Copyright
©2013 Baishideng Publishing Group Co.
World J Obstet Gynecol. Nov 10, 2013; 2(4): 143-152
Published online Nov 10, 2013. doi: 10.5317/wjog.v2.i4.143
Published online Nov 10, 2013. doi: 10.5317/wjog.v2.i4.143
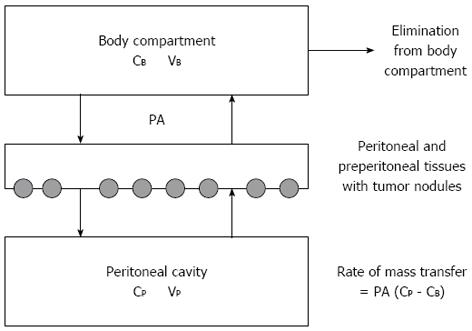
Figure 1 Three-compartment model of peritoneal transport in which transfer of a drug from the peritoneal cavity to the blood occurs across the peritoneal membrane and preperitoneal tissues.
In these tissues the peritoneal surface cancer nodules are located. The permeability-area product (PA) governs this transfer and can be calculated by measuring the rate of drug disappearance from the cavity and dividing by the overall concentration difference between the peritoneal cavity and the blood (B). CB: The free drug concentration in the blood (or plasma); VB: Volume of distribution of the drug in the body; CP: The free drug concentration in the peritoneal fluid; VP: Volume of the peritoneal cavity. Modified from Dedrick et al[16].
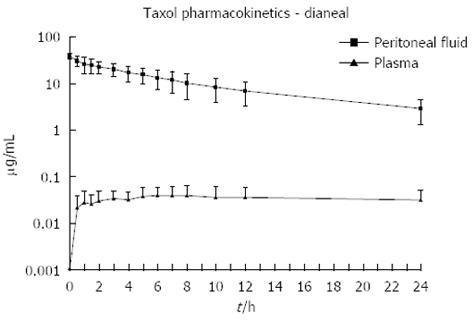
Figure 2 Pharmacokinetic study of concentration versus time for intraperitoneal paclitaxel.
The chemotherapy agent at 30 mg/m2 was instilled directly into the peritoneal cavity as rapidly as possible in a 1.5% dextrose peritoneal dialysis solution. The concentration of paclitaxel was determined in peritoneal fluid and in plasma for 24 h[51].
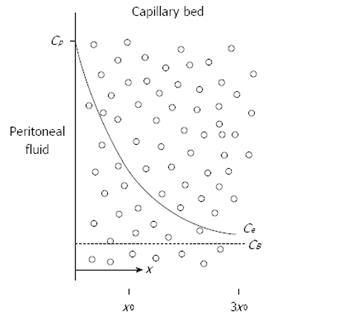
Figure 3 Conceptual diagram of tissue adjacent to the peritoneal cavity.
Solid line shows the exponential decrease in the free tissue interstitial concentration, Ce, as the drug diffuses down the concentration gradient and is removed by loss to the blood perfusing the tissue. Also shown are the characteristic diffusion length, x0, at which the concentration difference between the tissue and the blood has decreased to 37% of its maximum value, and 3x0, at which the difference has decreased to 5% of its maximum value. Cp: The free drug concentration in the peritoneal fluid; CB: The free drug concentration in the blood (or plasma). Modified from Dedrick et al[16].
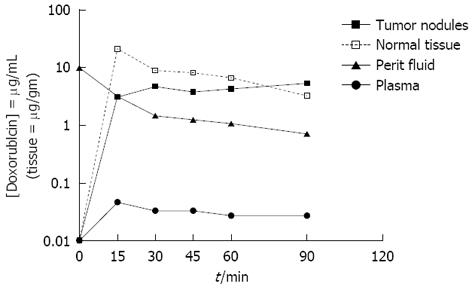
Figure 4 Doxorubicin levels in tumor nodules, normal adjacent tissues, peritoneal fluid and plasma during hyperthermic intraperitoneal peroperative chemotherapy over 90 min with 15 mg/m² doxorubicin intraperitoneal administration.
Modified from Van der Speeten et al[32].
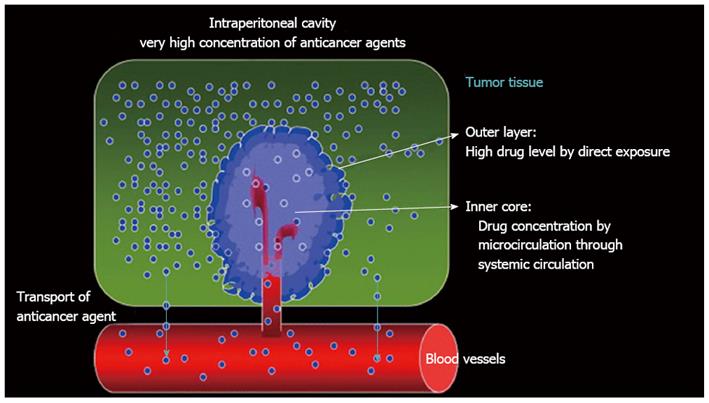
Figure 5 Pharmacologic concept of bidirectional intravenous and intraperitoneal chemotherapy.
Modified from Fujiwara et al[69].
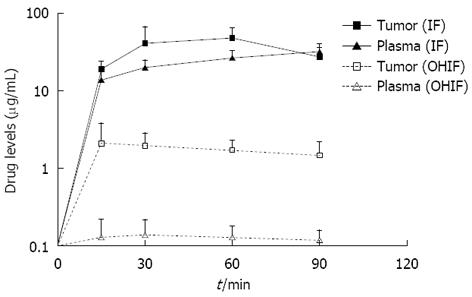
Figure 6 Comparison of ifosfamide and 4-hydroxyifosfamide concentrations in tumor nodules and in plasma during bidirectional intraoperative chemotherapy.
OHIF: 4-OH-ifosfamide.
- Citation: Speeten KVD, Stuart AO, Sugarbaker PH. Pharmacology of cancer chemotherapy drugs for hyperthermic intraperitoneal peroperative chemotherapy in epithelial ovarian cancer. World J Obstet Gynecol 2013; 2(4): 143-152
- URL: https://www.wjgnet.com/2218-6220/full/v2/i4/143.htm
- DOI: https://dx.doi.org/10.5317/wjog.v2.i4.143