Published online Jun 29, 2022. doi: 10.5317/wjog.v11.i3.33
Peer-review started: December 28, 2021
First decision: March 16, 2022
Revised: March 22, 2022
Accepted: May 28, 2022
Article in press: May 28, 2022
Published online: June 29, 2022
Processing time: 182 Days and 19.9 Hours
Hormones could play a role in the evolution of follicular thyroid cancer (FTC) for which we discuss an unusual presentation of FTC occurring during pregnancy.
A pregnant woman was admitted with FTC metastasis resulting in a gluteal mass. Preoperative abdominal computed tomography revealed liver metastasis for which the patient underwent total thyroidectomy and liver resection, oral radioiodine therapy and radiotherapy, followed by embolization of the pelvic mass. The patient died of cerebral hemorrhage 16 mo after the initial diagnosis.
Human chorionic gonadotropin and estrogen stimulation might have a role in cancer growth, especially during pregnancy. FTC management aims to stop disease progression and overcome hormonal imbalances after thyroidectomy thus reducing fetal complications. It is still under debate whether it is possible to combine optimal timing for treatment to ensure the best possible outcome with reduction of fetal complications and risk of cancer growth.
Core Tip: We discuss an uncommon presentation of follicular thyroid cancer occurring during pregnancy. Beta human chorionic gonadotropin and estrogens could take part in the progression of thyroid tumors.
- Citation: Spinelli C, Sanna B, Ghionzoli M, Micelli E. Therapeutic challenges in metastatic follicular thyroid cancer occurring in pregnancy: A case report. World J Obstet Gynecol 2022; 11(3): 33-39
- URL: https://www.wjgnet.com/2218-6220/full/v11/i3/33.htm
- DOI: https://dx.doi.org/10.5317/wjog.v11.i3.33
Thyroid cancer is reported as the second most common type of cancer diagnosed during pregnancy, followed by breast cancer[1]. Although the effects of pregnancy on the behavior of this tumor have already been widely discussed, the number of reported cases is too small to draw any conclusions. We assume that beta human chorionic gonadotropin (β-hCG) and estrogens could play a role in the progression and prognosis of the tumor. To date, few reports of follicular thyroid cancer (FTC) causing bone metastases[2-5] (skull[6], mandible[7], maxilla[8], spine[9] and orbit[10]) are described, whereas no cases of gluteus metastases have been reported.
Its management during pregnancy remains challenging. It is crucial to stop the disease progression as well as to overcome the hormonal imbalances after thyroidectomy to avoid fetal complications as a consequence of maternal hypothyroidism[11,12]. The actual standard of care for patients diagnosed with thyroid cancer is a total or near-total thyroidectomy either in the second trimester or after delivery. This treatment is followed by radioactive iodine administration (RAI), contraindicated during pregnancy, as an additional treatment for differentiated thyroid cancer (DTC)[13]. The RAI treatment, with the subsequent total loss of thyroid function and follow-up scintigraphy, is usually postponed to the neonatal period in order to avoid fetal congenital hypothyroidism. A deferred postpartum treatment does not seem to alter the prognosis of thyroid cancer. To our knowledge, patients who undergo postponed surgery should receive thyroid hormone suppression treatment (L-thyroxin) until the definitive surgical treatment[14,15]. It remains controversial to establish whether it is beneficial to postpone the treatment schedule in order to avoid early delivery or if a timely treatment should be mandatory.
Herein we report on an otherwise healthy pregnant woman who came to our attention with hip pain associated with a mass resulting as an FTC metastasis.
A 43-year-old pregnant woman, with no other comorbidities, was admitted at her 30th wk of gestation at our Institution for progressive pain in the right gluteal/iliac region.
Past history showed no smoking or alcohol consumption habits, neither allergies nor history of hypertension, diabetes mellitus, bronchial asthma, tuberculosis or neck swelling.
No hormonal fertility treatment had ever been performed on the patient who conceived naturally, carrying her first healthy pregnancy.
Physical examination showed a palpable lump of the right gluteus.
Blood tests revealed: thyroglobulin ≥ 10000 (normal value: 3-40 ng/mL), α-FP = 93.4 (normal value: < 6.0 ng/mL), β-hCG = 896 (post-partum) and calcitonin = 15.2 pg/mL (normal value: < 16 pg/mL).
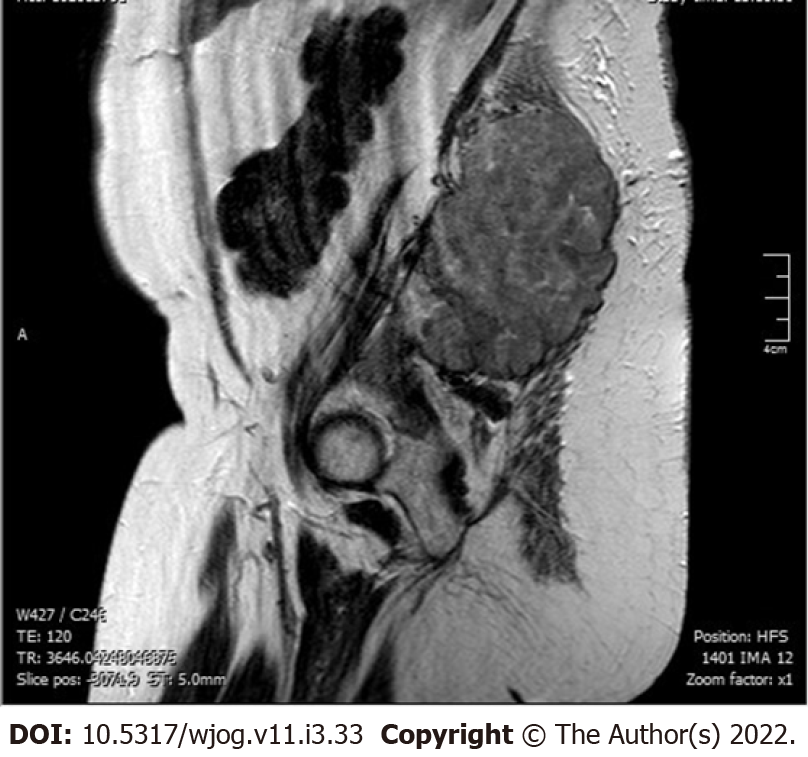
The abdominal and pelvic computed tomography (CT) scan and magnetic resonance imaging (Figure 1) revealed a solid polylobate mass of 7.3 cm × 7.9 cm × 11 cm with osteolytic involvement of the right portion of the sacrum, of the sacroiliac synchondrosis and of the contiguous iliac bone, extending to the soft tissues of the gluteus. The fetus was delivered via cesarean section at 35 wk of gestation without any issue reported concerning his wellbeing. After delivery, a total hysterectomy with bilateral adnexectomy and biopsies of the gluteal mass were performed.
Preoperative ultrasonography and CT scan showed a right thyroid lobe nodule with maximum axial diameter of 12 mm. No enlarged laterocervical, mediastinal, hilar and axillary lymph nodes were found whilst a 5.5 cm solid mass was detected within the liver parenchyma (4th and 8th segments).
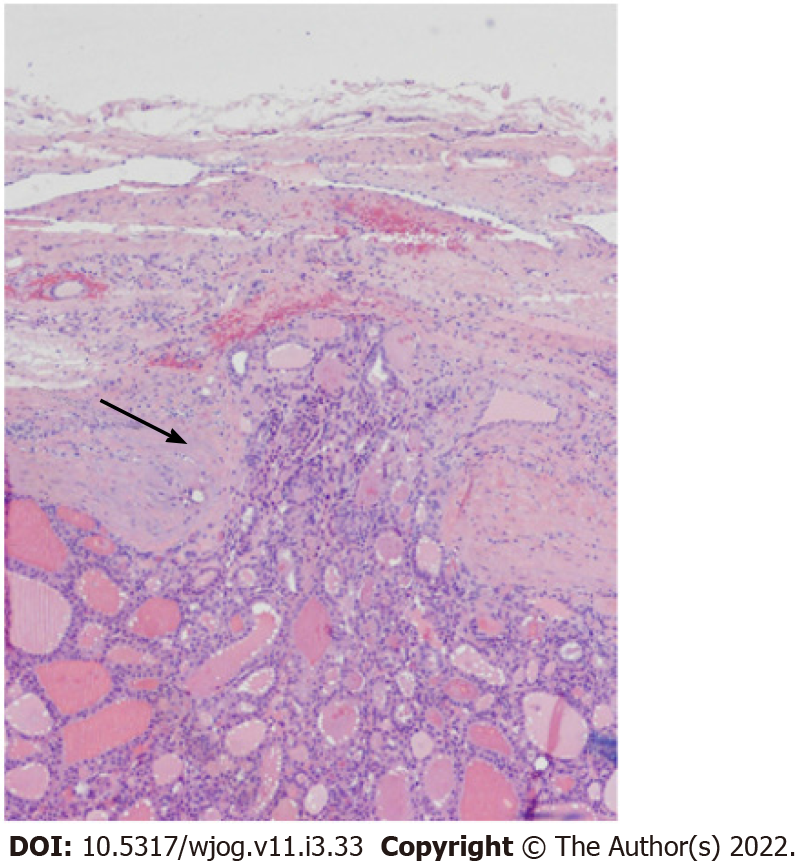
The histopathological examination confirmed differentiated epithelial follicular neoplasm by morphology and immunohistochemistry (CK+, TTF1+, thyroglobulin +) compatible with FTC metastasis (stage IV). To our knowledge, there have been no previous case reports of FTC in young pregnant patients presenting with gluteal and liver metastasis with no sign of thyroid symptoms.
Given these findings and the age of the patient, we opted for a total thyroidectomy and liver resection with cholecystectomy (Figure 2).
Because of the complex nature of the disease, 2 mo after the last surgery the patient underwent oral RAI. The first cycle (131-iodine, 3700 MBq dose) did not show the expected improvement. Therefore, it was decided to perform a second round of radioiodine treatment (131-iodine, 5550 MBq dose). Due to the non-resectability of the pelvic mass, 20 d after the RAI treatment, the patient underwent palliative radiotherapy with an external beam on D10 with a total dose of 2000 cGy in 5 fractions. Following radiotherapy, the right gluteal mass displayed an initial reduction with pain relief although after a few months relapsed. Therefore, it was considered to perform a vascular embolization leading to subtotal devascularization of the tumor.
A positron emission tomography (PET)/CT scan performed about 2 mo later showed the failure of this last procedure. Eventually, the patient died of cerebral hemorrhage 16 mo after the initial diagnosis.
Amongst all DTCs detected in women during their fertile age, about 10% are diagnosed during pregnancy or shortly after[16]. Female prevalence and increasingly age-specific incidence in women during the child-bearing period suggests a possible role of sexual hormones in the development of thyroid cancer, especially in cases of DTCs (papillary thyroid carcinoma and FTC). However, there is an ongoing debate about the role of pregnancy hormones with regard to the prognosis of DTC[17,18]. The pathophysiological framework of an increased risk of developing thyroid cancer and its progression in pregnant patients is still under debate. β-hCG and estrogen stimulation, increased vascularization and the absence of immune surveillance against cancer may be involved[19]. Hormonal stimulation during pregnancy might escalate the progression of thyroid cancer, suggesting that a more aggressive approach might be required in affected women[20-22]. Thyroid gland size normally increases by 30% during the first and third trimesters of pregnancy, and thyrotropin (TSH) levels fluctuate during pregnancy as they decrease during the first trimester to return to normal range during the following months[23].
β-hCG belongs to the subfamily of glycoprotein hormones, displaying a structural accordance both with TSH and its receptors. This similarity suggests the basis for β-hCG cross-reactivity with the TSH receptor[24]. β-hCG has a stimulating effect on the thyroid gland as it can be noted in gestational trophoblastic diseases that present with high levels of β-hCG and hyperthyroidism. Furthermore, β-hCG is the strongest stimulator of thyroid growth during the first trimester of pregnancy[25]. Therefore, in susceptible thyroid follicular cells (e.g., when BRAF and RAS mutations or RET/PTC and PAX8-PPAR-γ rearrangements occur), an excessive β-hCG stimulation may lead to rapid cancer progression[26].
Estrogen levels exert their effects through more complicated mechanisms. They have an indirect effect through increasing the serum thyroxine that binds globulin. A manifestation of their direct effect is estrogen receptor (ER) presentation on thyroid gland cells[27]. ERα and ERβ are intracellular nuclear receptors that exist in normal and neoplastic thyroid cells. When estradiol binds to ERα it enhances cell proliferation. On the contrary, ERβ inhibits these effects and leads to apoptosis[28,29]. Recent studies compared expression of ERα and ERβ in normal thyroid cells and malignant thyroid cells, revealing different levels of expression of ERα and a decreased ERβ activity in the latter[30,31].
The musculoskeletal system represents the most common localization for FTC metastases, which can develop in areas of high blood flow, like the red marrow of the axial skeleton, including the vertebrae (42%-52%), femur (9%-20%), skull (2%-16%) and pelvis (5%-13%)[32]. FTC usually presents itself as a single nodule, which can be either well defined or extensively infiltrating. Lymph node involvement is extremely rare[33]. Magnetic resonance imaging, CT, PET and scintigraphy could complete the diagnostic work-up to reveal metastases[34].
Surgery is the gold standard treatment for FTC. In all patients it is mandatory to balance risks against advantages of thyroid lobectomy with subsequent completion vs initial total thyroidectomy[33,35]. Thyroid cancer during pregnancy poses many challenges due to the need to carefully focus on both optimal timing for recommended treatments and the risks of cancer growth. The Endocrine Society recommends thyroidectomy following delivery for pregnancy-related DTC in patients showing no evidence of advanced disease or rapid progression. Meanwhile it is advisable to perform thyroidectomy during the second trimester of pregnancy in complicated cases. Lymph node dissection is not indicated in the absence of palpable lymph nodes[35-39]. Suppressive treatment with levothyroxine therapy is required after surgical treatment. Its aim is to keep TSH levels below 0.1-1 mU/L, with monthly monitoring of TSH and T4 levels.
However, if surgery is performed during pregnancy, levothyroxine therapy should promptly begin after surgery[39,40]. The post-surgical radio-ablation of the residual thyroid tissue facilitates the use of thyroglobulin detection and radioiodine scanning for long-term follow-up. Consequently, for patients at risk of recurrence and for those with known distant metastatic disease, 131I ablation may represent a valid therapeutic strategy[20]. Not all patients benefit from radioiodine therapy, and this treatment is contraindicated in pregnant and in breastfeeding women[30].
The presence of molecular pathway alterations in different DTC (RET/PTC rearrangements, RET mutations, BRAF mutations, RAS mutations and VEGFR-2 expression) has allowed the development of new selective drugs. Tyrosine kinase inhibitors are small organic compounds inhibiting tyrosine kinase autophosphorylation and activation; most of them are multikinase inhibitors. Tyrosine kinase inhibitors act on the aforementioned molecular pathways involved in growth, angiogenesis and local and distant spread of DTC and are emerging as a new approach for aggressive thyroid cancer[41].
β-hCG and estrogen stimulation might have a role in cancer growth, especially during pregnancy. FTC management aims to stop disease progression and overcome hormonal imbalances after thyroidectomy thus reducing fetal complications. It is still under debate whether it is possible to combine optimal timing for treatment to ensure the best possible outcome with reduction of fetal complications and risk of cancer growth.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chan SM, Taiwan; Tang J, China; Zhang YX, China; Zhao Y, China A-Editor: Liu X, China S-Editor: Zhang H L-Editor: Filipodia CL P-Editor: Zhang H
| 1. | Smith LH, Danielsen B, Allen ME, Cress R. Cancer associated with obstetric delivery: results of linkage with the California cancer registry. Am J Obstet Gynecol. 2003;189:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Budak A, Gulhan I, Aldemir OS, Ileri A, Tekin E, Ozeren M. Lack of influence of pregnancy on the prognosis of survivors of thyroid cancer. Asian Pac J Cancer Prev. 2013;14:6941-6943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Schlumberger M, Tubiana M, De Vathaire F, Hill C, Gardet P, Travagli JP, Fragu P, Lumbroso J, Caillou B, Parmentier C. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986;63:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 300] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 168] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Kim H, Kim HI, Kim SW, Jung J, Jeon MJ, Kim WG, Kim TY, Kim HK, Kang HC, Han JM, Cho YY, Kim TH, Chung JH. Prognosis of Differentiated Thyroid Carcinoma with Initial Distant Metastasis: A Multicenter Study in Korea. Endocrinol Metab (Seoul). 2018;33:287-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Ozdemir N, Senoğlu M, Acar UD, Canda MS. Skull metastasis of follicular thyroid carcinoma. Acta Neurochir (Wien). 2004;146:1155-8; discussion 1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Anil S, Lal PM, Gill DS, Beena VT. Metastasis of thyroid carcinoma to the mandible. Case report. Aust Dent J. 1999;44:56-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Hefer T, Manor R, Zvi Joachims H, Groisman GM, Peled M, Gov-Ari E, Laufer D. Metastatic follicular thyroid carcinoma to the maxilla. J Laryngol Otol. 1998;112:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Scarrow AM, Colina JL, Levy EI, Welch WC. Thyroid carcinoma with isolated spinal metastasis: case history and review of the literature. Clin Neurol Neurosurg. 1999;101:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Daumerie C, De Potter P, Godfraind C, Rahier J, Jamar F, Squifflet JP. Orbital metastasis as primary manifestation of thyroid carcinoma. Thyroid. 2000;10:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Mazzaferri EL. Approach to the pregnant patient with thyroid cancer. J Clin Endocrinol Metab. 2011;96:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Galofré JC, Riesco-Eizaguirre G, Alvarez-Escolá C; Grupo de Trabajo de Cáncer de Tiroides de la Sociedad Española de Endocrinología y Nutrición. Clinical guidelines for management of thyroid nodule and cancer during pregnancy. Endocrinol Nutr. 2014;61:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Khaled H, Al Lahloubi N, Rashad N. A review on thyroid cancer during pregnancy: Multitasking is required. J Adv Res. 2016;7:565-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Gibelli B, Zamperini P, Proh M, Giugliano G. Management and follow-up of thyroid cancer in pregnant women. Acta Otorhinolaryngol Ital. 2011;31:358-365. [PubMed] |
| 15. | Yu SS, Bischoff LA. Thyroid Cancer in Pregnancy. Semin Reprod Med. 2016;34:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Turner HE, Harris AL, Melmed S, Wass JA. Angiogenesis in endocrine tumors. Endocr Rev. 2003;24:600-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Kung AW, Chau MT, Lao TT, Tam SC, Low LC. The effect of pregnancy on thyroid nodule formation. J Clin Endocrinol Metab. 2002;87:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Sahin SB, Ogullar S, Ural UM, Ilkkilic K, Metin Y, Ayaz T. Alterations of thyroid volume and nodular size during and after pregnancy in a severe iodine-deficient area. Clin Endocrinol (Oxf). 2014;81:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Alves GV, Santin AP, Furlanetto TW. Prognosis of thyroid cancer related to pregnancy: a systematic review. J Thyroid Res. 2011;2011:691719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Longo DA, Anthony S. Fauci AS. Harrison's Principles of Internal Medicine. New York: McGraw-Hill, 2012. |
| 21. | Huang TC, Cheng YK, Chen TW, Hsu YC, Liu EW, Chen HH. A 'silent' skull metastatic follicular thyroid carcinoma mimicking as a benign scalp tumor in a pregnant woman. Endocrinol Diabetes Metab Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Yoshimura M, Hershman JM. Thyrotropic action of human chorionic gonadotropin. Thyroid. 1995;5:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 179] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | ACOG Practice Bulletin No. 144: Multifetal gestations: twin, triplet, and higher-order multifetal pregnancies. Obstet Gynecol. 2014;123:1118-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Dean DS, Hay ID. Prognostic indicators in differentiated thyroid carcinoma. Cancer Control. 2000;7:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Walkington L, Webster J, Hancock BW, Everard J, Coleman RE. Hyperthyroidism and human chorionic gonadotrophin production in gestational trophoblastic disease. Br J Cancer. 2011;104:1665-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Tafani M, De Santis E, Coppola L, Perrone GA, Carnevale I, Russo A, Pucci B, Carpi A, Bizzarri M, Russo MA. Bridging hypoxia, inflammation and estrogen receptors in thyroid cancer progression. Biomed Pharmacother. 2014;68:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Coelho RG, Fortunato RS, Carvalho DP. Metabolic Reprogramming in Thyroid Carcinoma. Front Oncol. 2018;8:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Gabriela V, Arciuch A, Di Cristofano A. Estrogen signaling and thyrocyte proliferation. In: Ward L. Thyroid and parathyroid diseases-new insights into some old and some new issues. London: IntechOpen, 2012. |
| 29. | Huang Y, Dong W, Li J, Zhang H, Shan Z, Teng W. Differential expression patterns and clinical significance of estrogen receptor-α and β in papillary thyroid carcinoma. BMC Cancer. 2014;14:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, Paschke R, Valcavi R, Vitti P; AACE/ACE/AME Task Force on Thyroid Nodules. American Association Of Clinical Endocrinologists, American College Of Endocrinology, And Associazione Medici Endocrinologi Medical Guidelines For Clinical Practice For The Diagnosis And Management Of Thyroid Nodules--2016 Update. Endocr Pract. 2016;22:622-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 758] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 31. | Zahid M, Goldner W, Beseler CL, Rogan EG, Cavalieri EL. Unbalanced estrogen metabolism in thyroid cancer. Int J Cancer. 2013;133:2642-2649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Mizoshiri N, Shirai T, Terauchi R, Tsuchida S, Mori Y, Saito M, Ueshima K, Kubo T. Metastasis of differentiated thyroid cancer in the subchondral bone of the femoral head: a case report. BMC Musculoskelet Disord. 2015;16:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Spinelli C, Rallo L, Morganti R, Mazzotti V, Inserra A, Cecchetto G, Massimino M, Collini P, Strambi S. Surgical management of follicular thyroid carcinoma in children and adolescents: A study of 30 cases. J Pediatr Surg. 2019;54:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1093] [Cited by in RCA: 999] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 35. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 9685] [Article Influence: 1076.1] [Reference Citation Analysis (1)] |
| 36. | Moosa M, Mazzaferri EL. Outcome of differentiated thyroid cancer diagnosed in pregnant women. J Clin Endocrinol Metab. 1997;82:2862-2866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 88] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Modesti C, Aceto P, Masini L, Lombardi CP, Bellantone R, Sollazzi L. Approach to thyroid carcinoma in pregnancy. Updates Surg. 2017;69:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Vannucchi G, Perrino M, Rossi S, Colombo C, Vicentini L, Dazzi D, Beck-Peccoz P, Fugazzola L. Clinical and molecular features of differentiated thyroid cancer diagnosed during pregnancy. Eur J Endocrinol. 2010;162:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Papini E, Negro R, Pinchera A, Guglielmi R, Baroli A, Beck-Peccoz P, Garofalo P, Pisoni MP, Zini M, Elisei R, Chiovato L; Italian Association of Clinical Endocrinologists; Italian Thyroid Association. Thyroid nodule and differentiated thyroid cancer management in pregnancy. An Italian Association of Clinical Endocrinologists (AME) and Italian Thyroid Association (AIT) Joint Statement for Clinical Practice. J Endocrinol Invest. 2010;33:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Messuti I, Corvisieri S, Bardesono F, Rapa I, Giorcelli J, Pellerito R, Volante M, Orlandi F. Impact of pregnancy on prognosis of differentiated thyroid cancer: clinical and molecular features. Eur J Endocrinol. 2014;170:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Ferrari SM, Fallahi P, Politti U, Materazzi G, Baldini E, Ulisse S, Miccoli P, Antonelli A. Molecular Targeted Therapies of Aggressive Thyroid Cancer. Front Endocrinol (Lausanne). 2015;6:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |