INTRODUCTION
The presence of oxidant and antioxidant systems in various reproductive tissues has evoked great interest in the role of oxidative stress (OS) in human reproduction. OS is defined as an elevation of levels of various reactive oxygen species (ROS) that exceeds the body’s antioxidant defenses[1]. It is generally accepted that an in vitro setup can never mimic the exact physiological conditions of an in vivo system. Multiple factors impinge on an in vitro fertilization (IVF) setting, leading to an increase in OS and suboptimal assisted reproductive technology (ART) outcome.
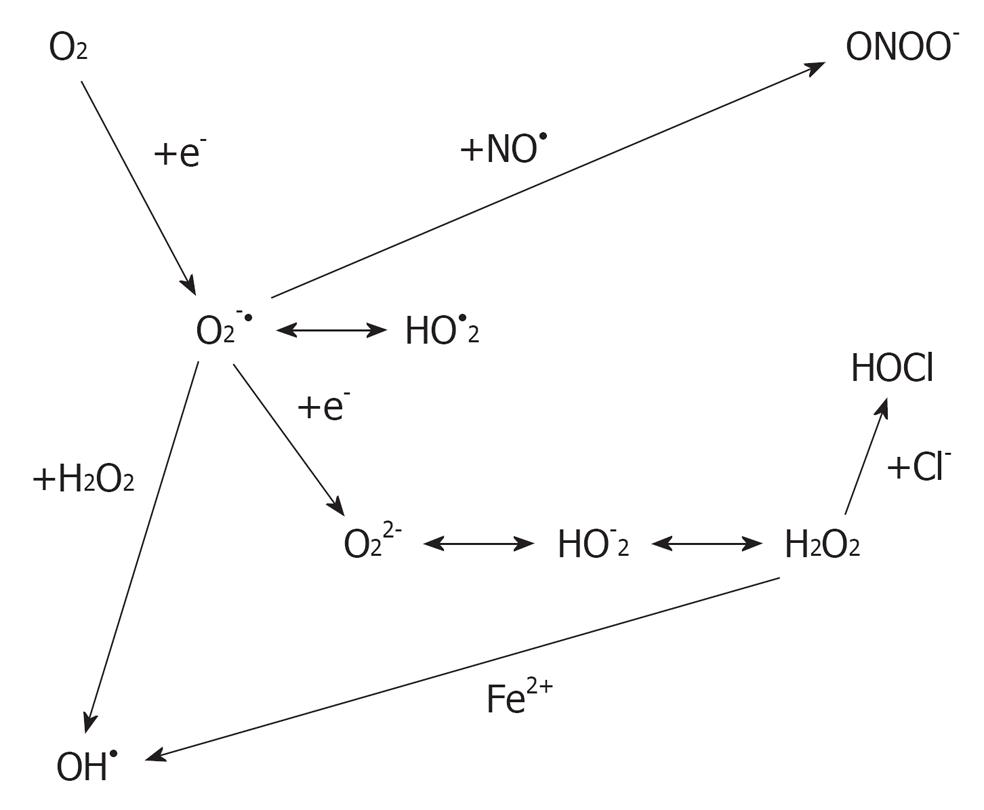
ROS are oxygen-derived molecules that act as powerful oxidants. Some of the ROS members, such as superoxide (O2.-), hydrogen peroxide (H2O2) and the hydroxyl radical (OH-) (Figure 1), are formed as intermediary products in low concentrations in the male and female genital tracts[2]. ROS has the ability to react with any molecule and modify it oxidatively, resulting in structural and functional alterations[3]. They need to be neutralized by an elaborate defense system consisting of enzymes, such as catalase, superoxide dismutase and glutathione peroxidase or reductase, and numerous non-enzymatic antioxidants, such as vitamin C, vitamin E, vitamin A, pyruvate, glutathione, taurine and hypotaurine[4].
Figure 1 Derivation of reactive oxygen species from oxygen.
The effects of OS in an ART setting may be amplified due to the lack of physiological defense mechanisms available and the number of potential sources of ROS at play. Sperm damage induced by OS includes membrane and DNA damage. The use in ART of spermatozoa that have been damaged may result in altered oocyte and/or embryo development.
ROS can be produced either intracellularly, originating from gametes, or extracellularly, from environmental factors. A potential source of ROS in the ART media is its generation during preparation of semen due to the activation of ROS production by immature spermatozoa by centrifugation, the absence of the antioxidant-rich seminal plasma, or contamination by leukocytes. Similarly, oocytes and embryos contribute to the increase in ROS levels because of their metabolism and the lack of the protective antioxidant mechanisms present in their natural habitat[5,6]. The external environment surrounding the ART procedure also plays an important role in the development of OS. The most important external factor that may affect gamete and embryo viability in vitro is oxygen partial pressure (pO2). Cells cultured in vitro are exposed to a relatively hypertoxic environment compared with the in vivo conditions. In addition to pO2, other physicochemical environmental factors such as temperature variation may also affect gamete and embryo development in vitro. Therefore, the external environment in the ART procedure can be a potential source of OS. Recently, studies have indicated the upper control limit of ROS in follicular fluid beyond which viable embryo formation is not favorable[7], as well as cut-off values for predicting semen quality and fertilization outcome[8].
Reactive nitrogen species such as nitric oxide (NO) have also been shown to play a role in reproduction. The production of NO is catalyzed by a family of NO synthase (NOS) enzymes[9]. NOS is responsible for the conversion of l-arginine to NO and l-citrulline[10]. Two NOS types have been identified in human spermatozoa: NOS, similar to the constitutively expressed brain neuronal NOS, and endothelial NOS[11]. The ability of human spermatozoa to synthesize NO has been demonstrated indirectly by measuring nitrite accumulation[12] and l-[3H] citrulline generation[13], or directly by means of an NO meter[11] and fluorescence activated cell sorting (FACS) analysis[14].
In vitro studies have shown contrasting results of exogenous NO yield on human sperm function, depending on the concentrations applied[15]. It has been reported that lower concentrations of NO are beneficial to human sperm function, whereas higher concentrations become detrimental[16]. This review is aimed at discussing sources of free radicals in an IVF setting and how to minimize their generation.
SOURCES OF FREE RADICALS IN AN IVF SETTING
Developing embryo
Like any other living aerobic cells, the developing embryo and the oocyte could be a major source of ROS because they use oxygen to produce energy through mitochondrial oxidative phosphorylation. This ROS is produced through several pathways, such as oxidative phosphorylation, nicotinamide adenine dinucleotide phosphate oxidase and xanthine oxidase systems[17]. Studies have reported that ROS production is increased in embryos cultured in vitro compared with those in vivo[5]. It is therefore recommended that embryo culture media should be supplemented with antioxidants that can scavenge the excess ROS that is generated by the developing embryo.
Spermatozoa and leukocytes
Spermatozoa and other cells in semen are huge sources of ROS. Morphologically abnormal and immature spermatozoa, together with the presence of leukocytes, can generate ROS in human ejaculates. The sperm generate ROS at the level of plasma membrane and mitochondria[18]. Studies have shown that human spermatozoa generate O2.-, which spontaneously dismutates to H2O2[19].
In the male genital tract and the ejaculate, ROS are not only derived from the spermatozoa but can also be generated by leukocytes, which physiologically produce up to 1000 times more ROS than spermatozoa[20,21]. This high ROS production by leukocytes plays a major role in infections, inflammation and cellular defense mechanisms. Basically, the cellular mechanisms for the generation of ROS in leukocytes and spermatozoa are the same, yet in leukocytes it is a physiological necessity to release large amounts of superoxide into phagocytic vesicles during the killing action of pathogens.
Considering the extraordinary high content of polyunsaturated fatty acids in their membrane, the sperm plasma membrane is particularly susceptible to OS and the double bonds of the membrane lipids can easily be oxidized by excessive ROS levels present in the sperm cells’ environment. These can either be produced in large amounts by leukocytes or the spermatozoa themselves. In the case of ROS attacking the plasma membrane lipids, a process called “lipid peroxidation” (LPO) is initiated. Ultimately, this process decreases membrane fluidity of both plasma and organelle membranes and, as a result, damages membrane function, ion gradients, receptor-mediated signal transduction, etc.[22]. Hence, with the loss of membrane function, spermatozoa lose the ability to function properly and therefore fertilization is impaired[23].
It is therefore advisable that patients with high leukocyte levels in their ejaculate (leukocytospermia) must have their spermatozoa quickly separated from the leukocytes to avoid ROS damage due to leukocyte activation. Supplementation with scavengers would also help to minimize OS.
Semen centrifugation
The removal of sperm from the seminal fluid is a very important step in the processing of semen during assisted reproductive procedures. Most sperm processing techniques employ the use of centrifugation to separate motile sperm from non-motile or dead sperm, as well as other contaminating cell debris[24]. However, sperm processing techniques that employ the use of centrifugation have their own disadvantages. Studies have shown that sperm preparation, processing and handling lead to increased free radical generation[25]. We have also shown in our previous study that centrifuging spermatozoa for more than 10 min led to an increase in ROS generation[26]. On the other hand, we demonstrated that 10 min of sperm centrifugation also increased NO generation, whereas 30 min of centrifugation led to a decrease in NO generation. The decrease in NO generation after 30 min of centrifugation was probably due to NOS enzyme down-regulation or loss. Alternatively, it could be that NO reacted with other members of the ROS family such as O2- to form peroxynitrite anion (ONOO-) since NO has been shown to possess antioxidant abilities[27].
It is therefore recommended that sperm separation techniques should avoid using centrifugation or prolonged centrifugation in assisted reproductive technologies. The addition of ROS scavengers prior to centrifugation could also help in reducing free radical generation and therefore improving sperm recovery.
Oxygen partial pressure
The pO2 in the laboratory environment is very different from that of the in vivo condition. It is higher in the in vitro laboratory setting; thus the higher pO2 activates various oxidase enzyme systems in the cells and contributes to increased ROS generation[28]. Gametes and the media in the IVF setting should therefore not be exposed to high pO2 to prevent excess ROS generation.
Light
Visible light has been reported to be another contributor that leads to increased ROS generation. Light induces photodynamic stress, leading to oxidative damage of unsaturated lipids and sterols within the membranes[29]. Studies have shown that exposing mouse embryos to transient visible light led to an increase in the generation of H2O2[5]. Therefore, gametes meant for use during assisted reproduction should not be exposed to direct light to avoid excess generation of free radicals.
Culture media
Different types of culture media are used in ART laboratories. These media have different ingredients which can lead to ROS generation. In some culture media there are metallic ions such as Fe2+ and Cu2+ which have the potential of accelerating ROS generation within the cell by participating in Fenton and Haber-Weiss reactions[17]. Furthermore, media additives such as serum albumin contain high levels of amine oxidase which leads to an increase in H2O2 production[30].
It is therefore recommended that culture media used in IVF settings should be supplemented with antioxidants. Antioxidants such as urate, isoflavones, ascorbic acid, taurine, hypotaurine, genistein and α-tocopherol can be used as culture media supplements to minimize the risk of OS and damage it causes to the gametes[4,31]. Vitamin E supplementation has also been shown to protect gametes from the LPO[32]. In order to prevent ROS generation by metallic ions found in culture media, metal chelators can be added to the media.
Cryopreservation-thawing
Many studies have reported that cryopreservation enhances LPO through ROS induced membrane lipid damage[33]. Cryopreserved sperm has been reported to be susceptible to ROS insults since they tend to lose their antioxidant defenses[34]. It is therefore recommended that improved cryopreservation protocols that optimize the use of cryoprotectants should be used to improve the quality of cryopreserved gametes.
MEASURING FREE RADICALS
There are different techniques that are employed to measure free radicals. An ideal assay for ROS detection should be sufficiently sensitive to ensure that measurements are within the linear range of the assay and well above the limits of detection. Preferably, it should be specific for one ROS, at least in physiological/pathophysiological concentrations. Its signal should be substantially inhibited by a specific scavenger of the ROS in question. It should be robust; that is, applicable to a wide variety of experimental conditions and comparable between these applications. However, ROS production can be highly localized and therefore most assays will not be able to detect ROS in all of the subcellular locations and extracellularly. Furthermore, the concentration of ROS at the site of production could be extremely high and this almost certainly will not be reflected by measurements obtained in intact tissues, cells or in the media in which tissues are incubated. Finally, it is very unlikely that any probe will react with all of the ROS produced, meaning that any assay cannot be viewed as completely quantitative.
Cytochrome C reduction
Oxygen is a diradical, possessing two unpaired electrons. When oxygen gains an additional electron, O2·- is formed. Superoxide is generally considered an oxidant because in most cases it extracts an electron from another molecule, leading to formation of H2O2. For other molecules with higher electron potentials, O2·- actually donates its extra electron. This is the case for ferricytochrome c, which is reduced to ferrocytochrome c by receiving an electron from O2·-. When ferricytochrome c is reduced, its spectrophotometric absorbance is altered in a very specific fashion. Absorbance at 550 nm is increased, whereas absorption at 540 and 560 nm remain unaltered and can serve as an isosbestic point. Unfortunately, ferricytochrome c can be directly reduced by electrons donated from enzymes and other molecules, so this change in absorbance is not specific for O2·-. For this reason, the assay must be performed in the presence and absence of SOD and only the SOD-inhibitable signal used to calculate the amount of O2·- formed.
Procedure: For measurement of O2·-, it is necessary to divide the cells in two aliquots of equal concentrations. Cells are placed in a buffer composed of (mmol/L): NaCl, 145; KCl, 4.86; NaH2PO4, 5.7; CaCl2, 0.54; MgSO4, 1.22; glucose, 5.5; deferoxamine mesylate, 0.1; and 50 μmol/L of acetylated ferricytochrome c. Another aliquot of cells is added to a similar buffer containing manganese superoxide dismutase (100 U/mL). The cells are incubated in a 96-well plate to minimize the volume and thus maximize the concentration of O2·- released from the cells exposed to ferricytochrome c. Acetylation of cytochrome c improves quantification and specificity for O2·- because it strongly inhibits direct enzymatic reduction, improves stability of the reduced cytochrome c, and has minimal effects on reaction with O2·-[35,36]. To further ensure that any reduced ferricytochrome c is not reoxidized, catalase (125 U/mL) is added to the reaction, which removes any H2O2 formed. The cells are incubated in these reaction mixtures for 30 min at 37 °C. The cells are then removed and the buffer absorbances measured at 540, 550 and 560 nm using a 96-well plate reader.
Chemiluminescence-based techniques
On exposure to O2·-, chemiluminescent probes release a photon, which in turn can be detected by a scintillation counter or a luminometer. Because most of these compounds are cell permeable, the O2·- measured reflects extracellular, as well as intracellular, O2·- production. The most commonly used chemiluminescence technique for measurement of O2·- is lucigenin-enhanced chemiluminescence.
Procedure: For the assay, a Krebs/HEPES buffer containing 5 μmol/lucigenin is prepared. It has been found that the results are most consistent if this buffer is “dark adapted.” The buffer is placed in either a luminometer or scintillation counter set to the out-of-coincidence mode and incubated until background counts have stabilized. The cells are then added to the reaction vial and, after 15 min of equilibration, counts are obtained every minute for the next 5 min and averaged. Counts can be expressed as chemiluminescence units.
This method has been widely used and an enormous amount of new information has been gained from its use. It is reasonably specific for O2·- and one does not need to routinely prepare a second sample with SOD to prove that the signal is derived from O2·-. If one chooses to use an O2·- scavenger, it should be kept in mind that lucigenin penetrates cells and therefore unmodified SOD, which is not cell permeable, will not reduce the lucigenin signal completely (usually ≤ 50%). The assay is also inexpensive and can be performed using equipment that is commonly available.
Detection of intracellular H2O2 with DCF-DA
One of the most commonly used probes for the detection of intracellular ROS formation is 2’7’-dichlorofluorescein diacetate (DCFH-DA)[37-39]. DCFH-DA is cell permeable and after uptake it is cleaved by intracellular esterases to 2’,7’-dichlorofluorescin (DCFH), trapped within the cells and oxidized to the fluorescent molecule 2’,7’-dichlorofluorescein (DCF) by a variety of ROS[40]. DCFH-DA is considered a general indicator of ROS, reacting with H2O2, ONOO-, lipid hydroperoxides and, to a lesser extent, O2·-. Because H2O2 is a secondary product of O2·-, DCF fluorescence has been used to implicate O2·- production.
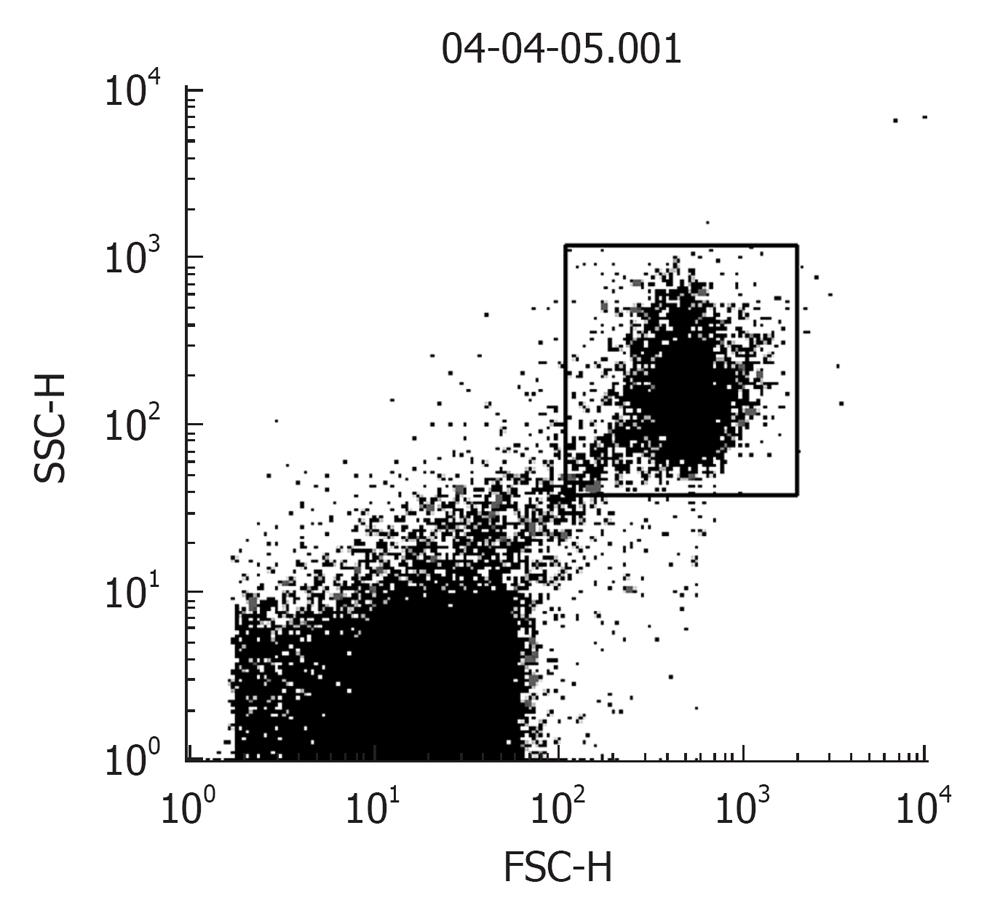
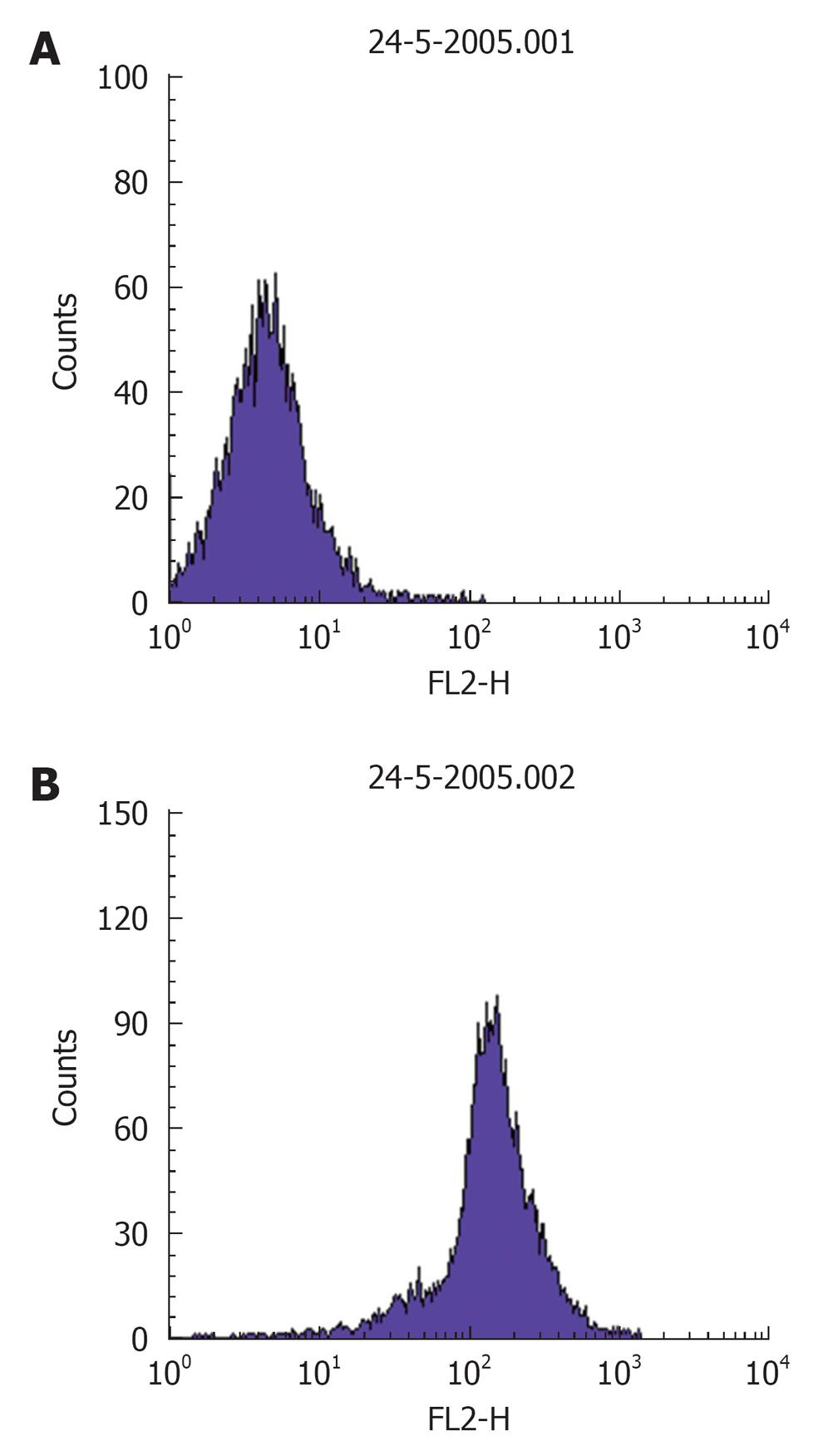
Procedure: For in situ localization of H2O2, cells are incubated with DCFH-DA (5 μmol/L; Molecular Probes)[41] for 30 min at 37 °C[42]. H2O2 detection is confirmed by simultaneously treating replicate cells with polyethylene glycol-catalase (350 U/mL). Cells incubated with vehicle can serve as negative controls. All of the images are acquired at identical settings. DCFH-DA fluorescence can also be measured using fluorescence-activated cell sorter analysis. A Becton Dickinson FACSCalibur™ analyzer can be used to quantify fluorescence at a single cell level and data analyzed by using CellQuest™. In each sample, the mean fluorescence intensity of the analyzed cells is determined after prior gating the cell population by forward and side light scatter signals, as recorded on the dot plot (Figure 2). In total, 100 000 events can be acquired but non-sperm particles and debris (located on the bottom left corner of the dot plot) are excluded by prior gating, thereby limiting undesired effects on overall fluorescence. The fluorescence signals are recorded on a frequency histogram (Figure 3A and B) using logarithmic amplification. Fluorescence data is expressed as mean fluorescence (percentage of control, control adjusted to 100%).
Figure 2 A dot plot of sperm cells showing the spread of the total recorded “events”.
Gated population (top right): sperm cells and bottom left: non-sperm particles, debris.
Figure 3 A representative frequency histogram showing baseline fluorescence (log) of DCFH on x-axis (A) and a shift to right depicting an increase in fluorescence intensity (B).
Detection of extracellular H2O2 with Amplex Red
The Amplex Red assay involves measurement of H2O2 by the horseradish peroxidase–catalyzed oxidation of the colorless and nonfluorescent molecule N-acetyl-3,7-dihydroxyphenoxazine (Amplex Red) to resorufin, which, when excited at 530 nm, strongly emits light at 590 nm[43].
Procedure: The cells are placed in the well of a 96-well plate and incubated with Amplex Red (10 μmol/L) and horseradish peroxidase (0.2 U/mL) for 60 min at 37 °C in Krebs Ringer’s phosphate glucose buffer (145 mmol/L of NaCl, 5.7 mmol/L of sodium phosphate, 4.86 mmol/L of KCl, 0.54 mmol/L of CaCl2, 1.22 mmol/L of MgSO4 and 5.5 mmol/L of glucose) protected from light. The cells are removed from the buffer and the buffer’s fluorescence is detected at 590 nm using an excitation 530 nm. Background fluorescence, determined using a reaction without cells, is subtracted from each value. H2O2 release, calculated using H2O2 standards or standard solutions of resorufin, is expressed as picomoles per concentration of cells[44]. In some experiments, SOD (40 U/mL) or catalase (200 U/mL) can be added to the cells.