Published online Feb 10, 2016. doi: 10.5317/wjog.v5.i1.73
Peer-review started: September 8, 2015
First decision: September 29, 2015
Revised: October 14, 2015
Accepted: December 9, 2015
Article in press: December 11, 2015
Published online: February 10, 2016
Processing time: 150 Days and 9.4 Hours
Application of vaginal mesh for stress urinary incontinence has seen widespread use due to its relatively short operative time in combination with its efficacy in treatment. However, vaginal mesh is not without its drawbacks and can lead to mesh erosion or extrusion, infection, dyspareunia, and recurrence of incontinence. Vaginal mesh complications can lead to feelings of hopelessness, isolation, shame, and emotional distress. Furthermore, failure to identify and address these complications in a timely manner can be permanently damaging to patient health. It is vital to be able to identify mesh complications early. Various imaging methodologies exist to visualize vaginal mesh placement and complications, including ultrasound, magnetic resonance imaging (MRI), and computed tomography (CT). This invited review paper focuses on the role of ultrasound in mesh visualization, mesh complication identification, and operative planning in the event of subsequent surgical mesh revision. Polypropylene mesh is echogenic on ultrasound, making it a useful tool for visualizing post-operative mesh placement. Transperineal, translabial and endovaginal ultrasound technique use has been described in the pre- and peri-operative setting to identify mesh in complex cases. Efficacy and practicality of CT and MRI use in identifying mesh in these cases is limited.
Core tip: Pelvic ultrasound is a valuable and inexpensive technique that can be used both for localization, diagnosis, preoperative planning, and intraoperative guidance when dealing with mesh complications.
- Citation: Singh W, Wadhwa H, Halgrimson W, Kocjancic E. Role of ultrasound imaging in advancing treatment of female patients with pelvic floor mesh complications. World J Obstet Gynecol 2016; 5(1): 73-77
- URL: https://www.wjgnet.com/2218-6220/full/v5/i1/73.htm
- DOI: https://dx.doi.org/10.5317/wjog.v5.i1.73
Urinary incontinence can affect up to 50% of women during their lifetime[1]. In the United States alone, the direct costs of urinary incontinence is upwards of $10 billion dollars per year with over $200 million directed towards surgical intervention[2,3]. Given the prevalence and cost of SUI, safe, effective and efficient treatment is imperative. Initial measures are generally conservative and involve some type of pelvic floor muscle training; other nonsurgical options include medical therapy, estrogen therapy, and injectable urethral bulking agents[4,5]. Begun in 1995, application of vaginal mesh for stress urinary incontinence has seen widespread use in recent years given its relatively short operative time in combination with its proven efficacy[6].
Vaginal mesh, although the preferred method of surgical intervention for stress urinary incontinence, is not without its drawbacks. In 2011, the Food and Drug Administration (FDA) issued an updated statement warning of potential side effects of vaginal mesh including mesh erosion or extrusion, infection, dyspareunia, and recurrence of incontinence[7]. Since the FDA warning, several studies have focused on mesh side effects and their etiologies.
Pain lasting longer than 6 wk after the initial operation is seen in about 2% of patients[8]. A retrospective study of 127 patients in 2009 showed transvaginal taping to have anywhere from 4%-10% incidence of mesh erosion (most commonly with anterior compartment repair)[9,10]. Mesh contraction (decreased mesh surface area) is another postoperative complication that occurs in roughly 5% of patients[11], though there is some evidence to suggest that this is actually due to mesh folding during surgical placement[12,13]. Commonly posited is the idea that surgeon skill is the most important factor in limiting mesh complications[13-15]. Regardless, reoperation for postoperative complications secondary to mesh insertion is not uncommon and has been estimated to occur at a rate of about 10% with some studies having reoperative rates as high as 29%[9,16,17]. Repeat operations can be very involved, ranging from mesh removal to abdominal cystorraphy or partial cystectomy depending on the exact mesh complications[18].
While initial physical symptoms such as irritation, vaginal or pelvic pain, and dyspareunia may be the first manifestations of complications, vaginal mesh complications in particular can lead to feelings of hopelessness, isolation, shame, and emotional distress. Failure to identify and address these complications in a timely manner can be permanently damaging to patient health[19]. Given the common nature of mesh side effects and high rate of surgical intervention for them, it is increasingly important to be able to identify mesh complications early. Various imaging methodologies exist to visualize vaginal mesh placement and complications, including ultrasound, magnetic resonance imaging (MRI), and computed tomography (CT). This paper focuses on the role of ultrasound in mesh visualization, mesh complication identification, and operative planning in the event of subsequent surgical mesh revision.
Common postoperative complaints such as dysuria, pelvic pain, and dyspareunia are generally elicited through patient history and physical examination. Clinical examination alone, however, can be insufficient in determining mesh-related complications[20,21]. Polypropylene mesh is echogenic on ultrasound, making it a useful tool in visualizing post-operative mesh placement[22,23]. Ultrasound can aid in the visualization of the pelvic floor, sling positioning, and urethral length as well as mesh length and thickening[13,24-28]. Various different methods of sonography have been used clinically with some success including transperineal, translabial, and endovaginal ultrasound. It should be noted that there is very little data comparing these different sonographical approaches; as such, they will be described independently here.
Fleischer et al[29] describe in detail a combined 2D and 3D transperineal ultrasound technique that is effective in visualizing mesh location and length in uncomplicated transvaginal tape (TVT) as well as urethral angulation or stenosis in patients with postoperative lower urinary tract symptoms. Eisenberg et al[12] described similar success with transperineal ultrasound using a combined 3D/4D technique in visualizing mesh location, anterior and posterior mesh arms, and mesh folding after abdominal sacrocolpopexy. Both transperineal techinques involve dynamic ultrasound, observing patients at rest and maximal Valsalva.
Denson et al[28] describe a 3D, endovaginal technique that allows for adequate visualization of mesh location. Their method is non-dynamic and has resulted in recognizable sonographical patterns associated with mesh contraction and extrusion allowing for easier identification of postoperative complications. Velemir et al[27] utilized a 2D endovaginal technique with dynamic imaging that also allowed for localization of mesh, measurement of mesh thickness, and identification of mesh contraction. Other endovaginal techniques exist including the use of 4D proprietary software to aid in imaging with similar reliability in observing mesh dimensions[13]. It should be noted, however, that endovaginal ultrasound can be insufficient when attempting to identify mesh arm dimensions depending on sling location[20].
Other sonographical techniques include introital and translabial ultrasound. Introital ultrasound - placing the transducer over the external urethral orifice - can be used to evaluate for urethral pathology as well as to image retropubic slings with or in place of endovaginal techniques[26,30]. A combined 3D/4D translabial ultrasound technique has also been shown to reliably assess mesh location, length, and erosion in transobturator slings. This technique may also have benefit in its ability to detail mesh arm location indirectly via measuring mesh axis rotation[31].
While clinical examination and ultrasound are the most commonly reported methods of postoperative assessment in TVT and transobturator tape (TOT), an emerging modality involves the use of dynamic MRI (dMRI) in pelvic floor disorders. dMRI can be used diagnostically to evaluate preoperative pelvic floor dysfunction and pelvic organ prolapse[32]. A 2006 study of 20 women also showed MRI to be useful postoperatively in evaluating the retropubic portion of vaginal tape[23]. Most types of mesh used for TVT are not routinely visible on MRI, however, and require novel techniques such as MRI-visible mesh implants for visualization[33,34]. Thus, the role of MRI in postoperative evaluation is to provide information on the pelvic floor itself. dMRI can be used to locate the postoperative position of the Pouch of Douglas as well as any changes in vaginal axis[35]. dMRI also has utility in evaluating for prolapse recurrence and has been shown to be more sensitive than quality of life questionnaires in at least one study[36]. And while MRI is incapable of providing adequate mesh visualization, it has been shown to detect postoperative mesh inflammation and fibrosis due to increased intensity transmitted by these processes[37,38].
Routine evaluation of postoperative complications has not been recommended, however, and there is little data to suggest its use for this purpose[23,38]. Given the cost, time commitment, and potential for patient anxiety with dMRI when compared to sonography, it is a second line imaging modality. Presently, dMRI is useful only in the immediate post-operative setting to visualize the pelvic floor itself and is not used routinely in management of mesh complications.
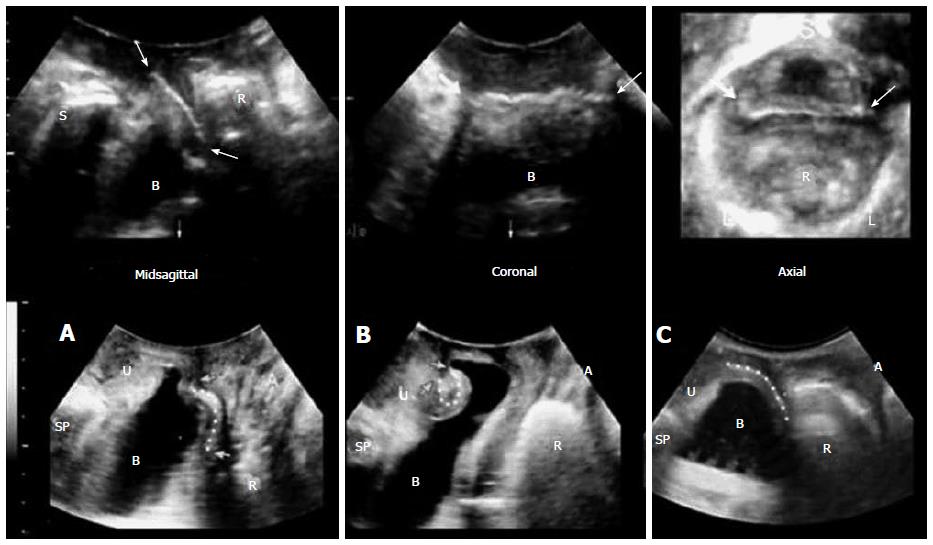
Presently there is no role for CT imaging in postoperative evaluation of TVT or TOT and there is little to no literature discussing it (Figure 1).
In addition to its diagnostic value, ultrasound can be used for operative planning in cases where mesh revision is necessary. For postoperative pain, conservative therapy with short term rest and pain management is a reasonable first step in treatment. Intractable pain, however, may require more invasive measures. Mesh incision, mesh excision, or obturator/pudendal neurolysis are all options depending on the etiology of the pain[15]. The ability to determine mesh dimensions with ultrasound can be used to assist in surgical decision making preoperatively[20,27].
Staack et al[21] first used preoperative translabial ultrasonography to determine sling type, location, and erosion into the urethra or bladder in 51 patients with previously placed suburethral slings and post-procedural lower urinary tract symptoms. Static, dynamic, and 3D techniques in conjunction allowed for visualization of the mesh in relation to the bladder neck and urethra, location of the mesh arms, and identification of urethral hypermobility and kinking or potential mesh folding. Sling location and type were then all confirmed intraoperatively. Thus, ultrasound had a 100% sensitivity in identifying sling location regardless of sling type even in patients without previous operative reports.
Intraoperative ultrasound is the next step in improving outcomes for patients with mesh complications. Though the literature is sparse, two case reports indicate that sonography could prove invaluable in difficult cases. Rostaminia et al[39] describes a case report of levator ani repair for a 33-year-old woman with bilateral levator ani separation after childbirth. With the aid of intraoperative 3D endovaginal ultrasound, the torn ends of the levator ani muscles were tagged with hooks to allow for identification and manipulation. Similarly, Mukati et al[40] reports the case of a 71-year-old woman with previous TVT surgery presenting 3 years later with incomplete bladder emptying requiring self-catheterization. Given the severity of voiding dysfunction, the patient underwent sling revision. As the previous sling could not be identified intraoperatively, a combined 3D-2D endovaginal ultrasound technique was used to localize and resect the sling. The patient’s symptoms resolved after mesh resection.
At our institution, we routinely use pelvic ultrasonography for preoperative diagnosis and operative planning as well as intraoperative guidance. We have found that pre- and intraoperative ultrasound use can be used in complex revision cases to better delineate mesh position and thus reducing the extent of resection required to correct mesh-related complications. Given the high success rate of ultrasonographic visualization of mesh location as well as the comprehensive picture provided by this technique, translabial ultrasonography can be very valuable in preoperative planning and intraoperative guidance for surgical correction of suburethral sling.
Meshes have a vital role in the treatment of female pelvic organ prolapse as well as urinary incontinence. Despite improvements in surgical techniques and available mesh products, there is still a significant morbidity associated with complications of mesh surgery; serious complications are not rare. These complications and their clinical manifestations such as pain, urinary tract dysfunction, or sexual dysfunction can be difficult to manage. Two major challenges are early recognition of complications and their subsequent surgical management. Delayed recognition leads to patient dissatisfaction; delayed surgical management may make cases much more difficult. As such, innovative techniques are desired in the armamentarium for surgeons treating these complications. Pelvic ultrasound is a valuable and inexpensive technique that can be used both for localization, diagnosis, preoperative planning, and intraoperative guidance when dealing with mesh complications.
P- Reviewer: Tsikouras P, Zafrakas M S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537-542. [PubMed] |
| 2. | Wilson L, Brown JS, Shin GP, Luc KO, Subak LL. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398-406. [PubMed] |
| 3. | Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001;98:646-651. [PubMed] |
| 4. | Dumoulin C, Hay-Smith EJ, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2014;5:CD005654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Bezerra CA, Bruschini H, Cody DJ. Traditional suburethral sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2005;CD001754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 470] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 7. | Food and Drug Administration. Urogynecologic surgical mesh: Update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse, 2011-07-13 [Accessed 2015 May 22]. Available from: http//www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. |
| 8. | Brubaker L, Norton PA, Albo ME, Chai TC, Dandreo KJ, Lloyd KL, Lowder JL, Sirls LT, Lemack GE, Arisco AM. Adverse events over two years after retropubic or transobturator midurethral sling surgery: findings from the Trial of Midurethral Slings (TOMUS) study. Am J Obstet Gynecol. 2011;205:498.e1-498.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Balchandra P, Marsh F, Landon C. Perioperative outcomes and prospective patient reported outcome measures for transvaginal mesh surgery. Arch Gynecol Obstet. 2015;292:875-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | de Tayrac R, Brouziyne M, Priou G, Devoldère G, Marie G, Renaudie J. Transvaginal repair of stage III-IV cystocele using a lightweight mesh: safety and 36-month outcome. Int Urogynecol J. 2015;26:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Eisenberg VH, Steinberg M, Weiner Z, Alcalay M, Itskovitz-Eldor J, Schiff E, Lowenstein L. Three-dimensional transperineal ultrasound for imaging mesh implants following sacrocolpopexy. Ultrasound Obstet Gynecol. 2014;43:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Svabík K, Martan A, Masata J, El-Haddad R, Hubka P, Pavlikova M. Ultrasound appearances after mesh implantation--evidence of mesh contraction or folding? Int Urogynecol J. 2011;22:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. |
Dietz HP, Erdmann M, Shek KL; Mesh contraction: myth or reality? |
| 15. | Mock S, Reynolds WS, and Dmochowski RR. Trans-vaginal mesh revision: A comprehensive review on etiologies and management strategies with emphasis on postoperative pain outcomes. LUTS: Lower Urinary Tract Symptoms. 2014;6:69-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Bartley JM, Sirls LT, Killinger KA, Boura JA. Secondary surgery after vaginal prolapse repair with mesh is more common for stress incontinence and voiding dysfunction than for mesh problems or prolapse recurrence. Int Urol Nephrol. 2015;47:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2007;CD004014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 18. | Padmanabhan P, Hutchinson RC, Reynolds WS, Kaufman M, Scarpero HM, Dmochowski RR. Approach to management of iatrogenic foreign bodies of the lower urinary tract following reconstructive pelvic surgery. J Urol. 2012;187:1685-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Dunn GE, Hansen BL, Egger MJ, Nygaard I, Sanchez-Birkhead AC, Hsu Y, Clark L. Changed women: the long-term impact of vaginal mesh complications. Female Pelvic Med Reconstr Surg. 2014;20:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Manonai J, Rostaminia G, Denson L, Shobeiri SA. Clinical and ultrasonographic study of patients presenting with transvaginal mesh complications. Neurourol Urodyn. 2015;Jan 25; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Staack A, Vitale J, Ragavendra N, Rodríguez LV. Translabial ultrasonography for evaluation of synthetic mesh in the vagina. Urology. 2014;83:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Dietz HP, Barry C, Lim YN, Rane A. Two-dimensional and three-dimensional ultrasound imaging of suburethral slings. Ultrasound Obstet Gynecol. 2005;26:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Schuettoff S, Beyersdorff D, Gauruder-Burmester A, Tunn R. Visibility of the polypropylene tape after tension-free vaginal tape (TVT) procedure in women with stress urinary incontinence: comparison of introital ultrasound and magnetic resonance imaging in vitro and in vivo. Ultrasound Obstet Gynecol. 2006;27:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Rodrigo N, Wong V, Shek KL, Martin A, Dietz HP. The use of 3-dimensional ultrasound of the pelvic floor to predict recurrence risk after pelvic reconstructive surgery. Aust N Z J Obstet Gynaecol. 2014;54:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Ng CC, Lee LC, Han WH. Use of three-dimensional ultrasound scan to assess the clinical importance of midurethral placement of the tension-free vaginal tape (TVT) for treatment of incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Tunn R, Picot A, Marschke J, Gauruder-Burmester A. Sonomorphological evaluation of polypropylene mesh implants after vaginal mesh repair in women with cystocele or rectocele. Ultrasound Obstet Gynecol. 2007;29:449-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Velemir L, Amblard J, Fatton B, Savary D, Jacquetin B. Transvaginal mesh repair of anterior and posterior vaginal wall prolapse: a clinical and ultrasonographic study. Ultrasound Obstet Gynecol. 2010;35:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Denson L, Shobeiri SA. Three-dimensional endovaginal sonography of synthetic implanted materials in the female pelvic floor. J Ultrasound Med. 2014;33:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Fleischer AC, Harvey SM, Kurita SC, Andreotti RF, Zimmerman CW. Two-/three-dimensional transperineal sonography of complicated tape and mesh implants. Ultrasound Q. 2012;28:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 30. | Tunn R, Petri E. Introital and transvaginal ultrasound as the main tool in the assessment of urogenital and pelvic floor dysfunction: an imaging panel and practical approach. Ultrasound Obstet Gynecol. 2003;22:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Shek KL, Dietz HP, Rane A, Balakrishnan S. Transobturator mesh for cystocele repair: a short- to medium-term follow-up using 3D/4D ultrasound. Ultrasound Obstet Gynecol. 2008;32:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Brocker KA, Alt CD, Rzepka J, Sohn C, Hallscheidt P. One-year dynamic MRI follow-up after vaginal mesh repair: evaluation of clinical, radiological, and quality-of-life results. Acta Radiol. 2015;56:1002-1008. [PubMed] |
| 33. | Ginath S, Garely AD, Luchs JS, Shahryarinejad A, Olivera CK, Zhou S, Ascher-Walsh CJ, Condrea A, Brodman ML, Vardy MD. Magnetic resonance imaging of abdominal versus vaginal prolapse surgery with mesh. Int Urogynecol J. 2012;23:1569-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Sindhwani N, Feola A, De Keyzer F, Claus F, Callewaert G, Urbankova I, Ourselin S, D’hooge J, Deprest J. Three-dimensional analysis of implanted magnetic-resonance-visible meshes. Int Urogynecol J. 2015;26:1459-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Kashihara H, Emmanuelli V, Poncelot E. Comparison of dynamic MRI vaginal anatomical changes after vaginal mesh surgery and laparoscopic sacropexy. Gynecological Surgery. 2014;11:249-256. [DOI] [Full Text] |
| 36. | Brocker KA, Alt CD, Corteville C, Hallscheidt P, Lenz F, Sohn C. Short-range clinical, dynamic magnetic resonance imaging and P-QOL questionnaire results after mesh repair in female pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2011;157:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | El Sayed RF. Commentary on “MRI and CT of sacrocolpopexy”. AJR Am J Roentgenol. 2013;200:938-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | van der Weiden RM, Rociu E, Mannaerts GH, van Hooff MH, Vierhout ME, Withagen MI. Dynamic magnetic resonance imaging before and 6 months after laparoscopic sacrocolpopexy. Int Urogynecol J. 2014;25:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Rostaminia G, Shobeiri SA, Quiroz LH. Surgical repair of bilateral levator ani muscles with ultrasound guidance. Int Urogynecol J. 2013;24:1237-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Mukati MS, Shobeiri SA. Transvaginal sling release with intraoperative ultrasound guidance. Female Pelvic Med Reconstr Surg. 2013;19:184-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |