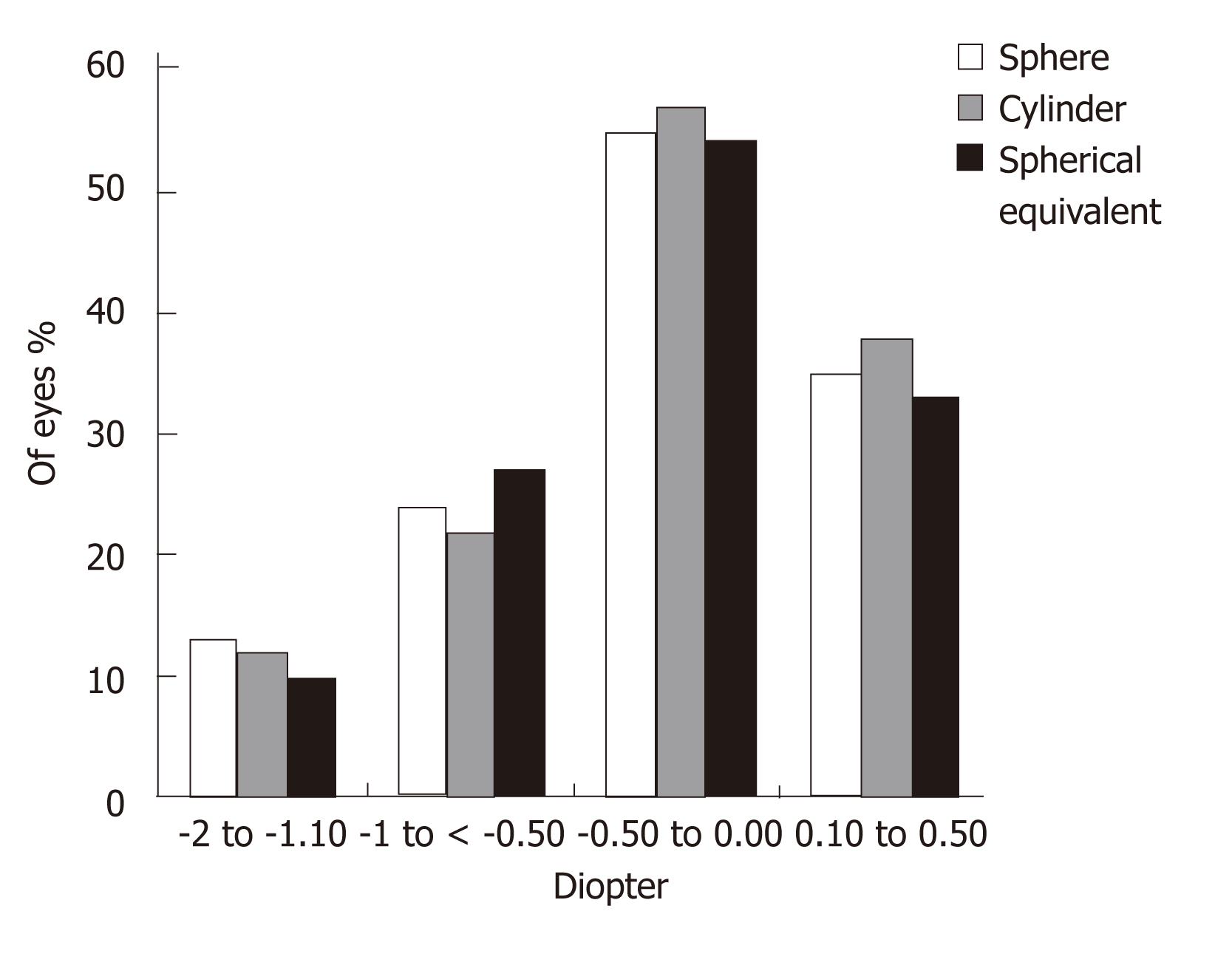
Figures 3 and 4 show the sphere, cylinder, and spherical equivalent in the thin and thick flap groups 12 mo postoperatively, respectively. No significant differences were found between the thin and thick flap groups.
The percentage of eyes that lost one or more lines of Landolt chart postoperatively was 7.3% (11 eyes). They were three eyes (4.0%) in the thin flap group (safety 94.8%) and 6 eyes (8.0%) in the thick flap group (safety 91.7%). The 11 eyes lost only one line of Landolt visual acuity chart. No eyes in the study lost two or more lines.
At 12 mo after LASIK, 65 eyes (86.6%) of the thin flap group and 59 eyes (78.7%) of the thick flap group were within ±1.0 D spherical equivalent (SE) of the attempted refraction. This difference was the measure of stability which was significantly higher in the thin flap group (90.1%) than in the thick flap group (86.3%).
Enhancement was performed in only eight eyes (5.3%) 12 mo after LASIK in the study. Though insignificant, the thin flap group had a lower rate of enhancement (3 eyes, 4.0%) than the thick flap group (5 eyes, 6.6%).
There were no intraoperative complications, especially flap-related problems such as free or incomplete flap or flaps with button-holes. No postoperative complications occurred. Subjective symptoms of dry eye such as dryness, blurring of vision, and foreign body sensation occurred in 20.7% (20) of eyes in the thin flap group and 33.3% (25) of eyes in the thick flap group. However, these symptoms were transient and disappeared after the use of lubricant eye drops and gel.
Flap thickness is a parameter in modern LASIK surgery. Innovations in excimer laser technology have improved the optical quality of the excimer laser profile. Together with the increasing use of the femtosecond laser, this has initiated the impact of flap thickness on the outcomes of LASIK.
Ectasia following LASIK surgery has been reported numerous times in ophthalmic literature, and predictability of flap thickness plays an important role in that risk[8,9]. Thin-flap LASIK is becoming more routine as refractive surgeons and patients continue to pursue the rapid healing advantages of LASIK but wish to minimize the risk for ectasia.
Studies evaluating the effect of corneal flap thickness on LASIK outcomes report conflicting results. Yi et al[10] found slightly better visual outcomes in the thick-flap group (> 165 μm) than in the thin-flap group (< 135 μm). Yeo et al[2] observed a higher incidence of central corneal opacity after LASIK with thin flaps (mean thickness 88.89 ± 8.07 μm). A possible explanation is injury to Bowman’s layer by the blade or a hidden masked buttonhole, camouflaged by intact epithelium, which may have caused central corneal scarring. The reason for the better outcomes achieved by thick-flap LASIK in these studies seems to be that the thin flap was an unintended complication; hence, it may have been irregular.
Recent retrospective studies evaluated the effect of intended thin flaps on the outcomes of LASIK 1, 3 and 6 mo after surgery and proposed that intended thin flaps for myopic LASIK were associated with better early visual and refractive results than thick flaps. Prandi et al[5] showed that thin flaps were associated with better UCVA at 3 mo and better residual spherical equivalent (SE) at 6 mo. Eleftheriadis et al[11] reported faster visual recovery (UCVA at 1 wk and 3 mo) and lower postoperative myopic SE in eyes with thinner flaps. Cobo-Soriano et al[3] found that patients with thin flaps achieved better contrast sensitivity and lower re-treatment rates.
The present study was in agreement with these studies. It shows better UCVA in the thin flap group with higher efficacy (94.8%) as compared to the thick flap group (90.8%) one week postoperatively. However, efficacy was 100% and equal in the two groups three months postoperatively. Degree of postoperative astigmatism at 12-mo follow-up was low (mean -0.76 ± 0.42) in the thin flap group and similar to that in the thick flap group. Furthermore, thin-flap LASIK was associated with significant ablation depth, hence permitting higher degrees of myopic correction.
Preoperatively the BCVA was 6/6 in 64 eyes (85.3%) in the thin flap group and in 62 eyes (82.7%) in the thick flap group. BCVA was 6/9 in 11 eyes (14.7%) in the thin flap group vs 14 eyes (18.7%) in the thick flap group. One week postoperatively, 62 eyes (82.7%) in the thin flap group and 55 eyes (73.3%) in the thick flap group had uncorrected visual acuity of 6/6. Ten eyes (13.7%) and 7 eyes (9.3%) respectively had UCVA of 6/9.
No intraoperative or postoperative complications occurred either in the thin or thick flap group. This was in accordance with the finding reported by Prandi et al[5], who showed that thin flaps were as safe as and did not create significant postoperative complications compared with conventional flaps. Also, Azar et al[6] reported no flap complications in their study. However, Esquenazi et al[7] showed an increased incidence of intraoperative and early postoperative complications in the thin-flap group compared with the medium- and thick-flap LASIK groups. Thin flaps were associated with buttonholes in 3% and epithelial defects in 7% of cases. These results suggested that surgeons performing thin flaps should follow up those patients more closely, especially in the first week after surgery.
Other advantages exist for thin-flap LASIK beyond the decreased risk for corneal ectasia. Anatomically, the anterior stroma is more compact[12], creating a smooth flap interface and anterior stromal bed surface, which is quite noticeable intraoperatively under the laser microscope. Thinner flaps have less edema[13] and are more easily stretched back into position to minimize or eliminate microstriae. This lessens the gap noted at the outer edge of the flap.
Theoretically, less flap bulk should allow better adherence of the flap created by the endothelial pumping mechanism, and this may lessen the risk for flap slippage and macrostriae. A smaller gap may also translate into less risk for epithelium in growth. Terminal corneal nerve bulbs are cut closer to the epithelial surface and they are fewer in number in thin flap, thus requiring less nerve regeneration and perhaps inducing fewer dry eye problems than in thick-flap LASIK[4].
Although corneal sensitivity was not measured in the present study, the incidence of subjective dry eye symptoms was lower in the thin flap group than in the thick flap group. Esquenazi et al[7] found no differences in these symptoms among their three groups.
Although Esquenazi et al[7] demonstrated a higher rate of complications in thin-flap LASIK compared to medium- and thick-flap LASIK, they stated that if no complications are encountered or if they are managed successfully, thin-flap LASIK has long term stability comparable to conventional flap thickness LASIK. Also, Azar et al[6] found that the stability of thin-flap LASIK was similar to that of conventional thick-flap LASIK in corneas with equivalent residual stromal bed thickness. In their study, there was a trend towards a lower re-treatment rate in the thin flap group than in the thick flap group. This is consistent with the present study which showed higher (90.1%) stability of thin-flap LASIK than thick-flap LASIK (87.3%) together with a lower rate of enhancement at six-month follow-up.
In summary, despite the conventional LASIK procedure used in this study, thin-flap LASIK is an effective and safe technique to correct myopic defects since it blends the advantages of surface procedures with the rapid and comfortable visual recovery of lamellar approaches. It achieves better visual results, more rapid visual postoperative recovery, stable postoperative refraction with better residual spherical equivalent, low degree of astigmatism, and a lower rate of enhancement than LASIK with thicker flaps. The accepted concept is that the main reason for creating thinner flaps is to have stronger corneas with wider ablations that provide higher vision quality and not to extend the range of power correction.
The authors thank Taha Baker for his care and diligence during writing the paper.
To evaluate the efficacy, safety, and stability of laser-assisted subepithelial keratectomy (LASIK) for myopic correction done under thin flaps and compare the results with those achieved with thick flaps. LASIK is still the main refractive procedure performed because of its rapid and comfortable visual rehabilitation, good refractive stability, and lower potential for haze formation.
Thin-flap LASIK is effective and safe to correct myopic defects. It achieves better visual results, rapid visual recovery, and stable postoperative refraction than LASIK with thicker flaps.
No intraoperative or postoperative complications occurred either in the thin or thick flap group. This finding is consistent with that reported by Prandi et al, who showed that thin flaps are as safe as and do not create significant postoperative complications compared with conventional flaps. Also, Azar et al reported no flap complications in their study.
Despite the conventional LASIK procedure used in this study, thin-flap LASIK is an effective and safe technique to correct myopic defects since it blends the advantages of surface procedures with the rapid and comfortable visual recovery of lamellar approaches. It achieves better visual results, more rapid visual postoperative recovery, stable postoperative refraction with better residual spherical equivalent, low degree of astigmatism, and a lower rate of enhancement than LASIK with thicker flaps. The accepted concept is that the main reason for creating thinner flaps is to have stronger corneas with wider ablations that provide higher vision quality and not to extend the range of power correction.
The paper is interesting and readable.
Peer reviewer: Alireza Ghaffarieh, MD, Research Fellowship, Department of Ophthalmology and Visual Sciences, University of Wisconsin School of Medicine and Public Health, Madison, WI 53726, United States
S- Editor Wu X L- Editor Wang TQ E- Editor Li JY
| 1. | 1 Rao SK, Srinivasan B, Sitalakshmi G, Padmanabhan P. Photorefractive keratectomy versus laser in situ keratomileusis to prevent keratectasia after corneal ablation. J Cataract Refract Surg. 2004;30:2623-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Yeo HE, Song BJ. Clinical feature of unintended thin corneal flap in LASIK: 1-year follow-up. Korean J Ophthalmol. 2002;16:63-69. [PubMed] |
| 3. | Cobo-Soriano R, Calvo MA, Beltrán J, Llovet FL, Baviera J. Thin flap laser in situ keratomileusis: analysis of contrast sensitivity, visual, and refractive outcomes. J Cataract Refract Surg. 2005;31:1357-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Duffey RJ. Thin flap laser in situ keratomileusis: flap dimensions with the Moria LSK-One manual microkeratome using the 100-microm head. J Cataract Refract Surg. 2005;31:1159-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Prandi B, Baviera J, Morcillo M. Influence of flap thickness on results of laser in situ keratomileusis for myopia. J Refract Surg. 2004;20:790-796. [PubMed] |
| 6. | Azar DT, Ghanem RC, de la Cruz J, Hallak JA, Kojima T, Al-Tobaigy FM, Jain S. Thin-flap (sub-Bowman keratomileusis) versus thick-flap laser in situ keratomileusis for moderate to high myopia: case-control analysis. J Cataract Refract Surg. 2008;34:2073-2078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Esquenazi S, Bui V, Grunstein L, Esquenazi I. Safety and stability of laser in situ keratomileusis for myopic correction performed under thin flaps. Can J Ophthalmol. 2007;42:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Binder PS. Ectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2003;29:2419-2429. [RCA] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Randleman JB, Russell B, Ward MA, Thompson KP, Stulting RD. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 487] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 10. | Yi WM, Joo CK. Corneal flap thickness in laser in situ keratomileusis using an SCMD manual microkeratome. J Cataract Refract Surg. 1999;25:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Eleftheriadis H, Prandi B, Diaz-Rato A, Morcillo M, Sabater JB. The effect of flap thickness on the visual and refractive outcome of myopic laser in situ keratomileusis. Eye (Lond). 2005;19:1290-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Müller LJ, Pels E, Vrensen GF. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 277] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Turss R, Friend J, Reim M, Dohlman CH. Glucose concentration and hydration of the corneal stroma. Ophthalmic Res. 1971;2:253-260. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 0.8] [Reference Citation Analysis (0)] |