INTRODUCTION
Though it is largely preventable, cervical cancer is an important cause of morbidity and mortality throughout the world. The age-adjusted incidence of cervical cancer is 14 cases per 100000 women worldwide. It is as high as 15.7 per 100000 in less developed areas of the world and 9.9 per 100000 in more developed areas. The age-standardized mortality rate for cervical cancer is 8.3 per 100000 for women in less developed regions, with a much lower rate of 3.3 per 100000 women in more developed areas[1]. The much lower rates in more developed areas underscore the importance of effective screening programs. In lesser developed regions with fewer healthcare resources, the lack of a reliable screening test and inadequate screening coverage result in more new cervical cancer cases and ultimately in more cervical cancer deaths[2].
Human papillomavirus (HPV) infection is now known to be a necessary cause of cervical cancer and as a result, testing women for high-risk subtypes of HPV is proving to be an effective method of screening. As the relative value of HPV testing in cervical cancer screening became more apparent, a variety of self-collection options were developed. Self-sampling options tend to be more acceptable to women because they overcome the previously identified barriers to cervical cancer screening. As a result, self-collection of HPV specimens will extend the reach of cervical cancer screening programs even in low-resource areas.
BARRIERS TO CERVICAL CANCER SCREENING
While every woman is an individual in terms of how personal characteristics and life circumstances affect her health care behaviors, women who are underscreened or unscreened for cervical cancer often experience one or more of a number of common barriers to participation. In a group of primarily urban minority women, the reluctant tended to possess a fatalistic attitude, believing that they are better off not knowing about their cancer or that cancer occurs in those who have bad luck. Additionally they reported a lack of family support and lack of understanding of the risk of cervical cancer[3]. Among 300 women in Botswana who answered questions about their perceptions of barriers to Papanicolau (Pap) testing, 32% found it embarrassing and 52% believed that getting a screen suggested a woman is sexually active. Many (63.3%) of the women who had never been screened and 51.7% of those who had been screened thought lack of information was a barrier for screening for cervical cancer. However, none of the barriers identified by the women was significantly associated with their screening behaviors[4]. In a study of 493 women in Brazil, 36.7% of women had adequate knowledge of cervical cancer, 67.2% had an appropriate attitude (recognized the importance of screening) and 69.6% reported having had a Pap in the past 3 years. The barriers to undergoing Pap testing with the highest scores were a lack of symptoms of cervical cancer and the embarrassment associated with the exam[5]. In a study 345 Appalachian women aged 40-64, questions regarding barriers were grouped according to the PRECEDE-PROCEED model as predisposing factor barriers, enabling factor barriers and reinforcing factor barriers. Barriers that were found among more than half of the women included: (1) worry (78%); (2) fear of cancer (67%); (3) embarrassment (56%); (4) the belief that cervical cancer (52%) and polyps (50%) would have symptoms; (5) unavailability of public transportation (71%); (6) preference for home screen (66%); (7) insurance coverage (65%); and (8) lack of choice of a male or female provider (62%)[6]. Among 21-65 year-old Malaysian women, 70% reported that cervical cancer screening is too embarrassing and almost half found the attitude of clinic staff, the lack of female healthcare providers, the worry associated with the outcome and the fear that she would no longer be a virgin after the test were important barriers[7]. In a review of the literature on cervical cancer screening in Asian women, barriers to screening could be grouped as cognitive, emotional, economic, logistic or social. Barriers to screening identified in each of these categories included a lack of understanding of the reason for or benefits of testing, fear, time away from work, lack of insurance, transportation and childcare issues, wait times in the clinic, and lack of support from family and healthcare clinic staff[8]. Lastly, a study of women with cervical abnormalities who were enrolled in a research program to help them navigate the healthcare system in multiple cities in the United States found that nearly half of the women experienced at least one barrier to care and some experienced as many as seven. Barriers that significantly delayed time to diagnosis included the presence of comorbidities, health insurance issues, minimization of the importance of treatment, out of town travel, and employment demands or healthcare system problems. Interestingly, the time from detection of the cervical abnormality to definitive diagnosis was not affected by fear, attitudes toward providers, perceptions about tests and treatments, quality of communication, ability to read and write, or language[9].
SELF-COLLECTED HUMAN PAPILLOMAVIRUS TEST
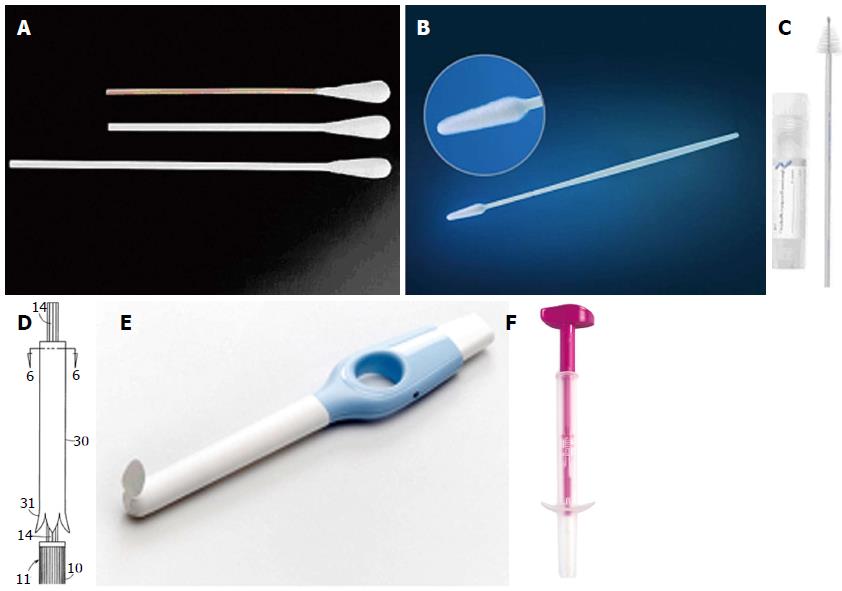
Self-collection of cervicovaginal HPV specimens is purported to be a viable alternative to Pap testing or clinician-collected HPV specimens that will overcome some of these barriers and extend the reach of screening in low-resource or underscreened populations. A variety of self-collection methods have been developed and tested around the world to determine their diagnostic accuracy (Figure 1). The available devices today include swabs, brushes, and lavage devices. In addition to the polyester (Dacron) tipped swab, flocked swabs are now available. The flocked swab is a variation on the polyester swab that comprises a solid plastic applicator with short nylon fibers attached perpendicularly to the tip. It is designed to allow the specimen to remain near the surface of the swab for ease of retrieval relative to the traditional cotton or Dacron swab. An additional variation on the swab includes the Fournier device. Its swab is wrapped in a sheath much like a tampon applicator. Upon insertion, the woman pushes the end of it to deploy the swab and after collection the swab retreats back into the sheath to prevent absorption of vaginal secretions when the device is removed. There are also a variety of brushes available including the cervical sampler brush and broom-shaped devices commonly used for clinician-collection of cervical cytology specimens. The Evalyn brush also offers an applicator to ease deployment of the brush. Following collection, the pink cap is snapped back onto the transparent applicator and the specimen is transported dry to the lab. Cervical lavage devices have also been developed. For the lavage, the woman inserts the device as she would a tampon and then pushes and holds a button for three seconds. During that time, a small amount of sterile fluid is released from the end near the proximal vagina and cervix. When she releases the button, the fluid flows back into the device along with cervical and vaginal cells.
The agreement of HPV self-collected specimens with clinician-collected specimens has been demonstrated to be strong in several studies[10-13]. The sensitivity of the self-collected studies has been consistently as high or higher than that of cervical cytology specimens for the detection of high-grade cervical intraepithelial neoplasia[10]. Self-collected HPV tests are emerging as an alternative to cervical cytology or even clinician-collected HPV tests because their diagnostic accuracy has been favorable and the self-collection kit can be distributed in person or by mail with the collection occurring almost anywhere. If HPV self-collection methods are acceptable to women and they are willing to collect, self-collection has the potential to extend the reach of screening to under- or unscreened women in high or low resource regions.
ACCEPTABILITY OF SELF-COLLECTION
The acceptability of self-collected HPV samples has been examined in a number of different ways. It is usually measured by an interview or a written questionnaire and compared with clinician-collected HPV or Pap test with acceptability parameters determined by the previously reported barriers to cervical cancer screening. Pain, discomfort, degree of embarrassment, level of privacy, ease of use, trust or confidence in the results are commonly measured parameters for acceptability. Other studies focused on women’s preferences for self-collection or clinician-collection and the reasons for their preferences.
Swabs
The acceptability of self-collection of HPV samples using some type of soft swab has been examined in various locations internationally. In Ontario, women’s responses to self-sampling were stratified by age and in both the younger (< 50 years old) and the older (> 50 years old) groups more than 45% of women preferred self-sampling to a clinician examination or had no preference[14]. A predominantly unscreened sample of women in India was invited to self-collect a HPV test either in the clinic or in their homes. Younger women (< 45 years old) and those invited for home collection were more likely to agree to participate. Among those who lived in remote areas where screening may only be possible once or twice in a lifetime, 71.5% said they would be willing to self-collect at home and 53.8% said they would be willing to go to a clinic to self-collect[15]. The response to self-collection in Uganda was very positive with 93.66% willing to self-collect a sample with no more than 5% of the sample concerned that the self-collection would be embarrassing or painful or too difficult to perform correctly. Most women were willing to either have the swab kit dropped off and picked up by a community health worker or to return the swab to the clinic themselves[16]. In a comparison of self-collection with a clinician collected Pap test, Mexican women reported the overall acceptability of the self-collection to be significantly greater than the Pap. They also reported less pain, less discomfort, less embarrassment and more privacy with self-collection than with the Pap. A majority (68%) preferred self-collection, citing greater comfort and less embarrassment as the primary reasons. Those who did prefer the Pap noted greater confidence in the results as the primary reason[17,18]. Similarly, in Puerto Rico[19] and Nicaragua[20], the overall acceptability of the self-collection was significantly higher. The individual acceptability parameters all scored higher for the self-collection with the exception of “comfort” in Nicaragua, seemingly because the women interpreted the question as comfort with the accuracy of the results rather than a measure of physical comfort. Nonetheless, in both studies more women preferred clinician-collection because they had more confidence in the accuracy of the results. The acceptability of self-collected swabs was also high in Ontario, London and in a predominantly Hispanic sample from New York City. In Ontario, two thirds of the sample found the swab easy and comfortable to self-collect and 87.7% were willing to perform self-collection again in the future[21]. In London the characteristics of the self-collected swab that appealed to most women were the lower levels of embarrassment, discomfort, anxiety and unpleasantness associated with it. Clinician-collection was preferred by some women because they had greater confidence in the results. There were some demographic differences in attitudes toward self-collection in that married women were more positive than single women and Asian women were more negative than women of other ethnicities[22]. One third of women in a New York City study preferred self-collection because they found it easy to use, less painful and more private than clinician collection. Almost two thirds could identify nothing unfavorable about self-collection. Once again, those who preferred the clinician collection did so because they had more confidence in the physician’s ability to do it properly. There were statistically significant differences in preference for ethnicity and education with non-Hispanic and more educated women preferring self-collection most[23]. A Cincinnati-based study asked adolescent women about their preferences before and after they performed self-collection. Their impressions of self-collection improved after they tried it themselves but even then more women preferred clinician-collection because they thought the results were more trustworthy[24]. A comparison of a polyester swab (dry transport) to a flocked swab (transported in liquid medium) in Switzerland found no difference between the two in overall acceptability though a few more women thought the wet transport system was slightly more complex[25]. In the northeastern United States, the acceptability of a self-collected swab was compared with that of a tampon with 90% of the respondents reporting they would be willing to self-collect in the future with either device. In a series of open-ended questions, respondents reported concern that the swab may break and that they would still want to have their annual physicals with a provider even if they self-collected their cervical cancer screen. There was a potential for bias in this study in that only 67 of 103 participants completed the questionnaire[26]. A Canadian study (n = 200) compared the acceptability of screening for HPV with vulvar and vaginal swabs as well as a urine specimen. In terms of overall acceptability, 88.2% of participants found a vaginal swab acceptable compared with 79% for the physician exam. In general they ranked the acceptability in order from furthest away to closest to the cervix. Of note the rank order of the sensitivities of the tests was the inverse[27]. In the Cameroon, all subjects agreed to self collect and self-collection scored more favorably than clinician collection for all parameters (embarrassment, pain, anxiety and ease of use) except confidence in the quality of collection, which scored much higher for the clinician collection. Women with a greater understanding of HPV and those who had been screened for cervical cancer in the past were significantly more likely to prefer self-collection[28]. Finally a qualitative study of African American women living in the Mississippi delta comprised focus groups with a total of 87 women exploring their HPV and cervical cancer knowledge as well as their attitudes toward self-collection. Of the 87 participants, 9 returned for a second phase to perform self-collection. Participants were willing to self-collect but had some concerns about accuracy, cost and the possibility of the specimen getting lost in the mail. They liked the privacy associated with home collection and avoiding the wait time associated with clinic appointments. Positive feedback from the nine who self collected included having female study personnel explain the collection and getting to handle a sample device during the explanation[29].
Brushes
The overall acceptability of self-collection with a cervical sampler brush was quite high (mean score 4.33 on 5-point scale) in a study in rural China, though 74% of women preferred clinician collection to self-collection. The primary reason was because they had greater confidence in the accuracy of the results. Among those who did prefer self-collection, there was substantial variation in the primary reason for their preferences including greater convenience, less embarrassment, less cost and greater comfort. There was no association of demographics with preferences[30]. Self-collection with a cytobrush was reviewed positively by a group (n = 435) of women in Munich. Nearly all of them said they were willing to self collect at home in the future and very few of them found the collection difficult to perform. When asked about their preference of self-collection or clinician-collection, 63% preferred them equally and 23% preferred self-collection[31]. Among 134 women in the Netherlands who self-collected with the Evalyn brush, 95% reported the experience, the instructions and the convenience as good, very good or excellent. Nearly all (95%) preferred self-sampling with the primary reasons that is simpler to use and less painful than clinician collection. Reliability of the result was the main reason for 6 of the 7 who preferred clinician collection[32]. A randomized trial in Holland demonstrated a significantly higher response rate to an invitation to self-collect with the brush kit mailed to their homes (30.8%) than to an invitation to come to the clinic for cytology (6.5%) among a group of Dutch women who had not responded to a reminder for their regular cervical cancer screen. The 29-33 years old age group had the lowest response rate in the self-sampling group[33]. Self-collection with a sampler brush was also reviewed favorably in the Netherlands with 70% of the 135 participants preferring self-collection to clinician-collection for their next exam and 91% reporting that the brush was easy to use[34].
Lavage devices
Lavage devices for self-collection have also been found to be highly acceptable to women. In Italy, 2480 women who had not previously responded to screening invitations were randomized to receive a letter of invitation for a Pap test, a letter of invitation for a clinic-based HPV test, a letter of invitation to request by phone a home HPV kit or a self-collection kit. The self-collection kit had the best response rate and the only rate that was significantly higher than the standard of care (letter of invitation for a Pap). Among the women who completed the questionnaires, 78.4% preferred the self-collection and the most commonly reported reasons were that they could do it themselves and it was more private[35]. A large Dutch study of nonresponders found that though adjusted response rate to an invitation to self collect with a lavage device was only 27.5%, it was significantly higher than the rate (16.6%) in the group receiving the standard Pap reminder letter[36]. In addition, in a pooled analysis of the Dutch brush and lavage studies, ethnicity, age and screening history predicted response rate with native Dutch, older age and previously screened women responding more often than immigrant, younger and underscreened or never-screened women[37]. In a similar study, 31.5% of Finnish nonresponders opted to participate in self-collection either by return-mailing a sample in the kit or presenting to clinic to self-collect a sample. The comparison group, sent a reminder card for clinician collection had a significantly lower response rate (25.9%)[38]. A group of 197 low-income women from New York City successfully self-lavaged a short time after their routine Pap test. A significantly higher percentage (96%) found the self-collection comfortable compared with the Pap (47%). Seventy-nine percent indicated they would prefer the self-collection with the lavage device for their next screening, largely because of the greater level of comfort and the convenience of the self-collection[39]. Among 354 Thai women who self-collected a cervicovaginal sample for cytology with the Kato device, more than 80% found it more convenient and less painful and 78.6% said they prefer it for their next cervical cancer screen. Though 94.3% of women were either satisfied or very satisfied with self-collection with this device, 57.6% thought the clinician collection was likely to produce more accurate results[40]. Though the Kato device was used to collect a cytology specimen, the process and likely the level of acceptability would be the same if the sample had been used to test for HPV. A London-based study involving focus groups (n = 28 total) explored Muslim women’s attitudes toward the thought of self-collection with a swab or a lavage device. The women were somewhat reluctant to endorse self-sampling though they all preferred the swab because it was smaller and seemed less messy to use[41]. In a group of 205 Italian women, 111 self-collected with a cervical sampler kit and the others used the self-lavage device. The entire group also underwent a pelvic exam with clinician-collected sample. Both self-collection methods were well accepted in terms of increased comfort and decreased embarrassment compared with clinician collection. However, the scores for overall acceptability and embarrassment were significantly better for the lavage device than for the cervical sampler. Among those in the lavage group, 77.6% preferred self-sampling to clinician-collection and in the cervical sampler group, 60.4% preferred self-sampling[42].
Other sampling devices
The acceptability of the Fournier device was examined in home collection in the Little Haiti section of Miami, Florida. More than 90% of women found it easy to use, were comfortable using it at home and said they would recommend it to a friend. Self-collection was preferred by 86.8% of the women who also had a Pap. As is common, the women who did not prefer self-collection expressed concern about having performed the collection correctly[43].
Intent to self sample
In addition to evidence generated through randomized trials and observational studies of self-collection compared with clinician-collection, women who have been educated about cervical cancer screening and HPV, and then surveyed regarding their preference for self-sampling as an alternative to a pelvic examination have responded in favor of self-collection. A large proportion of Kenyan women have stated they would be comfortable with self-collection (82%) and would prefer to collect at home rather than going to a clinic for an examination (84%)[44]. In a similar survey of willingness to self-collect, 80% of Ugandan women responded that they would be willing. An examination of the characteristics that predict a woman’s willingness to self-collect revealed that older age and a feeling of embarrassment with home-collection were negative predictors while a willingness to have a health worker deliver the swab to her home and go to a clinic for a pelvic examination if the HPV results were abnormal were positive predictors[45]. A study of personality characteristics predictive of willingness to self collect in college students found that women whose personality profiles ranked highly in extraversion, openness and conscientiousness were less likely to be deterred by the common barriers to self-collection[46].
CONCLUSION
Cervical cancer prevention is an important health priority around the world. Historically, cytology based screening programs have been effective in reducing morbidity and mortality but there are still significant numbers of unscreened or underscreened women in more developed as well as less developed countries. Barriers to cervical cancer screening range from personal issues such as the embarrassment and discomfort associated with the speculum exam and issues with being examined by a male provider to logistical concerns such as transportation to a clinic, childcare during the visit and the extended clinic wait times keeping women away from a job or family. Human papillomavirus testing, including self-sampling for HPV has been demonstrated to be as or more sensitive than cytology in the detection of high-grade cervical neoplasia. A number of different self-collection instruments including various brushes, swabs and lavage devices have been developed and found to be highly acceptable to women. The number of acceptability studies, conducted on at least five continents, continues to grow and the preponderance of the evidence indicates that women find the various types of self-collection instruments highly acceptable. Most women have indicated a preference for self-collection and willingness to self collect in the future. The most commonly occurring limiting factor to self-collection has been the woman’s confidence that she is collecting the specimen correctly. Another reason offered by women who preferred the clinician exam to self-collection despite a higher acceptability for self-collection was their concern that they would lose contact with their physicians. They preferred the clinician-collection because it provided an opportunity for somewhat regular interaction with the provider. These are important concerns that need to be considered in the development and implementation of large scale screening projects designed to draw unscreened or underscreened women by offering self-collection. Simple diagrams and written instructions for literate populations or clear oral instructions by culturally similar women who have used the device are likely to help overcome this barrier. As with any screening program, the systems-related barriers will need to be minimized for the extended reach of the screening program to have a meaningful impact on mortality and quality of life. With these caveats, self-collection of human papillomavirus specimens as a primary screen for cervical cancer seems to be highly acceptable to women and has the potential to extend the reach of screening programs, particularly in previously unscreened or underscreened women.