Published online May 10, 2014. doi: 10.5317/wjog.v3.i2.78
Revised: November 21, 2013
Accepted: January 13, 2014
Published online: May 10, 2014
Processing time: 318 Days and 11 Hours
AIM: To investigate the association between total bile acid (TBA) level during intrahepatic cholestasis of pregnancy (ICP) and fetal lung surfactant alteration.
METHODS: We recruited 42 ICP and 32 normal pregnancy women in this study. The maternal blood, fetal blood and amniotic fluid TBA level were detected using a circulating enzymatic method. Umbilical blood pulmonary surfactant protein A (SP-A) was evaluated with enzyme-linked immunosorbent assay. High performance liquid chromatography was used for the determination of phosphatidyl choline (PC), phosphatidyl inositol (PI), lysolecithin (LPC) and sphingomyelin (SM). Amniotic fluid lamellar body was counted with a fully automatic blood cell counter. Fetal lung area and fetal body weight were calculated from data obtained with an iu22 color supersonic diagnostic set. Clinical information of a nonstress test, amniotic fluid properties and neonatal Apgar score, and birth weight were recorded for review.
RESULTS: The TBA level in maternal blood, fetal blood and amniotic fluid in the ICP group were significantly higher than that in the control group (maternal blood: 34.11 ± 6.75 mmol/L vs 4.55 ± 1.72 mmol/L, P < 0.05; fetal blood: 11.9 ± 2.23 mmol/L vs 3.52 ± 1.56 mmol/L, P < 0.05; amniotic fluid: 3.89 ± 1.99 mmol/L vs 1.43 ± 1.14 mmol/L, P < 0.05). Amniotic fluid PC and PI in the ICP group were significantly lower than that in the control group (PC: 65.71 ± 7.23 μg/mL vs 69.70 ± 6.68 μg/mL, P < 0.05; PI: 3.87 ± 0.65 μg/mL vs 4.28 ± 0.74 μg/mL, P < 0.05). PC/LPC ratio of the ICP group was lower than that of the control group (14.40 ± 3.14 vs 16.90 ± 2.52, P < 0.05). Amniotic LB in the ICP group was significantly lower than that of the control group ((74.13 ± 4.37) × 109/L vs (103.0 ± 26.82) × 109/L, P < 0.05). Fetal umbilical blood SP-A level in the ICP group was significantly higher than that of the control group (30.26 ± 7.01 ng/mL vs 22.63 ± 7.42 ng/mL, P < 0.05). Fetal lung area/body weight ratio of the ICP group was significantly lower than that of the control group (5.76 ± 0.63 cm2/kg vs 6.89 ± 0.48 cm2/kg, P < 0.05). In the ICP group, umbilical cord blood TBA concentration was positively correlated to the maternal blood TBA concentration (r = 0.746, P < 0.05) and umbilical blood SP-A (r = 0.422, P < 0.05), but it was negatively correlated to the amniotic fluid lamellar corpuscle (r = 0.810, P < 0.05) and fetal lung area/body weight ratio (r = 0.769, P < 0.05). Furthermore, umbilical blood TBA showed a negative correlation to PC, SM and PI (rpc = 0.536, rsm = 0.438, rpi = 0.387 respectively, P < 0.05). The neonatal asphyxia, neonatal respiratory distress syndrome, fetal distress and perinatal death rates in the ICP group are higher than that of the control group.
CONCLUSION: ICP has higher TBA in maternal and fetal blood and amniotic fluid. The high concentration of TBA may affect fetal pulmonary surfactant production and fetal lung maturation.
Core tip: We studied total bile acid (TBA) concentration in maternal, fetal and amniotic fluid and its relationship with fetal surfactant, surfactant protein A, amniotic lamellar body and fetal lung development. Results demonstrated that intrahepatic cholestasis of pregnancy (ICP) has higher TBA in maternal and fetal blood and amniotic fluid. The high concentration of TBA may affect fetal pulmonary surfactant production and fetal lung maturation. It calls attention to delayed maturation of fetal lungs in ICP patients and to take steps to carefully check and improve fetal pulmonary maturity.
- Citation: Ding YL, Zhang LJ, Wang X, Zhou QC, Li N, Wang CX, Zhang XQ. Fetal lung surfactant and development alterations in intrahepatic cholestasis of pregnancy. World J Obstet Gynecol 2014; 3(2): 78-84
- URL: https://www.wjgnet.com/2218-6220/full/v3/i2/78.htm
- DOI: https://dx.doi.org/10.5317/wjog.v3.i2.78
Intrahepatic cholestasis of pregnancy is a maternal metabolic disease affecting up to 5% of pregnancies[1]. It is characterized by rising maternal serum bile acids and can be complicated by fetal distress, neonatal asphyxia and neonatal respiratory distress syndrome[2-4]. The etiology of intrahepatic cholestasis of pregnancy (ICP) is poorly understood but the perinatal complications are closely correlated with maternal total bile acid (TBA) level[5,6]. Savonius found that high TBA can cause neonatal lung injury but its mechanism is not clear[7]. In order to explore fetal lung alteration during ICP and its possible mechanisms, we investigated maternal and fetal TBA, fetal surfactant production and fetal lung development.
Protocols were approved by Central South University Xiangya Second Hospital Scientific Research Department. Informed consents were obtained from all patients involved in this study.
A total of 72 cases were recruited in this study during 2010 and 2011. It includes 40 ICP patients and 32 normal pregnant women with singleton pregnancy delivered using cesarean section. In the ICP group, the patients’ ages were from 18 to 40 years old and the average age was 27.7 ± 1.37 years. The gestational ages were from 33 wk to 41 wk + 5 d and the average gestational age was 37.25 ± 2.34 wk. In the normal pregnancy group, the patients’ ages were from 19 to 36 years old and the average age was 27.2 ± 4.67. The gestational ages were from 33 ± 2 to 40 ± 6 wk and the average gestational age was 37.5 ± 2.67 wk. There were no statistical differences between the ICP group and control group for maternal age, gestational age or pregnancy times. ICP was diagnosed with the diagnostic criteria referenced in the eighth edition of the national text book of obstetrics and gynecology[8]. Patients with liver disease, gall bladder disease, chronic vascular disease, gestational hypertension, gestational diabetes, anemia, kidney disease, heart disease or other pregnancy complications were excluded.
Maternal blood was collected at a fasting state before cesarean section. Fetal blood was collected through the umbilical artery immediately after delivery of the fetus during cesarean section. Amniotic fluid was collected with a syringe through the amniotic membrane just after cutting and separating the myometrium during cesarean section, with careful attention to avoid blood pollution.
Blood specimens were injected into a test tube dedicated with heparin immediately after being collected. After centrifuge (3000 r/min, 15 min), the supernatant was collected and stored at -20 °C for future experiments. For amniotic fluid, the upper solution was collected after centrifuge (3000 r/min, 15 min), then mixed 1:1 volume with methanol/chloroform. After centrifuge again (2500 r/min, 10 min), the lower liquid was extracted and mixed with a methanol-water extractor (1:1, v/v). The supernatant and interface impurities were discarded after centrifuge (2500 r/min, 10 min), 10 mL lower fluid was taken and sealed into a test tube, then stored for future tests at -20 °C. Before testing, a mobile phase containing chloroform was used to dissolve the samples.
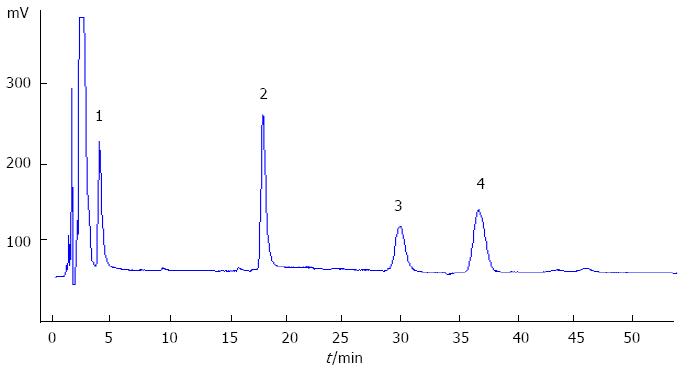
TBA was detected using the automatic biochemical analyzer (Hitachi 7060, Japan) with the TBA detection kit (Sigma, Shanghai Trading Co. Ltd.), following the instruction of the assay kit. The calibration was made each time using the standard calibrator. Surfactant protein A (SP-A) was detected with the SP-A detection kit using an enzyme-linked immunosorbent assay (Wuhan technology co., China and United States)[9]. Phospholipids phosphatidylcholine (PC), phosphatidylinositol (PI), lysolecithin (LPC) and sphingomyelin (SM) were detected with high-performance liquid chromatography (HPLC, Shanghai National Medicine Chemical Reagent Co. Ltd.) with the standard phospholipids (Sigma, Shanghai Trading Co. Ltd.). uPrasil column (300 mm × 4 mm, 5 μm) was used with a HW2000 chromatographic data station for data analysis. The procedures and steps were carried out accurately following the instructions of the agent kit and instrument. The phospholipid concentration results are shown in Figure 1. Amniotic fluid lamellar body was counted using a hematology analyzer (ABX-Pentra120, Diamond Diagnostics, United States).
Color ultrasonic diagnostic system (Philips iu22, United States, probe frequency 2.5-6.0 OMHZ) was used for fetal lung area and fetal body weight within 3 days of delivery. Fetal body weights were assessed and calculated by checking the fetal biparietal diameter, head circumference, abdominal circumference and femoral length. Fetal lung areas were calculated by measuring the fetal left and right lung area by freezing an image shot when the fetal heart was at the diastolic phase while the probe was parallel to the longitudinal line of the fetus. The area was digitally analyzed by the computerized system automatically. Data was taken by one professional individual using a mean of 3 measurements. Total lung area and lung area/body weight were digitally calculated[10].
Fetal heart rate patterns, amniotic fluid characteristics and neonatal Apgar score were recorded for evaluation. The situation of the neonates was also recorded for three days to evaluate the fetus and neonates.
Software SPSS13.0 was used for statistics. Student t-test was used for measurement data and χ2 test was used for numerous data. Correlation was analyzed using Pearson and Spearman correlation analysis.
The TBA concentration in maternal peripheral vein blood, fetal umbilical artery blood and amniotic fluid in the ICP group was 34.11 ± 6.76, 11.9 ± 2.23, and 3.89 ± 1.99 mmol/L respectively. They were significantly higher than that of the control group which were maternal: 4.55 ± 1.72 mmol/L, fetal: 3.52 ± 1.56 mmol/L, and amniotic fluid: 1.43 ± 1.14 mmol/L (P < 0.05 respectively). In addition, the TBA level in maternal serum was higher than that in fetal serum or amniotic fluid in both the ICP group and control group (Table 1).
| Control (n = 32) | Intrahepatic cholestasis of pregnancy (n = 40) | |
| Total bile acid (mmol/L) | ||
| Maternal serum | 4.55 ± 1.72 | 34.11 ± 6.75a |
| Umbilical artery serum | 3.52 ± 1.56 | 11.9 ± 2.23a |
| Amniotic fluid | 1.43 ± 1.14 | 3.89 ± 1.99a |
| Amniotic fluid phospholipids | ||
| PC (μg/mL) | 69.70 ± 6.68 | 65.71 ± 7.23a |
| PI (μg/mL) | 4.28 ± 0.74 | 3.87 ± 0.65a |
| LPC (μg/mL) | 4.21 ± 0.64 | 4.72 ± 0.86a |
| SM (μg/mL) | 3.95 ± 0.53 | 3.63 ± 0.66 |
| PC/LPC (μg/mL) | 6.90 ± 2.52 | 14.40 ± 3.14a |
| Lamellar body (× 109/L) | 103.0 ± 26.82 | 74.13 ± 4.37a |
| Perinatal outcomes | ||
| Fetal distress | 4 (12.4) | 13 (32.5) |
| Neonatal asphyxia | 1 (3.13) | 2 (5) |
| NRDS | 2 (6.25) | 6 (15) |
| Perinatal death | 0 | 1 (2.5) |
The PC and PI concentrations in amniotic fluid in ICP group were 65.71 ± 7.23 μg/mL and 3.87 ± 0.65 μg/mL respectively. They were evidently lower than that in the normal control group (69.70 ± 3.68, 4.28 ± 0.74 μg/mL respectively, P < 0.05). In the ICP group, LPC content in amniotic fluid was 4.72 ± 0.86 μg/mL, which was much higher than that in control group (4.21 ± 0.64 μg/mL, P < 0.05); the SM content in both groups had no statistical difference. The ratio of PC/LPC in the ICP group (14.40 ± 3.14) was much lower than that of the control group (16.90 ± 2.52, P < 0.05). The lamellar body in the ICP group was evidently lower than that of the control group (P < 0.05) (Table 1).
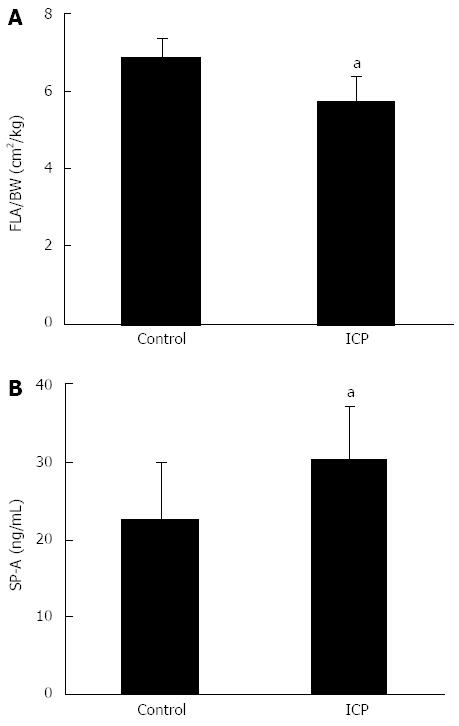
In the ICP group, fetal SP-A concentration was 30.26 ± 7.01 ng/mL, which is significantly higher than that of the control group, 22.63 ± 7.42 ng/mL (P < 0.05). The fetal lung area/body weight ratio of the ICP group was 5.76 ± 0.63 cm2/kg, while the control group was 6.89 ± 0.48 cm2/kg, which is a significant difference (P < 0.05) (Figure 2).
The maternal TBA concentration and fetal TBA level are positively correlated (r = 0.746, P < 0.05). Fetal TBA is positively correlated with fetal SP-A concentrations (r = 0.422, P < 0.05), but negatively correlated with amniotic fluid lamellar small mass (r = 0.810, P < 0.05) or fetal lung area/body weight ratio (r = 0.769, P < 0.05). Furthermore, fetal TBA is negatively correlated with amniotic fluid PC, SM and PI (rpc = 0.536, rsm = 0.438, rpi = 0.387, P < 0.05). In addition, amniotic fluid lamellar body are positively correlated with fetal lung area/body weight ratio (r = 0.929, P < 0.05).
The fetal distress, neonatal asphyxia, neonatal respiratory distress syndrome and perinatal death rates in the ICP and control group are shown in Table 1.
Intrahepatic cholestasis of pregnancy is a peculiar disease in middle-late pregnancy, with the pathological characteristics of hepatic capillary bile duct silts, causing increasing clinical bile components in peripheral blood and liver function damage[11,12]. High TBA has toxic cellular effects to many organs and mainly affects the fetus[13], leading to perinatal complications such as fetal distress, meconium inhaled syndrome and neonatal asphyxia[3,4]. The mechanism of ICP causing poor perinatal outcome has not yet been elucidated. Current studies suggest that maternal TBA level is the most sensitive index to diagnose ICP and predict the perinatal outcomes[4,14,15].
Fetal serum bile acid is synthesized from fetal liver, which increases with the gestational weeks. During late normal pregnancy, fetal blood bile acid concentration is higher than the maternal level[16,17]. Bile acid as fat soluble small molecules, diffuses through the placenta, then to maternal blood circulation and the normal liver system removes them from the body. During ICP, under the action of various factors, maternal bile acid levels increase, which damages the placenta, causing insufficiency of placental transferring, leading to fetal bile acid deposition in the body, and finally the fetal blood and the amniotic fluid bile acid levels become higher[18]. With the rise of maternal bile acid concentration, fetal blood bile acid increases and causes delay of fetal lung development[15].
Animal experiments and clinical studies have demonstrated that ICP leads to fetal and neonatal acute lung injury and causes bile acid pneumonia[19]. The cause of poor perinatal outcomes due to ICP is not very clear. Injection of cholic acids into the rabbit trachea induces dyspnea and respiratory failure[20]. The morphological changes are consistent with neonatal pulmonary hyaline membrane disease, decreasing of light transmittance, swelling, atelectasis and pulmonary hyaline membrane disease[6,14,21]. After giving pulmonary surfactant treatment, the symptoms and pathological changes reduce or disappear[22,23]. In a bronchoalveolar fluid study (BALF), it was found that the more bile acid content in BALF, the less production of the pulmonary surfactant A and D. It was demonstrated that the lung injury induced by bile acid is associated with pulmonary surfactant insufficiency[6]. Zecca et al[19] found that bile acids exist in all newborns in the BALF study on ICP.
Cholic acid can cause a dysfunction of surface active substances synthesis in the lung and induces an inflammatory reaction and chemical pneumonia. With bronchoalveolar lavage, Hills et al[24] found that the pulmonary phospholipid content is lower in sudden infant death syndrome than in normal cases, and bile acid content increased. It prompted the idea that bile acid may achieve the role of pulmonary surfactant to the lungs through acting on phospholipase[24]. In this study, umbilical cord blood SP-A in the ICP group is higher than that of the normal group and the umbilical cord blood total bile acid concentration is also higher. SP-A is the lung protein component of pulmonary surfactant, which is a hydrophilic multifunctional glycoprotein. Under normal circumstances, the alveolar capillary barrier is intact, which can prevent SP-A serum from entering the blood circulation. When the lungs are injured, the alveolar capillary permeability increases, then SP-A leaks from the alveolar cavity to the alveolar capillaries, which induces an increasing blood SP-A concentration[25]. We speculated that high amniotic bile acid concentrations can destroy the continuity of the pulmonary vascular endothelium, causing the fetus alveolar capillary damage and increasing alveolar capillary permeability. SP-A can damage the alveolar capillary membrane barrier, then get into the blood circulation, leading a SP-A rise in serum[26,27].
Pulmonary surfactant is synthesized in alveolar type II epithelial cells. When lung injury happens, the AT II cell synthesis ability decreases, which leads to the alveolar capillary permeability increasing and pulmonary surfactant decreasing[28,29]. Cholic acid can promote the secretion of phospholipase A2 and restrain and reduce the secretion of pulmonary surface active substance[20]. So, even although the amniotic fluid lecithin/sphingomyelin ratio (L/S) indicates mature lung, unusually high levels of cholic acid can still reverse the activity of phospholipase A2, causing a relative lack of lung surface. When using pulmonary surfactant to treat newborns diagnosed with bile acid pneumonia, Zecca found that clinical symptoms and signs obviously improved[19]. In our study, PC and PI levels in ICP amniotic fluid are lower than that in normal pregnancy. We speculate that there may be high concentrations of bile acids in the amniotic fluid and fetal circulation which work together in the respiratory tract and lungs of the fetus. A high level of bile acid has a cytotoxic effect in the lungs, destroying the AT-II cells and decreasing PS, PC and PI synthesis. Our results showed that the ICP’s LPC in amniotic fluid levels are higher than that of the normal group, which might be caused by the degradation in the amniotic fluid. As to what causes the degradation of the PC, further studies are needed. LPC has a direct toxic effect which may damage AT II cells, then affect the synthesis of PS. It can increase the damaging effect to the alveolar capillary system caused by TBA in fetal blood and amniotic fluid. This change may result in increasing cell membrane permeability and alveolar infiltrates.
The lamellar body is the special structure of lung surface active material stored in alveolar type II cellular cytoplasm which has a typical structure like an onion[30]. LB can be found in normal middle pregnancy and increases obviously at 34 to 36 gestational weeks. It is discharged by alveolar type II cells and attached to the alveolar surface, then contacts with amniotic fluid. Amniotic fluid LB increases gradually with the progress of pregnancy and fetal maturity. So, the LB measurement can predict fetal lung maturity[31]. Reports shows high bile acid can induce fetal rat alveolar type II epithelial cells to degenerate through necrosis, the cell surface microvilli structure disappears, the nucleus and mitochondria swells, the balloon sample changes and ridge cavitations disappear[25]. It can also result in the decrease of lamellar corpuscle numbers and the disappearing of the board layer structure.
In conclusion, our study demonstrated that maternal bile acid concentration is associated with fetal and amniotic fluid bile acid level. A maternal high blood bile acid level results in an increased fetal and amniotic bile acid level, which leads to a reduced synthesis of fetal pulmonary surfactant and delayed fetal lung development. High bile acid concentration has an increased perinatal morbidity and mortality. This study may help us to predict perinatal outcomes, to develop strategies improving the prenatal outcome, and to further study the mechanism of how fetal pulmonary AT-II cells are affected. It calls attention to delayed maturation of fetal lungs in ICP patients and to take steps to carefully check and improve fetal pulmonary maturity.
We thank the obstetric women for their participation in this study at Central South University Xiangya Second Hospital. We also gratefully acknowledge the assistance of medical and nursing staff. We appreciate English assistance from Christopher Leukel, a staff member from the University of Utah.
Intrahepatic cholestasis of pregnancy (ICP) can be complicated by fetal distress, neonatal asphyxia and neonatal respiratory distress syndrome. The etiology of ICP is poorly understood but the perinatal complications are closely correlated with maternal total bile acid (TBA) level. It is necessary to explore fetal lung alteration and development during ICP and its possible mechanisms affecting fetal pulmonary maturation.
Lung development in ICP is a research hotspot since neonatal respiratory distress syndrome is a serious complication which is usually related to the immaturity of fetal lungs. Finding out the relationship of TBA concentration in maternal, fetal and amniotic fluid and its association with fetal surfactant, surfactant protein A, amniotic lamellar body and fetal lung development will help us to predict and improve perinatal outcomes. It calls clinical attention to delayed maturation of fetal lungs in ICP and to improve fetal pulmonary maturity.
Previous studies have found that high TBA can cause neonatal lung injury but its mechanism is not clear. In order to explore fetal lung alteration during ICP and its possible mechanisms, the authors investigated maternal and fetal TBA, fetal surfactant production, fetal surfactant protein level and fetal lung development. The study demonstrated that a maternal high blood bile acid level results in an increased fetal and amniotic bile acid level, which leads to a reduced synthesis of fetal pulmonary surfactant and delayed fetal lung development.
This study may help to predict perinatal outcomes, to develop strategies improving the perinatal outcome, and to further study the mechanism of how fetal pulmonary AT-II cells are affected. It calls attention to delayed maturation of fetal lungs in ICP patients and to take steps to carefully check and improve fetal pulmonary maturity.
ICP: It is also called obstetric cholestasis, jaundice of pregnancy, or pruritus of pregnancy. It is a medical condition during pregnancy in which hepatic capillary bile duct silt. It typically presents with itching and can lead to complications for both mother and fetus. Pulmonary surfactant: It is a surface-active lipoprotein complex formed by type II alveolar cells which reduces surface tension. Mature surfactant in the fetus is very important for neonates to start normal breathing after birth.
The manuscript has new information on lung volume and levels of surfactant phospholipid and surfactant protein A in ICP. This information advances to understanding fetal lung injury in ICP.
P- Reviewers: Boggaram V, Eberlein M, Van Haute L S- Editor: Zhai HH L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | Abedin P, Weaver JB, Egginton E. Intrahepatic cholestasis of pregnancy: prevalence and ethnic distribution. Ethn Health. 1999;4:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Zecca E, De Luca D, Baroni S, Vento G, Tiberi E, Romagnoli C. Bile acid-induced lung injury in newborn infants: a bronchoalveolar lavage fluid study. Pediatrics. 2008;121:e146-e149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Pathak B, Sheibani L, Lee RH. Cholestasis of pregnancy. Obstet Gynecol Clin North Am. 2010;37:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Zecca E, De Luca D, Marras M, Caruso A, Bernardini T, Romagnoli C. Intrahepatic cholestasis of pregnancy and neonatal respiratory distress syndrome. Pediatrics. 2006;117:1669-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Rioseco AJ, Ivankovic MB, Manzur A, Hamed F, Kato SR, Parer JT, Germain AM. Intrahepatic cholestasis of pregnancy: a retrospective case-control study of perinatal outcome. Am J Obstet Gynecol. 1994;170:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 177] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Savonius H, Riikonen S, Gylling H, Haukkamaa M. Pregnancy outcome with intrahepatic cholestasis. Acta Obstet Gynecol Scand. 2000;79:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Feng YJ, Shen J. Obstetrics and Gynecology. Beijing: People Health Press 2011; 137-139. |
| 9. | Chaiworapongsa T, Hong JS, Hull WM, Romero R, Whitsett JA. Amniotic fluid concentration of surfactant proteins in intra-amniotic infection. J Matern Fetal Neonatal Med. 2008;21:663-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Liang Q, Zhou QC, Peng QH, Zhang M, Sun W, Cao DM, Ding YL. [Comparison of five different ultrasonographic parameters for diagnosis of lethal fetal pulmonary hypoplasia]. Zhonghua Fu Chan Ke Zazhi. 2008;43:332-335. [PubMed] |
| 11. | Li MK, Crawford JM. The pathology of cholestasis. Semin Liver Dis. 2004;24:21-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Favre N, Bourdel N, Sapin V, Abergel A, Gallot D. [Importance of bile acids for intra-hepatic cholestasis of pregnancy]. Gynecol Obstet Fertil. 2010;38:293-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Zhang XQ, Ding YL, Zhang LJ. Why more attentions to fetus in cases of intrahepatic cholestasis of pregnancy? World J Obstet Gynecol. 2013;2:62-64. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Zhou L, Qi HB, Luo X. [Analysis of clinical characteristics and perinatal outcome of early-onset intrahepatic cholestasis of pregnancy]. Zhonghua Fu Chan Ke Zazhi. 2013;48:20-24. [PubMed] |
| 15. | Smolarczyk R, Grymowicz M, Sienko J, Czajkowski K. Successful perinatal outcome in an early onset intrahepatic cholestasis of pregnancy with extremely high serum hepatic function tests. Gynecol Endocrinol. 2009;25:475-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Serrano MA, Brites D, Larena MG, Monte MJ, Bravo MP, Oliveira N, Marin JJ. Beneficial effect of ursodeoxycholic acid on alterations induced by cholestasis of pregnancy in bile acid transport across the human placenta. J Hepatol. 1998;28:829-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Howard PJ, Murphy GM. Bile acid stress in the mother and baby unit. Eur J Gastroenterol Hepatol. 2003;15:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Ding YL, Tang LL. [Stereological study on syncytial cell of human placenta and determinations of total bile acid in cord blood of intrahepatic cholestasis of pregnancy]. Zhonghua Fu Chan Ke Zazhi. 2005;40:453-456. [PubMed] |
| 19. | Zecca E, Costa S, Lauriola V, Vento G, Papacci P, Romagnoli C. Bile acid pneumonia: a “new” form of neonatal respiratory distress syndrome? Pediatrics. 2004;114:269-272. [PubMed] |
| 20. | Henderson RD, Fung K, Cullen JB, Milne EN, Marryatt G. Bile aspiration: an experimental study in rabbits. Can J Surg. 1975;18:64-69. [PubMed] |
| 21. | Grabowski M, Kasran A, Seys S, Pauwels A, Medrala W, Dupont L, Panaszek B, Bullens D. Pepsin and bile acids in induced sputum of chronic cough patients. Respir Med. 2011;105:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Zecca E, De Luca D, Barbato G, Marras M, Tiberi E, Romagnoli C. Predicting respiratory distress syndrome in neonates from mothers with intrahepatic cholestasis of pregnancy. Early Hum Dev. 2008;84:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Gilson SD, Stone EA. Sinus mucocele secondary to craniofacial trauma in a dog. J Am Vet Med Assoc. 1991;198:2100-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Hills BA, Chen Y, Masters IB, Hills YC. Raised bile acid concentrations in SIDS lungs at necropsy. Arch Dis Child. 1997;77:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Bersten AD, Hunt T, Nicholas TE, Doyle IR. Elevated plasma surfactant protein-B predicts development of acute respiratory distress syndrome in patients with acute respiratory failure. Am J Respir Crit Care Med. 2001;164:648-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Gnadt M, Kardziev B, Schmidt M, Högger P. Surfactant protein A (SP-A) and angiotensin converting enzyme (ACE) as early biomarkers for pulmonary edema formation in ventilated human lung lobes. Lung. 2012;190:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Sone K, Akiyoshi H, Shimizu J, Cao Z, Li Y, Tanaka T, Hayashi A, Sugii S, Ohashi F. Surfactant protein-A concentration in sera from dogs with pulmonary parenchymal diseases. J Vet Med Sci. 2013;75:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Mura M, Binnie M, Han B, Li C, Andrade CF, Shiozaki A, Zhang Y, Ferrara N, Hwang D, Waddell TK. Functions of type II pneumocyte-derived vascular endothelial growth factor in alveolar structure, acute inflammation, and vascular permeability. Am J Pathol. 2010;176:1725-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Lucas R, Verin AD, Black SM, Catravas JD. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol. 2009;77:1763-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 30. | Zhang L, Yu K, Robert KW, DeBolt KM, Hong N, Tao JQ, Fukuda M, Fisher AB, Huang S. Rab38 targets to lamellar bodies and normalizes their sizes in lung alveolar type II epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;301:L461-L477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Lockwood CM, Crompton JC, Riley JK, Landeros K, Dietzen DJ, Grenache DG, Gronowski AM. Validation of lamellar body counts using three hematology analyzers. Am J Clin Pathol. 2010;134:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |