Published online May 10, 2013. doi: 10.5317/wjog.v2.i2.8
Revised: April 18, 2013
Accepted: May 8, 2013
Published online: May 10, 2013
Processing time: 149 Days and 0.7 Hours
In the past decades, a lot of advances in understanding the biochemistry and physiology of the pineal gland have been made. There is evidence that it interacts with many endocrine as well as non-endocrine tissues to influence their metabolic activity modulating many organs and functions. Melatonin is secreted by the pineal gland in the brain and plays an important role in regulating the neuroendocrine system. This hormone is one of the major role players in the regulation of the circadian sleep-wake cycle. It is normally released from the pineal gland during the night in response to environmental changes in light. Studies have shown that melatonin plays a role in the regulation of many reproductive processes such as puberty, gonadal function, and pregnancy. Beside these, melatonin has been shown to be able to directly neutralize a number of free radicals and reactive oxygen and nitrogen species. The main objective of this review is to provide comprehensive information about the new developments in melatonin research regarding its role in reproduction. A review of international scientific literature was done and a question-and-answer format was used in an attempt to convey comprehensive information in a simple manner. This review discusses evidence currently available relating to the effect of melatonin on reproductive processes. It deliberates the mechanism of action of melatonin, its effect on puberty, testicular and ova function, pregnancy, and oxidative stress. A growing body of scientific evidence is suggesting that melatonin plays an important role in reproductive function. It is therefore imperative to highlight the beneficial effects of this hormone in improving the reproductive processes.
Core tip: In recent years, many studies have been focusing on the role melatonin plays in the process of reproduction. The low success rate in assisted reproductive technologies due to the detrimental effects of oxidative stress has led to studies investigating the potency of melatonin as an antioxidant. Studies have shown that melatonin reduces oxidative stress and contributes to oocyte maturation, embryo development, and luteinization of granulosa cells. Clinical studies have demonstrated that melatonin treatment for infertile women increases intra-follicular melatonin concentrations, reduces intra-follicular oxidative damage, and increases the chances of pregnancy. This review highlights the effects of melatonin in reproduction.
- Citation: Lampiao F, Du Plessis SS. New developments of the effect of melatonin on reproduction. World J Obstet Gynecol 2013; 2(2): 8-15
- URL: https://www.wjgnet.com/2218-6220/full/v2/i2/8.htm
- DOI: https://dx.doi.org/10.5317/wjog.v2.i2.8
In the past few decades, a lot of studies regarding the biochemistry and physiology of a hormone called melatonin (N-acetyl-5-methoxytryptamine) have taken place. This hormone is secreted during the dark hours at night by the pineal gland and is responsible for the regulation of a variety of important central and peripheral actions related to circadian rhythms and reproduction[1]. Although melatonin is primarily synthesized and secreted by the pineal gland, it is has been reported that it is also formed in tiny amounts by other organs such as the retina, harderian gland, gastrointestinal tract, lymphocytes, and the skin[2-5]. The role of melatonin in other animal species is related to seasonal reproductive cycles. In humans, melatonin secretion levels by the pineal gland can regulate the reproductive neuroendocrine axis[6]. The increase in reactive oxygen species (ROS) generation in in vitro fertilization (IVF) settings has been reported to negatively affect the success rate of IVF outcomes[7-9].
Melatonin has also been reported to have free radical scavenging properties[10,11] as well as stimulating several other antioxidant enzymes[12]. Can melatonin supplementation during assisted reproductive technologies increase the success rate of these procedures Since the body is capable of producing melatonin does endogenous melatonin production or exogenous melatonin supplementation has any effect on the reproductive processes of humans and animals
This review will provide comprehensive information about the new developments in melatonin research specifically regarding its role in the process of reproduction of both humans and animals. It will discuss the mechanism of action of melatonin, its effect on puberty, testicular and ovarian function, pregnancy, and oxidative stress.
There is accumulation of evidence suggesting that the pattern of melatonin secretion, which is mediated by photoperiod, directly influences reproductive function. Much of the evidence has been generated from seasonally breeding mammals[13-16]. Long-day breeding animals such as rodents have been shown to be depressed during winter months (when elevated melatonin levels are at their longest nocturnal duration). The reproductive quiescent period was also prevented by surgical removal of the pineal gland[17]. On the other hand, short-day breeders such as sheep, and white-tailed deer were shown to be sexually very active and capable during the shortest days of the year, when melatonin levels are highest in terms of their nocturnal duration[18,19]. These observations suggest that melatonin is neither antigonadotrophic nor progonadotrophic. Thus, the changing duration of the nocturnal melatonin message is a passive signal that provides the hypothalamo-pituitary-gonadal (HPG)-axis information as to the time of year[20].
In a study involving male and female Syrian hamsters which were maintained under naturally occurring short days and reduced temperatures, it was observed that they developed gonadal regression. This regression was reversed by surgical removal of the pineal gland[21]. This is evidence that the reproductive axis obviously uses the seasonally dependent melatonin rhythm to adjust testicular and ovarian physiology accordingly.
Investigations using long-day and short-day breeding animals have enormously contributed to the understanding of the mechanisms whereby day length and melatonin govern seasonal reproduction. These findings have led to the successful use of melatonin as a pharmacological agent to advance the breeding season of sheep and to induce estrous cycles and increase lambing during the interval when these animals would normally be experiencing seasonal anestrus[22-24].
Some studies have demonstrated that melatonin may be involved in the selection of sexual partner. It was observed that administering melatonin to male zebra finches in the drinking fluid in combination with carotenoids enhanced the brightness of the carotenoid-based pigmentation in their bills[25]. Since males with brighter coloured bills are more likely to be selected as a mate by females, melatonin may aid in the selection of a mate. Colourful plumage generally signals superior genetic quality and is common ploy used by many bird species as a sexual attractant[26].
More evidence of the role of melatonin on the selection of sexual mate has been demonstrated by the two-spotted goby fish[27]. Treating the skin explants of gobies with a combination of either melatonin and melanocyte-stimulating hormone or melatonin and prolactin, led to an exaggerated orange colouration and transparency of the belly skin. This colouration change induced by melatonin and other hormones would presumably benefit the individual in terms of attracting a sexual mate.
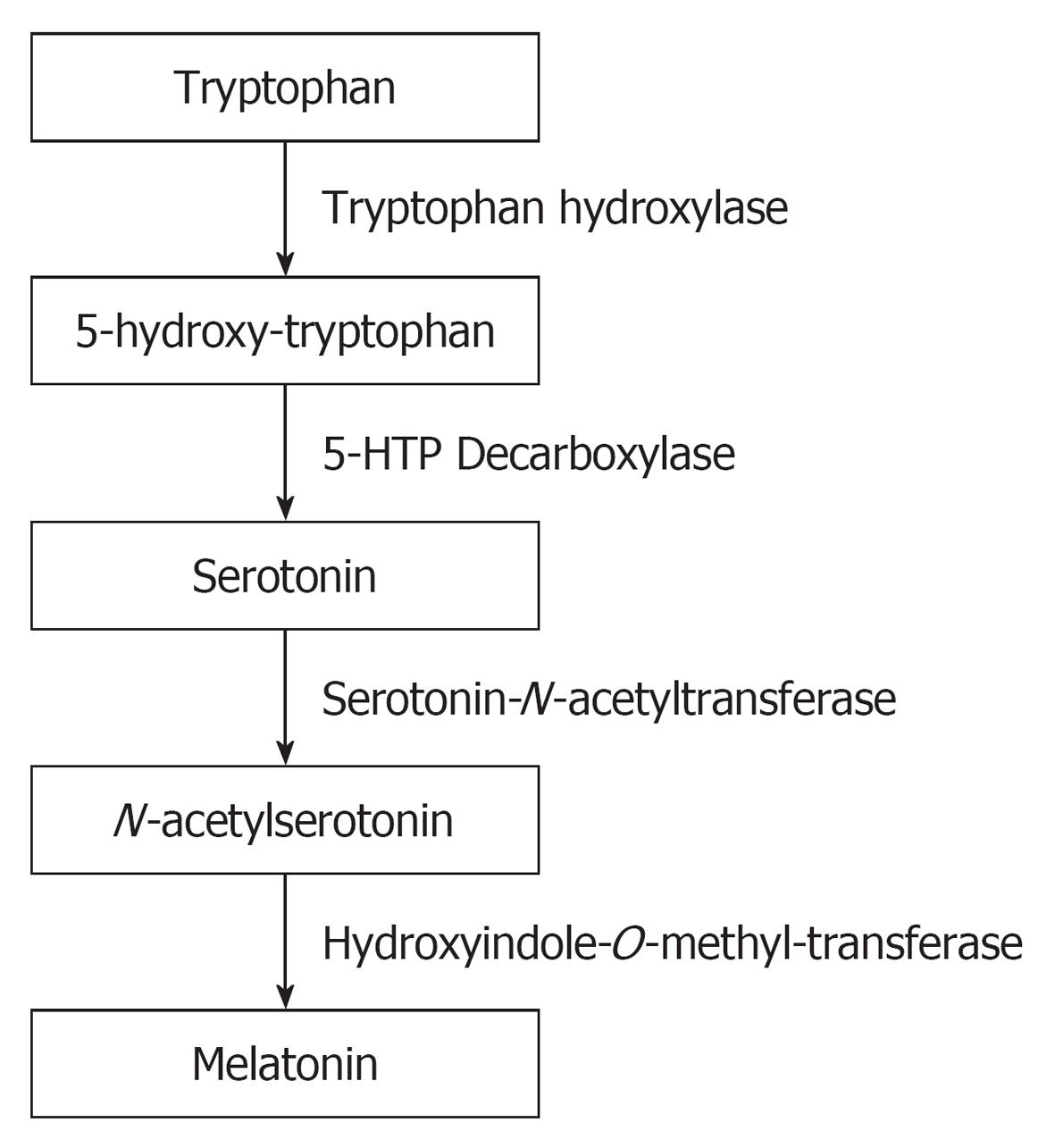
The production of melatonin by the pineal gland exhibits a circadian rhythm with low level of production during day time and high levels during the night[28,29]. During the process of melatonin synthesis, Tryptophan is hydroxylated to 5-hydroxy-tryptophan and subsequently into serotonin. Serotonin is acetylated to form N-acetylserotonin and then converted into melatonin (Figure 1). The suprachiasmatic nucleus (SCN) which is the major circadian oscillator that receives light input from the retina through the retino-hypothalamic tract is the one that regulate the circadian melatonin production[30]. When melatonin is formed in the pineal gland, it is not stored there, but released immediately into the blood or into the cerebrospinal fluid. It is metabolized mainly in the liver.
Melatonin exerts its actions through two types of receptors belonging to the super-family of G-protein coupled receptors. These receptors contain seven typical transmembrane domains and are called the MT1 and MT2[31,32]. The MT1 and MT2 are membrane bound receptors which are widely distributed in different organs of the body, including the brain and other peripheral organs.
When these receptors are activated they cause inhibition of adenyl cyclase activity[33] and inhibition of forskolin-induced cyclic adenosine monophosphate (cAMP) formation which result in the reduction in activated protein kinase[34]. In mammals melatonin has been reported to affect the reproductive function by activation of melatonin receptor sites within the HPG-axis[35].
Neonatal pituitary cells have been shown to express MT1 and MT2 subtype of melatonin receptors. These receptors when activated lead to a decrease in cAMP production and activity of protein kinase A, and attenuation of gonadotropic releasing hormone (GnRH)-induced gonadotropin secretion[36].
During fetal life and the first year of life, the HPG-axis is active, but becomes quiescent thereafter until around 10 years. Its reactivation depends on the progressive increase in the levels of GnRH which subsequently lead to the pulsatile secretion of luteinizing hormone (LH) and follicle stimulating hormone[37]. It has been reported that melatonin secretion has an inhibitory influence on the hypothalamic secretion of GnRH in humans[38]. It is therefore speculated that before puberty, melatonin concentrations are too high thus inhibiting the hypothalamic activation. But prior to puberty, the levels of melatonin decline below the threshold value thus forming the trigger signals of GnRH from the hypothalamus which leads to the onset of pubertal changes[39]. Therefore, it is the decline of melatonin levels that trigger puberty. Studies have demonstrated that high nocturnal melatonin secretion in children delays puberty[40] whereas low levels of melatonin have been shown to be associated with precocious puberty[41].
The mechanism by which the HPG-axis is inhibited by melatonin after the first year of life until puberty is not well elucidated. However, there are reports that point to the influence of melatonin on the HPG-axis. These include, the evidence that melatonin is involved in the control of pulsatile secretion of LH[42] and that there is a negative correlation between nocturnal melatonin and LH concentrations[43]. Furthermore, high levels of serum melatonin in women have been shown to be associated with amenorrhea accompanied with decreased GnRH/LH pulsatile secretion[44,45]. Similarly, increases in nocturnal peak amplitude and duration of melatonin were reported in amenorrhoeic athletes who displayed irregularities in hypothalamic-pituitary-ovarian-axis functioning[46,47]. In vitro studies have demonstrated that melatonin leads to the down-regulation of the GnRH gene expression in a cell line containing GnRH secreting neurons[48].
In animal studies, it has been shown that melatonin may modulate testicular function. In mice and rats it was reported that melatonin has an inhibitory effect on Leydig cells[49,50]. The Leydig cells are responsible for the production of testosterone. Mel1a and Mel1b receptor mRNAs are expressed in epithelial cells of rat epididymis suggesting that melatonin has a role in the regulation of epididymal physiology[51]. The epididymis is important for the maturation and storage of spermatozoa before they are ejaculated into the female reproductive tract.
There are contradictory reports concerning the effect of melatonin on spermatozoa function. It has been reported that long term administration of melatonin to healthy men is associated with decreased semen quality[52]. Sperm concentration, motility as well as testosterone levels were found to be significantly decreased in healthy men administered with melatonin. On the other hand, an in vitro study demonstrated that administration of melatonin to human spermatozoa improved progressive motility and reduced the number of static cells[53]. In another study in which melatonin levels were measured in fertile and infertile men, it was found that serum and seminal melatonin levels in infertile men were significantly reduced compared with the levels in the fertile men[54]. This demonstrated that melatonin may be involved in the modulation of the reproductive neuroendocrine axis in male infertility.
The role of melatonin in the production of female gametes is focused on its direct actions in the ovary. It is able to pass through all cell membranes and enter all tissues because of its lipophilic property, however, it specifically concentrates in the ovary when injected systemically[55]. Studies have shown that high levels of melatonin are found in human preovulatory follicular fluid at concentrations which are much higher than those in serum[56,57]. It has been reported that the follicular fluid melatonin levels depend on the follicular growth[58]. The larger the follicle the higher the melatonin concentration. When oocytes are incubated in medium with melatonin supplementation during in vitro maturation, they have lower levels of ROS than control (without melatonin treatment) oocytes[59]. The ability of melatonin to promote embryo development in different species has correspondingly been reported. When mouse embryos were cultured in medium containing melatonin, increased blastocyst development rates were observed[60]. This suggests that melatonin may be involved in embryo development.
People living in the Arctic region have shown that their pituitary-gonadal function and conception rates are lower in the dark winter months than in the summer[61]. It has been further observed that during these dark periods of the winter season, the increases in serum melatonin concentration correlate with reduced activity of the anterior pituitary-ovarian axis[62]. The precise role of melatonin in human pregnancy is not clear. However, it has been reported that serum melatonin levels are higher during pregnancy than in nonpregnant women[63]. Moreover, twin pregnancies have been reported to yield higher nocturnal melatonin levels than singleton pregnancies[63]. This suggests that melatonin might have a role to play in human pregnancy. Clinical studies have demonstrated that melatonin treatment for infertile women increases intra-follicular melatonin concentrations, reduces intra-follicular oxidative damage and elevates fertilization and pregnancy rates[8].
Because melatonin is a small molecule, it gets transferred from the maternal circulation into that of the fetus through the placenta[64]. This means that the fetal circulation mirrors a circadian rhythm of plasma melatonin similar to that of the mother[65]. It has also been reported that there are melatonin receptors in the human fetal SCN. It is believed that melatonin is involved in the regulation of the circadian rhythm in the fetus. It has been observed that if maternal melatonin is suppressed, both MT1 gene and clock genes are affected, suggesting that maternal melatonin has a role in modulating fetal clock gene function[66]. The generation as well as maintenance of circadian clock function depends on clock genes[67].
In some mammals such as rats, parturition occurs during daytime[68]. Continuous darkness abolishes the photoperiodic timing of parturition[69]. If the pineal gland is removed in rats, the daytime delivery birth pattern is abolished and melatonin replacement therapy restores it[70]. It is well documented that the human myometrium has functional melatonin receptors[71]. Administration of melatonin has been shown to modulate the strength of affinity of gap junctions found in the myometrium[72,73]. These gap junctions serve to coordinate individual myometrial cell contractions into powerful labor inducing forces[72], thus implicating melatonin as a possible role player in the mechanism underlying the initiation of parturition.
In females ROS is locally produced during the rupturing of the follicle at the time of ovulation[74]. It has been suggested that the ROS are involved in the ovulation process. There is a surge of LH during ovulation which induces dissolution of the basement membrane between the granulosa and theca internal layers and an expansion of the theca capillaries into the avascular granulosa cell layer to form a dense network of capillaries. These endothelial cell capillaries contribute to the generation of the free radicals[74]. Neutrophils and macrophages are also reported to reside in follicles[75]. These macrophages and neutrophils produce tremendous amounts of free radicals. The locally produced free radicals seems to have an important role on follicle rupture, since ROS have been shown to act as second messengers modulating the expression of genes that govern physiological processes of oocyte maturation[76,77]. However, excess ROS is responsible for oxidative stress which can damage molecules and structures of oocyte and granulosa cells within the follicle. Hence the ROS must be continuously scavenged to keep only small amounts necessary to maintain normal cell function.
In the male reproductive system, the cellular component of semen is a huge source of ROS. Morphologically abnormal and immature spermatozoa together with the presence of leukocytes can generate ROS in human ejaculates. Spermatozoa do generate ROS at the level of the plasma membrane and mitochondria[78]. Studies have shown that human spermatozoa generate superoxide (O2.-), which spontaneously dismutates to hydrogen peroxide (H2O2)[79].
In the male genital tract and the ejaculate, ROS are not only derived from the spermatozoa, but can also be generated by leukocytes, which physiologically produce even up to 1000 times more ROS than spermatozoa[80,81]. This high ROS production by leukocytes plays a major role in infections, inflammation and cellular defense mechanisms. Basically, the cellular mechanisms for the generation of ROS in leukocytes and spermatozoa are the same, yet in leukocytes it is a physiological necessity to release large amounts of O2.- into phagocytic vesicles during the killing action of pathogens.
Considering the extraordinary high content of polyunsaturated fatty acids in their membrane, the sperm plasma membrane is particularly susceptible to oxidative stress and the double bonds of the membrane lipids can easily be oxidized by excessive ROS levels present in the sperm cells' environment. These can either be produced in large amounts by leukocytes or the spermatozoa themselves. In case of ROS attacking the plasma membrane lipids, a process called “lipid peroxidation” is initiated. Ultimately, this process decreases membrane fluidity of both plasma and organelle membranes and, as a result, damages membrane function, ion gradients, receptor-mediated signal transduction, etc[82]. Hence, with the loss of membrane function, spermatozoa lose the ability to function properly and therefore, fertilization is impaired[83].
Usually melatonin exerts its effects through its receptors, but it can also act directly as a powerful free radical scavenger by detoxifying the highly reactive hydroxyl radical[84,85]. There are numerous other reports confirming the scavenging abilities of melatonin on ROS and reactive nitrogen species[86,87]. Some of the free radicals scavenged by melatonin include O2-, H2O2, hydrochlorous acid, nitric oxide and the peroxynitrite anion[88-91]. The antioxidant properties of melatonin as a cell protector have been extensively studied and its scavenging ability have been reported to be higher than that of well known scavengers such as vitamin C and vitamin E[86]. Apart from scavenging free radicals directly, melatonin has a high capability to detoxify ROS and suppress its oxidative effects indirectly by enhancing the production of endogenous antioxidants. Melatonin has been shown to stimulate the scavenging activities and mRNA levels of antioxidant enzymes including superoxide dismutase, glutathione peroxidase, and catalase[92,93].
In recent years, a lot of research focused on the effect of melatonin as a direct free radical scavenger. This has greatly broadened our understanding of its multiple physiological roles. Melatonin’s role in the regulation of reproductive physiology has been demonstrated in photoperiod dependent breeding mammals, and it seems to be receptor mediated mechanism in hypothalamus and pituitary gland. Currently, most of the research on melatonin is focusing on its local role as an antioxidant. The intra-follicular role of melatonin in the ovary has been demonstrated. Melatonin, secreted by the pineal gland, has been reported to be taken up into the follicular fluid from the blood. The free radicals produced within the follicles, especially during the ovulation process, are scavenged by melatonin, and reduced oxidative stress may be involved in oocyte maturation and embryo development. Evidence is pointing to the fact that melatonin treatment for infertility in women increases intra-follicular melatonin concentrations which subsequently reduces intra-follicular oxidative damage and elevates fertilization and pregnancy rates. The safety of exogenous melatonin treatment has been demonstrated in many studies[94,95]. Animal studies have also shown that melatonin has no detrimental effects on mouse and rat embryo development both in vitro and in vivo[96,97]. Future studies will indicate whether melatonin treatment could become a new cure for improving oocyte and sperm quality in infertile patients.
P- Reviewers Dane C, Martins WP, Zhang QX S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Aasada H, Yamagata Y. The role of melatonin as an antioxidant in the follicle. J Ovarian Res. 2012;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, Slominski RM, Tobin DJ. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 4. | Carrillo-Vico A, Calvo JR, Abreu P, Lardone PJ, García-Mauriño S, Reiter RJ, Guerrero JM. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004;18:537-539. [PubMed] |
| 5. | Cardinali DP, Rosner JM. Metabolism of serotonin by the rat retina in vitro. J Neurochem. 1971;18:1769-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Anderson RA, Lincoln GA, Wu FC. Melatonin potentiates testosterone-induced suppression of luteinizing hormone secretion in normal men. Hum Reprod. 1993;8:1819-1822. [PubMed] |
| 7. | Eryilmaz OG, Devran A, Sarikaya E, Aksakal FN, Mollamahmutoğlu L, Cicek N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J Assist Reprod Genet. 2011;28:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Asada H, Yamagata Y. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J. 2013;60:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Kim MK, Park EA, Kim HJ, Choi WY, Cho JH, Lee WS, Cha KY, Kim YS, Lee DR, Yoon TK. Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS. Reprod Biomed Online. 2013;26:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Webb SM, Puig-Domingo M. Role of melatonin in health and disease. Clin Endocrinol (Oxf). 1995;42:221-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Zang LY, Cosma G, Gardner H, Vallyathan V. Scavenging of reactive oxygen species by melatonin. Biochim Biophys Acta. 1998;1425:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 147] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7:444-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 779] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 13. | Reiter RJ, Hester RJ. Interrelationships of the pineal gland, the superior cervical ganglia and the photoperiod in the regulation of the endocrine systems of hamsters. Endocrinology. 1966;79:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 110] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Reiter RJ. Comparative physiology: pineal gland. Annu Rev Physiol. 1973;35:305-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 125] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Stetson MH, Elliott JA, Menaker M. Photoperiodic regulation of hamster testis: circadian sensitivity to the effects of light. Biol Reprod. 1975;13:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Turek FW, Desjardins C, Menaker M. Melatonin-induced inhibition of testicular function in adult golden hamsters. Proc Soc Exp Biol Med. 1976;151:502-506. [PubMed] |
| 17. | Wagner GC, Johnston JD, Clarke IJ, Lincoln GA, Hazlerigg DG. Redefining the limits of day length responsiveness in a seasonal mammal. Endocrinology. 2008;149:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Coelho LA, Rodrigues PA, Nonaka KO, Sasa A, Balieiro JC, Vicente WR, Cipolla-Neto J. Annual pattern of plasma melatonin and progesterone concentrations in hair and wool ewe lambs kept under natural photoperiod at lower latitudes in the southern hemisphere. J Pineal Res. 2006;41:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Chemineau P, Guillaume D, Migaud M, Thiéry JC, Pellicer-Rubio MT, Malpaux B. Seasonality of reproduction in mammals: intimate regulatory mechanisms and practical implications. Reprod Domest Anim. 2008;43 Suppl 2:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Reiter RJ. The melatonin rhythm: both a clock and a calendar. Experientia. 1993;49:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 695] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 21. | Reiter RJ. Pineal control of a seasonal reproductive rhythm in male golden hamsters exposed to natural daylight and temperature. Endocrinology. 1973;92:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 99] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Haresign W, Peters AR, Staples LD. The effect of melatonin implants on breeding activity and litter size in commercial sheep flocks in the UK. Anim Prod. 1990;50:111-121. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Haresign W. Manipulation of reproduction in sheep. J Reprod Fertil Suppl. 1992;45:127-139. [PubMed] |
| 24. | Abecia JA, Valares JA, Forcada F, Palacín I, Martín S, Martino A. The effect of melatonin on the reproductive performance of three sheep breeds in Spain. Small Rumin Res. 2007;69:10-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Bertrand S, Faivre B, Sorci G. Do carotenoid-based sexual traits signal the availability of non-pigmentary antioxidants. J Exp Biol. 2006;209:4414-4419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Roulin A, Jungi TW, Pfister H, Dijkstra C. Female barn owls (Tyto alba) advertise good genes. Proc Biol Sci. 2000;267:937-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 134] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Sköld HN, Amundsen T, Svensson PA, Mayer I, Bjelvenmark J, Forsgren E. Hormonal regulation of female nuptial coloration in a fish. Horm Behav. 2008;54:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 995] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 29. | Pang SF, Li L, Ayre EA, Pang CS, Lee PP, Xu RK, Chow PH, Yu ZH, Shiu SY. Neuroendocrinology of melatonin in reproduction: recent developments. J Chem Neuroanat. 1998;14:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2253] [Cited by in RCA: 2035] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 31. | Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 761] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 32. | Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci USA. 1995;92:8734-8738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 631] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 33. | von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 335] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 34. | Vanecek J, Klein DC. Melatonin inhibits gonadotropin-releasing hormone-induced elevation of intracellular Ca2+ in neonatal rat pituitary cells. Endocrinology. 1992;130:701-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Malpaux B, Migaud M, Tricoire H, Chemineau P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J Biol Rhythms. 2001;16:336-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 242] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 36. | Balík A, Kretschmannová K, Mazna P, Svobodová I, Zemková H. Melatonin action in neonatal gonadotrophs. Physiol Res. 2004;53 Suppl 1:S153-S166. [PubMed] |
| 37. | Sizonenko PC. Physiology of puberty. J Endocrinol Invest. 1989;12:59-63. [PubMed] |
| 38. | Buchanan KL, Yellon SM. Delayed puberty in the male Djungarian hamster: effect of short photoperiod or melatonin treatment on the GnRH neuronal system. Neuroendocrinology. 1991;54:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Silman R. Melatonin and the human gonadotrophin-releasing hormone pulse generator. J Endocrinol. 1991;128:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Cohen HN, Hay ID, Annesley TM, Beastall GH, Wallace AM, Spooner R, Thomson JA, Eastwold P, Klee GG. Serum immunoreactive melatonin in boys with delayed puberty. Clin Endocrinol (Oxf). 1982;17:517-521. [PubMed] |
| 41. | Cavallo A. Melatonin and human puberty: current perspectives. J Pineal Res. 1993;15:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Brzezinski A, Lynch HJ, Wurtman RJ, Seibel MM. Possible contribution of melatonin to the timing of the luteinizing hormone surge. N Engl J Med. 1987;316:1550-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Waldhauser F, Weiszenbacher G, Frisch H, Zeitlhuber U, Waldhauser M, Wurtman RJ. Fall in nocturnal serum melatonin during prepuberty and pubescence. Lancet. 1984;1:362-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 172] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Berga SL, Mortola JF, Yen SS. Amplification of nocturnal melatonin secretion in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1988;66:242-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 87] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Brzezinski A, Lynch HJ, Seibel MM, Deng MH, Nader TM, Wurtman RJ. The circadian rhythm of plasma melatonin during the normal menstrual cycle and in amenorrheic women. J Clin Endocrinol Metab. 1988;66:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 108] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Laughlin GA, Loucks AB, Yen SS. Marked augmentation of nocturnal melatonin secretion in amenorrheic athletes, but not in cycling athletes: unaltered by opioidergic or dopaminergic blockade. J Clin Endocrinol Metab. 1991;73:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Aleandri V, Spina V, Morini A. The pineal gland and reproduction. Hum Reprod Update. 1996;2:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Roy D, Belsham DD. Melatonin receptor activation regulates GnRH gene expression and secretion in GT1-7 GnRH neurons. Signal transduction mechanisms. J Biol Chem. 2002;277:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Ng TB, Lo LL. Inhibitory actions of pineal indoles on steroidogenesis in isolated rat Leydig cells. J Pineal Res. 1988;5:229-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Persengiev S, Kehajova J. Inhibitory action of melatonin and structurally related compounds on testosterone production by mouse Leydig cells in vitro. Cell Biochem Funct. 1991;9:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Shiu SY, Li L, Wong JT, Pang SF. Biology of G protein-coupled melatonin receptors in the epididymis and prostate of mammals. Chin Med J (Engl). 1997;110:648-655. [PubMed] |
| 52. | Luboshitzky R, Shen-Orr Z, Nave R, Lavi S, Lavie P. Melatonin administration alters semen quality in healthy men. J Androl. 2002;23:572-578. [PubMed] |
| 53. | Ortiz A, Espino J, Bejarano I, Lozano GM, Monllor F, García JF, Pariente JA, Rodríguez AB. High endogenous melatonin concentrations enhance sperm quality and short-term in vitro exposure to melatonin improves aspects of sperm motility. J Pineal Res. 2011;50:132-139. [PubMed] |
| 54. | Awad H, Halawa F, Mostafa T, Atta H. Melatonin hormone profile in infertile males. Int J Androl. 2006;29:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Wurtman RJ, Axelrod J, Potter LT. The uptake of H3-melatonin in endocrine and nervous tissues and the effects of constant light exposure. J Pharmacol Exp Ther. 1964;143:314-318. [PubMed] |
| 56. | Brzezinski A, Seibel MM, Lynch HJ, Deng MH, Wurtman RJ. Melatonin in human preovulatory follicular fluid. J Clin Endocrinol Metab. 1987;64:865-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 182] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Rönnberg L, Kauppila A, Leppäluoto J, Martikainen H, Vakkuri O. Circadian and seasonal variation in human preovulatory follicular fluid melatonin concentration. J Clin Endocrinol Metab. 1990;71:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Nakamura Y, Tamura H, Takayama H, Kato H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil Steril. 2003;80:1012-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 59. | Jahnke G, Marr M, Myers C, Wilson R, Travlos G, Price C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol Sci. 1999;50:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 60. | Ishizuka B, Kuribayashi Y, Murai K, Amemiya A, Itoh MT. The effect of melatonin on in vitro fertilization and embryo development in mice. J Pineal Res. 2000;28:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 61. | Sawhney RC, Malhotra AS, Prasad R, Pal K, Kumar R, Bajaj AC. Pituitary-gonadal hormones during prolonged residency in Antarctica. Int J Biometeorol. 1998;42:51-54. [PubMed] |
| 62. | Kauppila A, Kivelä A, Pakarinen A, Vakkuri O. Inverse seasonal relationship between melatonin and ovarian activity in humans in a region with a strong seasonal contrast in luminosity. J Clin Endocrinol Metab. 1987;65:823-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Nakamura Y, Tamura H, Kashida S, Takayama H, Yamagata Y, Karube A, Sugino N, Kato H. Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J Pineal Res. 2001;30:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 64. | Yellon SM, Longo LD. Effect of maternal pinealectomy and reverse photoperiod on the circadian melatonin rhythm in the sheep and fetus during the last trimester of pregnancy. Biol Reprod. 1988;39:1093-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Muñoz-Hoyos A, Jaldo-Alba F, Molina-Carballo A, Rodríguez-Cabezas T, Molina-Font JA, Acuña-Castroviejo D. Absence of plasma melatonin circadian rhythm during the first 72 hours of life in human infants. J Clin Endocrinol Metab. 1993;77:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Torres-Farfan C, Rocco V, Monsó C, Valenzuela FJ, Campino C, Germain A, Torrealba F, Valenzuela GJ, Seron-Ferre M. Maternal melatonin effects on clock gene expression in a nonhuman primate fetus. Endocrinology. 2006;147:4618-4626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 67. | Hawkins GA, Meyers DA, Bleecker ER, Pack AI. Identification of coding polymorphisms in human circadian rhythm genes PER1, PER2, PER3, CLOCK, ARNTL, CRY1, CRY2 and TIMELESS in a multi-ethnic screening panel. DNA Seq. 2008;19:44-49. [PubMed] |
| 68. | Boer K, Lincoln DW, Swaab DF. Effects of electrical stimulation of the neurohypophysis on labour in the rat. J Endocrinol. 1975;65:163-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Lincoln DW, Porter DG. Photoperiodic dissection of endocrine events at parturition. Anim Reprod Sci. 1979;2:97-115. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Takayama H, Nakamura Y, Tamura H, Yamagata Y, Harada A, Nakata M, Sugino N, Kato H. Pineal gland (melatonin) affects the parturition time, but not luteal function and fetal growth, in pregnant rats. Endocr J. 2003;50:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Schlabritz-Loutsevitch N, Hellner N, Middendorf R, Müller D, Olcese J. The human myometrium as a target for melatonin. J Clin Endocrinol Metab. 2003;88:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Tamura H, Nakamura Y, Terron MP, Flores LJ, Manchester LC, Tan DX, Sugino N, Reiter RJ. Melatonin and pregnancy in the human. Reprod Toxicol. 2008;25:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 73. | Olecese J. Melatonin effects on uterine physiology. Melatonin: from molecules to therapy. New York: Nova Science publishers 2007; 205-225. |
| 74. | Brännström M, Norman RJ. Involvement of leukocytes and cytokines in the ovulatory process and corpus luteum function. Hum Reprod. 1993;8:1762-1775. [PubMed] |
| 75. | Nakamura Y, Smith M, Krishna A, Terranova PF. Increased number of mast cells in the dominant follicle of the cow: relationships among luteal, stromal, and hilar regions. Biol Reprod. 1987;37:546-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47-95. [PubMed] |
| 77. | Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 684] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 78. | Gavella M, Lipovac V. NADH-dependent oxidoreductase (diaphorase) activity and isozyme pattern of sperm in infertile men. Arch Androl. 1992;28:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987;8:338-348. [PubMed] |
| 80. | Plante M, de Lamirande E, Gagnon C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil Steril. 1994;62:387-393. [PubMed] |
| 81. | de Lamirande E, Gagnon C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic Biol Med. 1995;18:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 153] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 82. | Sikka SC, Rajasekaran M, Hellstrom WJ. Role of oxidative stress and antioxidants in male infertility. J Androl. 1995;16:464-468. [PubMed] |
| 83. | Riffo MS, Parraga M. Study of the acrosome reaction and the fertilizing ability of hamster epididymal cauda spermatozoa treated with antibodies against phospholipase A2 and/or lysophosphatidylcholine. J Exp Zool. 1996;275:459-468. [PubMed] |
| 84. | Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J Pineal Res. 1993;14:151-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 357] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 85. | Schindler AE, Christensen B, Henkel A, Oettel M, Moore C. High-dose pilot study with the novel progestogen dienogestin patients with endometriosis. Gynecol Endocrinol. 2006;22:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001;34:237-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 87. | Tan DX, Manchester LC, Reiter RJ, Plummer BF, Limson J, Weintraub ST, Qi W. Melatonin directly scavenges hydrogen peroxide: a potentially new metabolic pathway of melatonin biotransformation. Free Radic Biol Med. 2000;29:1177-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 320] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 88. | Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 89. | Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, Livrea MA. The chemistry of melatonin’s interaction with reactive species. J Pineal Res. 2003;34:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 513] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 90. | Reiter RJ, Tan DX, Gitto E, Sainz RM, Mayo JC, Leon J, Manchester LC, Vijayalaxmi E, Kilic U. Pharmacological utility of melatonin in reducing oxidative cellular and molecular damage. Pol J Pharmacol. 2004;56:159-170. [PubMed] |
| 91. | Tan DX, Manchester LC, Sainz RM, Mayo JC, Leon J, Hardeland R, Poeggeler B, Reiter RJ. Interactions between melatonin and nicotinamide nucleotide: NADH preservation in cells and in cell-free systems by melatonin. J Pineal Res. 2005;39:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Mayo JC, Sainz RM, Antoli I, Herrera F, Martin V, Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci. 2002;59:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 93. | Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1419] [Cited by in RCA: 1454] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 94. | Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, Vohra S, Klassen TP, Baker G. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ. 2006;332:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 281] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 95. | Carr R, Wasdell MB, Hamilton D, Weiss MD, Freeman RD, Tai J, Rietveld WJ, Jan JE. Long-term effectiveness outcome of melatonin therapy in children with treatment-resistant circadian rhythm sleep disorders. J Pineal Res. 2007;43:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Chan WY, Ng TB. Development of pre-implantation mouse embryos under the influence of pineal indoles. J Neural Transm Gen Sect. 1994;96:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 97. | McElhinny AS, Davis FC, Warner CM. The effect of melatonin on cleavage rate of C57BL/6 and CBA/Ca preimplantation embryos cultured in vitro. J Pineal Res. 1996;21:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |