Published online May 10, 2016. doi: 10.5317/wjog.v5.i2.187
Peer-review started: December 4, 2015
First decision: December 28, 2015
Revised: February 26, 2016
Accepted: March 14, 2016
Article in press: March 16, 2016
Published online: May 10, 2016
Processing time: 157 Days and 10.3 Hours
AIM: To examine the influence of gynecologic oncologists (GO) in the United States on surgical/chemotherapeutic standard of care (SOC), and how this translates into improved survival among women with ovarian cancer (OC).
METHODS: Surveillance, Epidemiology, and End Result (SEER)-Medicare data were used to identify 11688 OC patients (1992-2006). Only Medicare recipients with an initial surgical procedure code (n = 6714) were included. Physician specialty was identified by linking SEER-Medicare to the American Medical Association Masterfile. SOC was defined by a panel of GOs. Multivariate logistic regression was used to determine predictors of receiving surgical/chemotherapeutic SOC and proportional hazards modeling to estimate the effect of SOC treatment and physician specialty on survival.
RESULTS: About 34% received surgery from a GO and 25% received the overall SOC. One-third of women had a GO involved sometime during their care. Women receiving surgery from a GO vs non-GO had 2.35 times the odds of receiving the surgical SOC and 1.25 times the odds of receiving chemotherapeutic SOC (P < 0.01). Risk of mortality was greater among women not receiving surgical SOC compared to those who did [hazard ratio = 1.22 (95%CI: 1.12-1.33), P < 0.01], and also was higher among women seen by non-GOs vs GOs (for surgical treatment) after adjusting for covariates. Median survival time was 14 mo longer for women receiving combined SOC.
CONCLUSION: A survival advantage associated with receiving surgical SOC and overall treatment by a GO is supported. Persistent survival differences, particularly among those not receiving the SOC, require further investigation.
Core tip: A significant survival advantage is associated with receiving surgical standard of care (SOC), yet still some women had lower odds of receiving surgical SOC.
- Citation: Rim SH, Hirsch S, Thomas CC, Brewster WR, Cooney D, Thompson TD, Stewart SL. Gynecologic oncologists involvement on ovarian cancer standard of care receipt and survival. World J Obstet Gynecol 2016; 5(2): 187-196
- URL: https://www.wjgnet.com/2218-6220/full/v5/i2/187.htm
- DOI: https://dx.doi.org/10.5317/wjog.v5.i2.187
Women in the United States with advanced stage epithelial ovarian cancer (OC) have an overall 5-year survival rate of about 30%[1]. As with many cancers, survival is closely linked with the stage of diagnosis, such that women with localized (stage I) disease have a relative 5-year survival rate of 92%; the prognosis however declines with late stage disease and metastases[2]. Without an adequate early detection strategy, ensuring that women receive appropriate, standard of care (SOC) treatment is a very important intervention that has demonstrated reduction in OC mortality[3].
National Comprehensive Cancer Control Network (NCCN) current treatment recommendations for women with epithelial OC include an evaluation prior to initiating chemotherapy along with accurate surgical staging and primary debulking surgery/cytoreduction performed by a gynecologic oncologist (GO)[3]. In most but not all cases, at least six cycles of platinum and taxane-based chemotherapy administration is recommended for advanced epithelial OCs[3]. Appropriate care not only constitutes the receipt of SOC treatment, but also quality care from an experienced GO, who is trained to both perform the surgery and administer chemotherapy[3,4]. The evidence supporting better guideline-adherent care and outcomes among patients seen by a GO has been previously examined[5-8], and prior studies suggest only 30%-40% of women with OC are treated by a GO[5,9-11]. While NCCN cancer center patients tend to receive guideline-adherent care[12], there is potential in exploring whether differences in SOC treatment are affected across patient-level demographic and clinical subgroups.
To date, few studies have jointly considered surgical and chemotherapeutic SOC indicators in examining survival in OC patients[13-17]. In this study, we examine predictors of both SOC receipt (surgical and chemotherapeutic) and adherence to these treatments among women treated by GOs compared to non-GOs. We further quantified the survival advantage of SOC treatment receipt among OC patients.
The study included all women in the Surveillance, Epidemiology, End Results (SEER)-Medicare database[18] diagnosed with OC from January 1, 1992 to December 31, 2006 (n = 38972). We excluded women who did not have a primary epithelial OC diagnosis (n = 6175); were Medicare age-ineligible (age < 66) at date of diagnosis (n = 11716); had an invalid month of diagnosis (n = 166); had diagnoses based on autopsy or death certificate only (n = 543); had a non-epithelial ovarian malignancy (n = 3198); and were not continuously enrolled in both Medicare Part A and B or were enrolled in an Health Maintenance Organization plan during the course of treatment (n = 5486). A total of 11688 OC patients met the inclusion criteria for the study.
Patient-level covariates included age, race, stage at diagnosis, marital status, year of diagnosis, geographic region of SEER registry, and cancer histology. The Charlson-Klabunde comorbidity index score was determined using Medicare claims data for 12 mo prior to and 4 mo after cancer diagnosis date, per prior studies[19,20].
We examined all procedure codes in the Medicare claims data falling within a treatment window (defined as two months prior to and one year after the diagnosis date) to determine if a patient received surgical or chemotherapeutic SOC. Since only month and year of diagnosis are reported in the SEER database, the 15th day of the month was assigned as the day of diagnosis for each patient.
Per recommendation from an experienced group of GOs, consulted specifically for this project (W. Brewster, R.E. Bristow and D.K. Singh), the International Federation of Gynecologists and Obstetricians (FIGO) stage of disease categories were grouped as: IA/IB, IC/II, IIIA/IIIB and IIIC/IV based on similarities in current surgical and chemotherapeutic treatment regimens. FIGO stage III NOS and stage IV were grouped into stage IIIC/IV group, given that a high proportion of all stage III cases were stage IIIC.
Among the women who met the inclusion criteria (n = 11688), we examined receipt of SOC among women receiving any initial surgical care. Thus, we further excluded women who received treatment outside of the treatment window (n = 28), those who had no procedure codes of interest for any surgical care (n = 2464), and women who received neoadjuvant chemotherapy (n = 2482) (given the difficulty of cancer staging for women who are eligible for neoadjuvant chemotherapy) to examine differences in guideline-adherent treatment and survival. We also excluded all OC patients diagnosed with stage I NOS or who were unstaged at diagnosis since minimum SOC parameters are not well defined for these groups.
The GO group defined minimum surgical SOC as lymph node dissection, omentectomy and oophorectomy for all patients with FIGO stage IA/IB, IC/II or IIIA/IIIB at diagnosis, but omentectomy and oophorectomy only for women with stage IIIC/IV at diagnosis. Minimum chemotherapy SOC definition depended on: (1) stage of disease at diagnosis; (2) number of chemotherapy cycles received; and (3) type of chemotherapy agent received. For analysis, chemotherapy SOC was defined as an individual receiving the defined number of cycles (three cycles of chemotherapy for stage IC/II and six cycles for stage III/IV), with at least one multi-agent cycle (defined as one platinum based and one non-platinum based agent) using either intravenous or intraperitoneal modes of administration. One cycle of chemotherapy was equal to three weeks of treatment, given that chemotherapy is usually administered every 3-4 wk[3,21]. Patients were documented as receiving overall SOC if they received both surgical and adjuvant chemotherapeutic SOC.
The GO group recommended surgical and chemotherapy procedure codes for use in determining SOC for each FIGO stage category. Procedure codes included both International Classification of Diseases, Ninth revision, clinical modification codes and American Medical Association (AMA) Current Procedural Terminology codes.
Self-reported, physician specialty information from the SEER-Medicare claims file was linked with and verified against the AMA Physician Masterfile using the unique provider identification number (UPIN) for physicians performing (or those in attendance) of an OC procedure of interest. If the operating physician UPIN was not available, but the attending physician UPIN was available, AMA specialty was assigned to the attending physician. If the UPIN for an operating and attending physician was unavailable, the self-reported physician specialty variable found in the Medicare data set was used to define specialty. When a patient received treatment from multiple physicians, care was attributed to the most specialized physician (most to least specialized: GO, gynecologist, general surgeon, and other physician). For analytic purposes, physician specialty was grouped as GO and non-GO.
We examined predictors associated with receipt of surgical and chemotherapeutic SOC. A forward selection logistic regression model was used to examine each question. Comparisons of the distribution of OC patients receiving the SOC by physician specialty was examined using the Pearson χ2 test.
Cox proportional hazard methods were used to determine differences in survival time from date of OC diagnosis to date of death. The proportional hazards assumption was examined by testing interactions between time and each covariate in the model. The final models (Model 1 and 2) exclude women (n = 1003) who died within 4.5 mo after diagnosis (i.e., women who did not live long enough to receive chemotherapy SOC). Due to a common category in the chemotherapy variables (chemotherapy SOC and chemotherapy physician specialty), we examined two different models. The first model (Model 1) examined surgery physician specialty and receipt of both SOC measurements, while the second model (Model 2) examined both surgery and chemotherapy physician specialty and receipt of surgery SOC, adjusting for patient-level and clinical factors. All final models were adjusted for covariates that had a statistically significant association from the bivariate analysis or were of importance in the literature. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, United States).
Among the 11688 OC patients, 57.4% (n = 6714) received an initial surgical procedure code of interest. Table 1 shows the patient and tumor characteristics by the type of physician performing the initial surgery. The mean age of patients was mid to late-70s; most women were white, married or widowed, had no comorbidities, had FIGO stage IIIC/IV disease, and serous histology. More women received an initial surgical procedure from OB/GYNs (n = 3088) than GOs (n = 2254), general surgeons (n = 914), or other non-GO/unknown specialties (n = 419).
| Characteristic | Surgeon specialty1 | |||
| GO | Non-GO | |||
| OBGYN | General surgeon | Other2 | ||
| No. of patients | 2254 | 3088 | 914 | 419 |
| Mean age at diagnosis (stddev) | 74.6 (5.9) | 74.8 (6.1) | 77.0 (6.8) | 75.5 (6.2) |
| Race n (%) | ||||
| White | 1995 (88.5) | 2844 (92.1) | 827 (90.5) | 379 (90.5) |
| African American | 121 (5.4) | 104 (3.4) | 49 (5.4) | 26 (6.2) |
| Hispanic | 35 (1.6) | 31 (1.0) | 3 | 3 |
| Asian | 53 (2.4) | 66 (2.1) | 3 | 3 |
| Other4 | 47 (2.1) | 37 (1.2) | 3 | 3 |
| Marital status | ||||
| Married | 1052 (46.7) | 1424 (46.1) | 327 (35.8) | 170 (40.6) |
| Single | 159 (7.1) | 221 (7.2) | 53 (5.8) | 31 (7.4) |
| Divorced | 148 (6.6) | 166 (5.4) | 58 (6.3) | 29 (6.9) |
| Widowed | 799 (35.4) | 1168 (37.8) | 458 (50.1) | 176 (42.0) |
| Separated/unknown | 96 (4.2) | 109 (3.5) | 3 | 3 |
| Charlson-Klabunde comorbidity score | ||||
| 0 | 1521 (67.5) | 2133 (69.1) | 605 (66.2) | 266 (63.5) |
| 1 | 498 (22.1) | 644 (20.9) | 188 (20.6) | 93 (22.2) |
| 2 | 175 (7.8) | 189 (6.1) | 78 (8.5) | 38 (9.1) |
| 3 | 45 (2.0) | 80 (2.6) | 29 (3.2) | 3 |
| 4 or more | 3 | 42 (1.4) | 3 | 3 |
| FIGO treatment stage | ||||
| IA/IB | 200 (8.9) | 383 (12.4) | 66 (7.2) | 43 (10.3) |
| IC/II | 276 (12.2) | 516 (16.7) | 90 (9.8) | 40 (9.5) |
| IIIA/IIIB | 119 (5.3) | 179 (5.8) | 59 (6.5) | 3 |
| IIIC/IV | 1580 (70.1) | 1898 (61.5) | 660 (72.2) | 308 (73.5) |
| Unstaged/NOS | 79 (3.5) | 112 (3.7) | 39 (4.2) | 3 |
| Histology | ||||
| Serous | 1460 (64.8) | 1897 (61.4) | 554 (60.6) | 254 (60.6) |
| Endometrioid | 238 (10.6) | 381 (12.3) | 73 (8.0) | 46 (11.0) |
| Mucinous | 129 (5.7) | 235 (7.6) | 79 (8.6) | 25 (6.0) |
| Clear cell | 84 (3.7) | 127 (4.1) | 3 | 3 |
| Adenocarcinoma | 275 (12.2) | 344 (11.1) | 175 (19.1) | 66 (15.8) |
| Other5 | 68 (3.1) | 104 (3.3) | 20 (2.2) | 3 |
Among women treated by a GO, 79.2% received the surgical SOC and 52.8% received the chemotherapy SOC (Figure 1). Regardless of stage at diagnosis, women more frequently received surgical and chemotherapeutic SOC from a GO than from a non-GO.
Table 2 reports the factors associated with receipt of surgical SOC after adjusting for other covariates. Surgery performed by a GO was strongly associated with receiving surgical SOC [odds ratio (OR) for GO = 2.35; 95%CI: 2.03-2.71]. Other factors associated with greater odds of surgical SOC receipt included: More advanced stage of disease, white vs African-American race, younger age at diagnosis, serous vs adenocarcinoma not otherwise specified histologic type, being married vs not married, and diagnosis during the later years of the study period.
| Surgical standard of care2 | Chemotherapeutic standard of care2 | |||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | |
| Physician specialty3 | ||||
| Gynecologic oncologist | 2.35 (2.03-2.71) | < 0.01 | 1.25 (1.07-1.47) | 0.006 |
| Non-gynecologic oncologist | 1.00 | 1.00 | ||
| Age at diagnosis | ||||
| 66-69 | 1.00 | 1.00 | ||
| 70-74 | 0.80 (0.67-0.96) | 0.017 | 0.93 (0.78-1.09) | 0.393 |
| 75-79 | 0.83 (0.69-1.0) | 0.053 | 0.79 (0.66-0.94) | 0.008 |
| 80-84 | 0.58 (0.47-0.71) | < 0.01 | 0.61 (0.48-75) | < 0.001 |
| ≥ 85 | 0.40 (0.31-0.51) | < 0.01 | 0.31 (0.21-0.48) | < 0.001 |
| Race4 | ||||
| White | 1.00 | |||
| African American | 0.67 (0.50-0.91) | 0.01 | - | |
| Other | 0.83 (0.62-1.10) | 0.208 | - | |
| Treatment stage5 | ||||
| IA/IB | 0.08 (0.07-0.10) | < 0.01 | NA | NA |
| IC/II | 0.08 (0.07-0.10) | < 0.01 | 3.46 (2.86-4.18) | < 0.001 |
| IIIA/IIIB | 0.05 (0.04-0.07) | < 0.01 | 0.83 (0.64-1.09) | 0.182 |
| IIIC/IV | 1.00 | 1.00 | ||
| Charlson-Klabunde comorbidity score | ||||
| 0 | 1.00 | 1.00 | ||
| 1 | 0.84 (0.72-0.98) | 0.029 | 0.84 (0.71-0.99) | 0.029 |
| 2 | 0.81 (0.63-1.02) | 0.084 | 0.78 (0.60-1.03) | 0.078 |
| 3 | 0.65 (0.44-0.97) | 0.039 | 0.49 (0.31-0.80) | 0.005 |
| 4 or more | 1.09 (0.60-1.97) | 0.771 | 0.63 (0.29-1.37) | 0.247 |
| Histology | ||||
| Serous | 1.00 | 1.00 | ||
| Endometrioid | 1.10 (0.90-1.35) | 0.356 | 0.70 (0.56-0.89) | 0.003 |
| Mucinous | 0.95 (0.74-1.35) | 0.67 | 0.49 (0.34-0.70) | < 0.001 |
| Clear cell | 1.29 (0.93-1.78) | 0.13 | 0.62 (0.41-0.93) | 0.026 |
| Transitional | 0.70 (0.27-1.79) | 0.454 | 0.76 (0.30-1.97) | 0.572 |
| Adenocarcinoma (NOS) | 0.44 (0.37-0.54) | < 0.001 | 1.04 (0.86-1.27) | 0.695 |
| Other | 1.05 (0.70-1.56) | 0.813 | 0.74 (0.47-1.13) | 0.168 |
| Marital status | ||||
| Married | 1.00 | 1.00 | ||
| Not married | 0.83 (0.72-0.95) | 0.007 | 0.75 (0.66-0.86) | < 0.001 |
| Unknown | 1.03 (0.69-1.52) | 0.87 | 0.73 (0.48-1.09) | 0.127 |
| Year of diagnosis | ||||
| 1993-1997 | 0.62 (0.52-0.73) | < 0.01 | 0.28 (0.23-0.33) | < 0.001 |
| 1998-2002 | 0.79 (0.68-0.92) | 0.003 | 1.09 (0.94-1.26) | 0.261 |
| 2003-2006 | 1.00 | 1.00 | ||
| SEER region4 | ||||
| Northeast | - | 1.00 | ||
| Midwest | - | 0.76 (0.62-0.93) | 0.009 | |
| South | - | 1.09 (0.88-1.37) | 0.424 | |
| West | - | 0.93 (0.78-1.10) | 0.391 | |
Table 2 also reports factors associated with receipt of the minimum chemotherapy SOC after adjusting for other covariates. Women who obtained chemotherapy from a GO had a higher odds of receiving chemotherapeutic SOC (OR = 1.25, 95%CI: 1.07-1.47). Other statistically significant factors for higher odds of chemotherapeutic SOC included: Less advanced stage of disease, younger age at diagnosis, histologic type (serous compared with endometrioid/mucinous/clear cell), being married compared with unmarried, living in the SEER Midwest region (compared to the SEER Northeast), and diagnosis during more recent years.
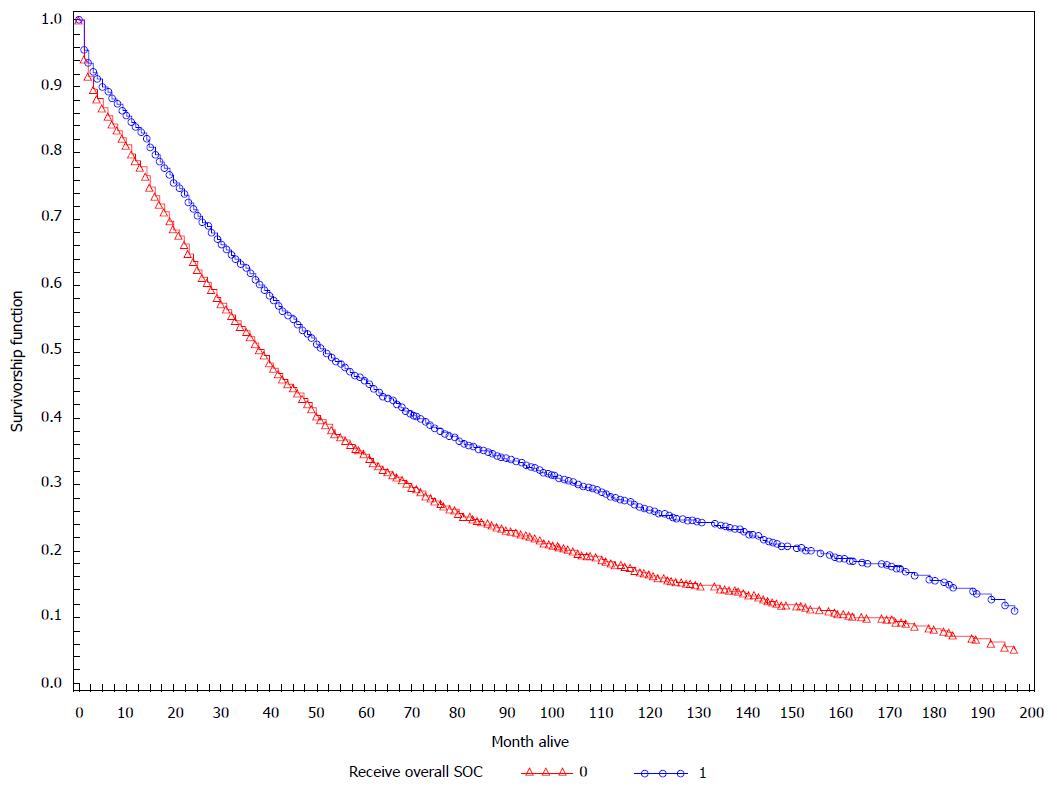
Table 3 shows the Cox regression model of time to death among the sample of OC patients who received a primary surgery procedure who did not die within 4.5 mo after diagnosis. In Table 3 (Model 1), women who did not receive surgery SOC had increased mortality compared to women who did [hazard ratio 1.22 (95%CI: 1.12-1.33)]. Similarly, women who did not receive any chemotherapy SOC had a higher risk of earlier death compared to women who received the full contingent of chemotherapy [hazard ratio 1.29 (95%CI: 1.14-1.46)]. Increasing age, late stage disease, higher number of comorbidities, and mucinous histology compared to serous histology were all associated with increased death (Table 3, Model 1). Similar patterns were observed in Table 3, Model 2 after controlling for chemotherapy physician specialty (as opposed to chemotherapy SOC). For Model 2, women who received surgery from a GO had better survival. Although there was no significant difference in survival between chemotherapy treatment from a GO compared to non-GO, those not receiving any chemotherapy had a significantly shorter survival time (Table 3, Model 2). The median survival time for women who received the overall SOC was 52 mo compared to 38 mo for women that did not receive the overall surgical and chemotherapeutic SOC (Figure 2).
| Predictor | Model 11 | Model 21 | ||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Received surgery SOC2 | ||||
| Yes | 1.00 | 1.00 | ||
| No | 1.22 (1.12-1.33) | < 0.01 | 1.21 (1.11-1.31) | < 0.01 |
| Received chemotherapy SOC2 | 4 | |||
| Yes | 1.00 | 4 | ||
| No, but received some chemotherapy | 0.95 (0.89-1.02) | 0.18 | 4 | |
| Received no chemotherapy | 1.29 (1.14-1.46) | < 0.01 | 4 | |
| Age at diagnosis | ||||
| 66-69 | 1.00 | 1.00 | ||
| 70-74 | 1.07 (0.98-1.17) | 0.13 | 1.05 (0.97-1.15) | 0.24 |
| 75-79 | 1.23 (1.12-1.34) | < 0.01 | 1.21 (1.10-1.32) | < 0.01 |
| 80-84 | 1.52 (1.37-1.69) | < 0.01 | 1.48 (1.33-1.65) | < 0.01 |
| ≥ 85 | 1.96 (1.70-2.26) | < 0.01 | 1.92 (1.67-2.21) | < 0.01 |
| Race | ||||
| White | 1.00 | 1.00 | ||
| African American | 1.11 (0.95-1.29) | 0.18 | 1.13 (0.97-1.32) | 0.12 |
| Other | 0.90 (0.78-1.05) | 0.17 | 0.88 (0.75-1.02) | 0.09 |
| Year of diagnosis | ||||
| 1993-1997 | 1.27 (1.17-1.38) | < 0.01 | 1.24 (1.14-1.35) | < 0.01 |
| 1998-2002 | 1.18 (1.09-1.27) | < 0.01 | 1.17 (1.08-1.27) | < 0.01 |
| 2003-2006 | 1.00 | 1.00 | ||
| Treatment stage | ||||
| IA/IB | 0.20 (0.18-0.23) | < 0.01 | 0.17 (0.15-0.20) | < 0.01 |
| IC/II | 0.35 (0.32-0.40) | < 0.01 | 0.36 (0.32-0.40) | < 0.01 |
| IIIA/IIIB | 0.61 (0.53-0.71) | < 0.01 | 0.62 (0.54-0.71) | < 0.01 |
| IIIC/IV | 1.00 | 1.00 | ||
| Charlson-Klabunde comorbidity score | ||||
| 0 | 1.00 | 1.00 | ||
| 1 | 1.28 (1.18-1.38) | < 0.01 | 1.26 (1.17-1.36) | < 0.01 |
| 2 | 1.38 (1.22-1.56) | < 0.01 | 1.37 (1.21-1.55) | < 0.01 |
| 3 | 1.64 (1.34-2.00) | < 0.01 | 1.64 (1.34-2.01) | < 0.01 |
| ≥ 4 | 2.33 (1.73-3.15) | < 0.01 | 2.27 (1.67-3.09) | < 0.01 |
| Histology | ||||
| Serous | 1.00 | 1.00 | ||
| Endometrioid | 0.76 (0.68-0.85) | < 0.01 | 0.75 (0.68-0.84) | < 0.01 |
| Mucinous | 1.22 (1.06-1.41) | < 0.01 | 1.22 (1.06-1.41) | < 0.01 |
| Clear cell | 0.83 (0.69-1.00) | 0.05 | 0.83 (0.69-1.00) | 0.05 |
| Transitional | 0.79 (0.47-1.31) | 0.36 | 0.79 (0.48-1.32) | 0.37 |
| Adenocarcinoma (NOS) | 1.07 (0.98-1.18) | 0.14 | 1.07 (0.97-1.17) | 0.2 |
| Other | 1.02 (0.82-1.28) | 0.85 | 1.02 (0.82-1.28) | 0.85 |
| Marital status | ||||
| Married | 1.00 | 1.00 | ||
| Not Married | 1.07 (1.00-1.14) | 0.05 | 1.07 (1.00-1.14) | 0.05 |
| Unknown | 1.00 (0.82-1.23) | 0.97 | 0.99 (0.80-1.21) | 0.89 |
| Surgeon specialty3 | ||||
| Non-GO | 1.00 | 1.00 | ||
| GO | 0.90 (0.84-0.96) | < 0.01 | 0.90 (0.84-0.97) | < 0.01 |
| Chemotherapy specialty3 | ||||
| Non-GO | 4 | 1.00 | ||
| GO | 4 | 0.98 (0.89-1.08) | 0.68 | |
| Did not receive chemotherapy | 4 | 1.33 (1.19-1.47) | < 0.01 | |
Our findings show that among OC patients receiving initial surgical treatment, only 25% of women received the overall SOC as defined by our panel of GOs. Few women (approximately one-third of women receiving a surgical procedure) had a GO involved at any point during their care. Women who obtained surgery from a GO however, were more likely to receive the surgical SOC and chemotherapeutic SOC than women who obtained treatment from a non-GO. The median survival time was 14 mo longer for women who received the overall SOC compared to women who did not receive overall SOC.
Our results are consistent with prior studies that suggest that appropriate surgical treatment in the United States is more frequently performed when a GO is the treating physician[5]. Data from a single state cancer registry study by Chan et al[14] showed that women with OC under the care of GOs were more likely to receive appropriate staging and chemotherapy treatments, controlling for age, stage, and grade of disease. Also similar to previous studies, our results suggest that greater utilization of GOs in the care of OC patients would be beneficial[22]. Although the level of detail in our analysis is unable to discriminate the factors underlying the low utilization, it is likely that our results reflect a complex interaction of both preference and access-relevant effects, such as the influence of a patient’s choice in receipt of GO care vs a shortage of available GOs in some areas.
While patient treatment preferences can independently and significantly affect chemotherapy receipt[23], geographic access may also play an important role in (both chemotherapeutic or surgical) treatment receipt from a GO. For example, a previous analysis reported on the unequal distribution of GOs in the United States[24]. A recently published study suggested that OC mortality may be a function of distance to a practicing GO as counties located more than 50 miles from a gynecologic oncology practice had almost 60% increased likelihood of OC mortality than those physically closer to a practice location[25]. While earlier research efforts have indicated that treatment of OC can be improved by early referral to a GO[5,10], referral and consultation from GOs have generally been low, with only about 39% of family physicians and 51% of general internists self-reporting referrals to a GO[26]. Given that surgery is an important determinant of outcomes for OC patients, receiving surgery/treatment from surgeons with specialized training in pelvic surgery (i.e., GOs)[27], who see a high volume of cases[10,28] at high volume facilities treating more than 20 OC cases per year[28,29], might help improve outcomes.
It is important to note that there are still subgroups that require further research. Although African-American women were more likely than their white counterparts to receive their initial surgical procedure from a GO (data not shown), they had lower odds of receiving the surgical SOC and there was no difference in survival after adjusting for physician specialty, surgical SOC, and other tumor and sociodemographic characteristics. The increased risk of death among African American women noted in other studies, when controlling for receipt of chemotherapeutic SOC, suggests that there may be some important nuanced differences in the definitions of chemotherapeutic SOC[30,31], chemotherapeutic agents, and/or interaction effects between age, comorbidity, stage, and race that have not been adequately explored. Bristow et al[32] have previously suggested similar differences in survival between African-American and white OC patients and the complexity of examining race-based survival associations[33,34].
The findings in this study should be considered in light of several limitations: (1) our analysis was focused on fee for service Medicare; women who received treatment under managed care were not included because the managed care cases did not include codes to identify specific treatment procedures; (2) neoadjuvant chemotherapy cases, which could have later received surgical SOC, were excluded; and (3) it is a challenge to operationalize NCCN recommendations into an analytic/computer program because the recommendations are relatively complex, and some information required for the NCCN decision algorithms is not available in claims data. However, our panel of experienced GOs developed a simpler, but accurate definition of the SOC so that recommendations could be converted into analytic code. Similarly, since SOC definitions were varied for each stage at diagnosis, if claims data were not available for the full contingency of treatment procedures, it is possible that there was an underestimation of patients identified as receiving overall SOC in that subgroup. Fourth, given the limitations of Medicare data, inaccuracies or incomplete data in billing, drug, or procedure codes could have resulted in an underestimate or overestimate of the total number of surgeries and/or chemotherapy procedures performed, thus biasing the estimate. Previous studies have noted some concerns in the validation of chemotherapeutic agents within Medicare claims data[30,31]. Fifth, there is potential for misclassification of physician specialty, given the use of multiple data sources including operating physician, attending physician, and self-reported physician specialty[35]. Furthermore, in our analysis, receipt of treatment from a GO was designated as such if a GO had been seen at any point during the care. Lastly, since we assumed each cycle of treatment lasted three weeks, we calculated that it would take at least 4.5 mo for women diagnosed with stage IIIC or IV to complete the chemotherapy SOC as defined in our study. Thus, women who died within five months of the diagnosis date would not have had the opportunity to receive chemotherapy SOC. Our definition of chemotherapy SOC may have been too rigorous and potentially introduce selection or survival bias.
Our study showed that GOs more often provided the surgical and chemotherapeutic SOC. The receipt of surgical standards was associated with better survival outcomes, even after adjusting for provider specialty. As such, these two NCCN-recommendations (i.e., treatment from a GO and receipt of SOC) continue to be critical points of intervention for improving survival time and reducing deaths from OC. Although it is difficult to determine when adjuvant chemotherapy is warranted based on sound clinical judgement (i.e., taking into consideration the patient’s comorbidities, toxicities, age, etc.) or patient refusal, one area that has not been carefully examined is the potential that race/ethnicity-based differences in patient and caregiver preferences may have for OC care. Future research may further explore this and the interaction effects of race, age, comorbidities on survival.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Ovarian cancer (OC) is the deadliest gynecologic cancer among women. Standard treatment for OC consists of extensive surgery and chemotherapy. Gynecologic oncologists (GOs) more often adhere to standard treatment guidelines among OC patients, resulting in longer patient survival.
Low survival rates from OC may be related to lack of GO involvement in surgery or chemotherapy and/or lack of standard treatment receipt. Research measuring receipt of standard of care and its effect on survival can help reveal areas for intervention to improve OC mortality.
These results among over 6000 OC patients aged 65 and older indicate that a low proportion received standard treatment. Not having seen a GO, African American race, and being older (80+) were associated with not receiving standard treatment. Women who received standard treatment survived over one year longer than those who did not receive standard treatment.
Ensuring appropriate referral of OC patients to GOs for treatment will likely increase survival rates from OC. Education of primary care providers and/or health systems changes that promote referral would be beneficial to increase referral rates. Research is needed into patient factors and other potential reasons underlying lack of referral.
GOs are subspecialists trained to administer both surgical and chemotherapeutic treatment to OC patients.
The authors investigated the influence of GO in the United States on surgical/chemotherapeutic SOC, and how this translates into improved survival among women with OC. The authors claimed that a survival advantage is associated with receiving surgical SOC and overall treatment by a GO. This manuscript provides useful information to the medical students, clinicians, and researchers in this field.
P- Reviewer: Cosmi E, Sonoda K, Tan GC, Yokoyama Y S- Editor: Song XX L- Editor: A E- Editor: Jiao XK
| 1. | Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. . |
| 2. | Chan JK, Kapp DS, Shin JY, Osann K, Leiserowitz GS, Cress RD, O’Malley C. Factors associated with the suboptimal treatment of women less than 55 years of age with early-stage ovarian cancer. Gynecol Oncol. 2008;108:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | National Comprehensive Cancer Network, 2014 Epithelial Ovarian Cancer/Fallopian Tube Cancer/Primary Peritoneal Cancer. 2014. . |
| 4. | Bristow RE, Zahurak ML, Diaz-Montes TP, Giuntoli RL, Armstrong DK. Impact of surgeon and hospital ovarian cancer surgical case volume on in-hospital mortality and related short-term outcomes. Gynecol Oncol. 2009;115:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Earle CC, Schrag D, Neville BA, Yabroff KR, Topor M, Fahey A, Trimble EL, Bodurka DC, Bristow RE, Carney M. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006;98:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 313] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Eisenkop SM, Spirtos NM, Montag TW, Nalick RH, Wang HJ. The impact of subspecialty training on the management of advanced ovarian cancer. Gynecol Oncol. 1992;47:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 188] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Kehoe S, Powell J, Wilson S, Woodman C. The influence of the operating surgeon’s specialisation on patient survival in ovarian carcinoma. Br J Cancer. 1994;70:1014-1017. [PubMed] |
| 8. | Mayer AR, Chambers SK, Graves E, Holm C, Tseng PC, Nelson BE, Schwartz PE. Ovarian cancer staging: does it require a gynecologic oncologist? Gynecol Oncol. 1992;47:223-227. [PubMed] |
| 9. | Vernooij F, Heintz P, Witteveen E, van der Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecol Oncol. 2007;105:801-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 186] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Mercado C, Zingmond D, Karlan BY, Sekaris E, Gross J, Maggard-Gibbons M, Tomlinson JS, Ko CY. Quality of care in advanced ovarian cancer: the importance of provider specialty. Gynecol Oncol. 2010;117:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Chan JK, Kapp DS, Shin JY, Husain A, Teng NN, Berek JS, Osann K, Leiserowitz GS, Cress RD, O’Malley C. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet Gynecol. 2007;109:1342-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Erickson BK, Martin JY, Shah MM, Straughn JM, Leath CA. Reasons for failure to deliver National Comprehensive Cancer Network (NCCN)-adherent care in the treatment of epithelial ovarian cancer at an NCCN cancer center. Gynecol Oncol. 2014;133:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121:1226-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 14. | Chan J, Kapp D, Shin J, Husain A, Teng N, Berek J, Osann K, Leiserowitz G, Cress R, O’Malley C. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet Gynecol. 2007;109:1342-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Engelen MJ, Kos HE, Willemse PH, Aalders JG, de Vries EG, Schaapveld M, Otter R, van der Zee AG. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer. 2006;106:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Goff BA, Matthews BJ, Larson EH, Andrilla CH, Wynn M, Lishner DM, Baldwin LM. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer. 2007;109:2031-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Howell E, Egorova N, Hayes M, Wisnivesky J, Franco R, Bickell N. Racial disparities in the treatment of advanced epithelial ovarian cancer. Obstet Gynecol. 2013;122:1025-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | National Cancer Institute. About the SEER-Medicare Database Factsheet. Available from: http://seer.cancer.gov/about/factsheets/. |
| 19. | National Cancer Institute. SEER-Medicare: Calculation of Comorbidity Weights. [updated 2013 Oct]. . |
| 20. | O’Malley CD, Shema SJ, Cress RD, Bauer K, Kahn AR, Schymura MJ, Wike JM, Stewart SL. The implications of age and comorbidity on survival following epithelial ovarian cancer: summary and results from a Centers for Disease Control and Prevention study. J Womens Health (Larchmt). 2012;21:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Thrall MM, Gray HJ, Symons RG, Weiss NS, Flum DR, Goff BA. Trends in treatment of advanced epithelial ovarian cancer in the Medicare population. Gynecol Oncol. 2011;122:100-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Austin S, Martin M, Kim Y, Funkhouser E, Partridge E, Pisu M. Disparities in use of gynecologic oncologists for women with ovarian cancer in the United States. Health Serv Res. 2013;48:1135-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Mandelblatt J, Faul L, Luta G, Makgoeng S, Isaacs C, Taylor K, Sheppard V, Tallarico M, Barry W, Cohen H. Patient and physician decision styles and breast cancer chemotherapy use in older women: Cancer and Leukemia Group B protocol 369901. J Clin Oncol. 2012;30:2609-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Stewart SL, Rim SH, Richards TB. Gynecologic oncologists and ovarian cancer treatment: avenues for improved survival. J Womens Health (Larchmt). 2011;20:1257-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Stewart SL, Cooney D, Hirsch S, Westervelt L, Richards TB, Rim SH, Thomas CC. Effect of gynecologic oncologist availability on ovarian cancer mortality. World J Obstet Gynecol. 2014;3:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Goff BA, Miller JW, Matthews B, Trivers KF, Andrilla CH, Lishner DM, Baldwin LM. Involvement of gynecologic oncologists in the treatment of patients with a suspicious ovarian mass. Obstet Gynecol. 2011;118:854-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Roland PY, Kelly FJ, Kulwicki CY, Blitzer P, Curcio M, Orr JW. The benefits of a gynecologic oncologist: a pattern of care study for endometrial cancer treatment. Gynecol Oncol. 2004;93:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Bristow RE, Chang J, Ziogas A, Randall LM, Anton-Culver H. High-volume ovarian cancer care: survival impact and disparities in access for advanced-stage disease. Gynecol Oncol. 2014;132:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Wright JD, Neugut AI, Lewin SN, Lu YS, Herzog TJ, Hershman DL. Trends in hospital volume and patterns of referral for women with gynecologic cancers. Obstet Gynecol. 2013;121:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Du XL, Key CR, Dickie L, Darling R, Geraci JM, Zhang D. External validation of medicare claims for breast cancer chemotherapy compared with medical chart reviews. Med Care. 2006;44:124-131. [PubMed] |
| 31. | Lund JL, Stürmer T, Harlan LC, Sanoff HK, Sandler RS, Brookhart MA, Warren JL. Identifying specific chemotherapeutic agents in Medicare data: a validation study. Med Care. 2013;51:e27-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Bristow RE, Powell MA, Al-Hammadi N, Chen L, Miller JP, Roland PY, Mutch DG, Cliby WA. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013;105:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 33. | Bristow RE, Zahurak ML, Ibeanu OA. Racial disparities in ovarian cancer surgical care: a population-based analysis. Gynecol Oncol. 2011;121:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | McGuire V, Herrinton L, Whittemore AS. Race, epithelial ovarian cancer survival, and membership in a large health maintenance organization. Epidemiology. 2002;13:231-234. [PubMed] |
| 35. | Pollack LA, Adamache W, Eheman CR, Ryerson AB, Richardson LC. Enhancement of identifying cancer specialists through the linkage of Medicare claims to additional sources of physician specialty. Health Serv Res. 2009;44:562-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |