Peer-review started: August 15, 2022
First decision: October 21, 2022
Revised: November 1, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 15, 2023
Processing time: 149 Days and 8.4 Hours
The sympathetic nervous system makes medium and large peripheral arteries smaller to slow the blood flowing through them.
To observe brachial artery sympathetic innervation.
We developed a neurophysiological autonomous test that measured the effects of peripheral sympathetic fibres on peripheral arteries. Our specific objective was to find the sympathetic innervation of the brachial artery. To accomplish this purpose, the brachial artery baseline diameter and flow rate were measured in the right arm of the patients. Afterwards, electrical stimulus was applied to the medial nerve for 5 s. Through electrical sympathetic activation, the vessel diameter and overall flow rate will decrease. After 7 d, a similar experiment was repeated using the ulnar nerve.
The differences in diameter and flow rate of the brachial artery in response to median and ulnar nerve activation were compared. In the total group, no significant difference in diameter was seen between medial and ulnar nerve stimulation (P = 0.648). The difference in absolute slowdown of flow rate between median nerve stimulation and ulnar nerve stimulation was not statistically significant for the entire group (P = 0.733).
As a target organ, the brachial artery receives an equal amount of sympathetic innervation from the median and the ulnar nerves.
Core Tip: These observations imply that a peripheral nerve injury causes vasomotor paralyses and higher blood flow in large and medium arteries of extremities. Conversely, physiological activation of a peripheral nerve causes lower blood flow. Our study depended on this pathophysiological background and aimed to investigate the anatomical innervation of the brachial artery by utilizing a neurophysiological experiment. As a target organ, the brachial artery receives an equal amount of sympathetic innervation from the median and ulnar nerves. Expanding similar neurophysiological research to other arteries in the upper and lower limbs will contribute to the advancement of the field of functional neuroanatomy.
- Citation: Ege F, Kazcı O. Brachial arteries sympathetic innervation: A contribution to anatomical knowledge. World J Neurol 2023; 9(1): 1-7
- URL: https://www.wjgnet.com/2218-6212/full/v9/i1/1.htm
- DOI: https://dx.doi.org/10.5316/wjn.v9.i1.1
The sympathetic nervous system is primarily responsible for controlling the peripheral arteries[1]. However, there is insufficient information to determine which artery receives more postganglionic sympathetic innervation from which peripheral nerve. To our knowledge, there are only two post-mortem reports that compare the sympathetic fibre intensity of the median and ulnar nerves. The first report by Morgan et al[2] was a dark fluid fluorescence microscopic study; according to them, the sympathetic fibre density of the median nerve was higher than the ulnar nerve, particularly at the elbow level. The second report by Balogh et al[3] was a immunohistochemistry-assisted investigation; their findings indicated that there was no substantial variation in sympathetic fibre distribution between the median and ulnar nerves. While these two studies examined the density of sympathetic fibres of median and ulnar nerves, they could not make a claim about the effects of these fibres on target organs because such evidence (e.g., specific proof intended for postganglionic innervation of the brachial artery) can only be demonstrated through functional autonomic tests.
The data presented here was composed of a subgroup from a larger unpublished and ongoing study where we are assessing the sensitivity of the brachial artery to sympathetic nervous system activity. It is well known in this field that the sympathetic nervous system narrows the diameter of medium and large peripheral arteries and decreases their blood flow[4]. Based on this knowledge, we developed a model that was analogous to the sympathetic skin response, in which the sympathetic sudomotor response was assessed via electrical stimulation of a peripheral nerve[5]. However, unlike the sympathetic skin response, this study did not assess the sudomotor but rather the vasomotor response by utilizing a Doppler ultrasound and focal electrical stimulation of a peripheral nerve.
With the aid of a neurophysiological autonomous test, our primary objective was to determine which peripheral nerve the brachial artery received more intense sympathetic innervation from.
This study conformed to the current version of the Declaration of Helsinki, and the protocols were authorized by the Clinical Research Ethics Committee at Ankara City Hospital No. 2 (Ethics approval No. E2-22-1307). This report followed the guidelines that are described in the “Preliminary Minimum Reporting Requirements for In-Vivo Neural Interface Research: Implantable Neural Interfaces”[6].
Subjects with polyneuropathy, median or ulnar neuropathy, peripheral artery diabetes mellitus, essential hypertension, and cardiac disease were excluded. Individuals experiencing numbness, paraesthesia, or discomfort in their hands were checked for a peripheral nerve injury 24 h prior to the test using a nerve conduction study in the electrophysiology laboratory. Subjects with abnormal values were also excluded. All of the study participants signed an informed consent.
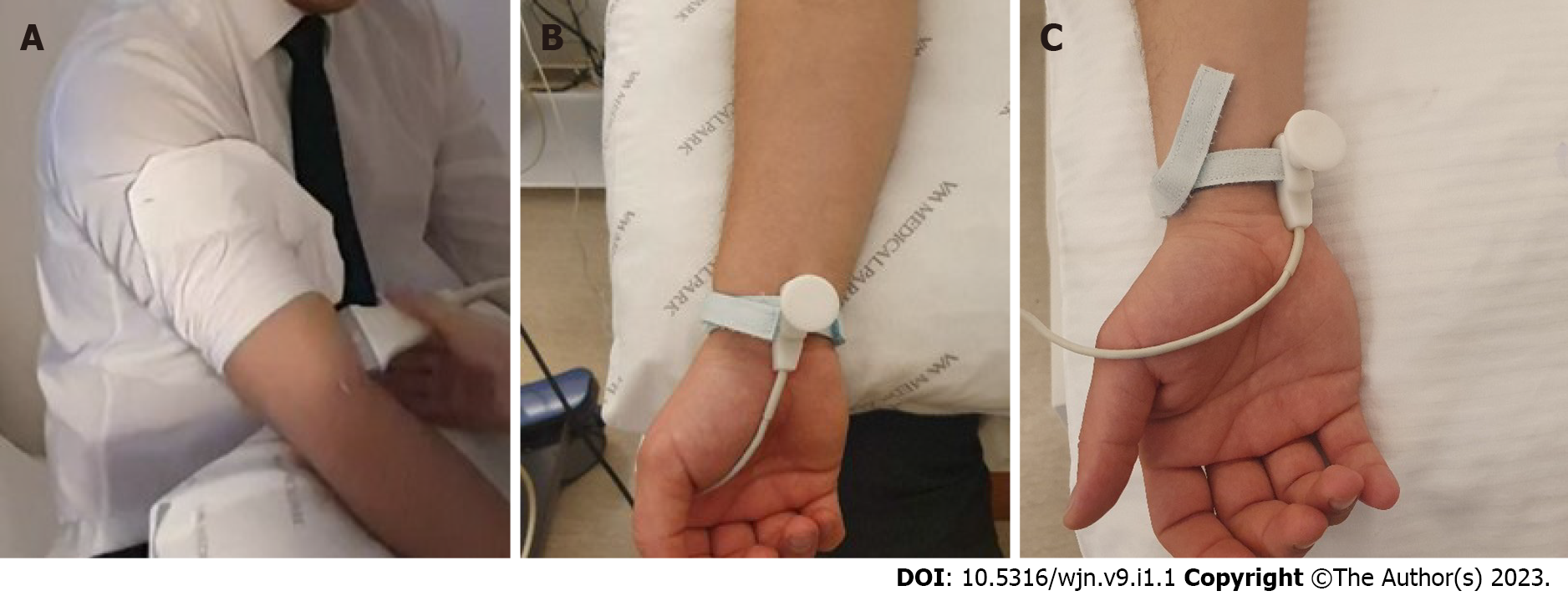
Nicotine, caffeine, alcohol, and exercise were restricted for 6 h prior to the test. The study was conducted at room temperature (22 °C-24 °C). For 5 min, participants rested in a seated position with their forearms supinated. Afterwards, blood pressure, heart rate, and body temperature were measured; subjects who had abnormal values were excluded at this stage, including 1 male subject with high blood pressure (> 135/85 mmHg). A radiologist with 6 years of expertise in the field of Doppler imaging measured the right brachial artery baseline diameter and flow rate 2 cm above the antecubital fossa using a 9 Hz linear probe from a General Electric LOQIC P2 USG Doppler equipment (Figure 1A). The position of the probe was not changed, and images were continually obtained. Then, for 5 s, a 1 Hz electrical stimulus with an intensity of 10 mA was applied to the median nerve at the wrist level in the direction of the sensory fibres (orthodromic way) using the bipolar stimulus electrode from the Nihon Kohden MEB-9400A EMG/EP system (Figure 1B). On the fifth second of the electrical current, the diameter and flow rate of the brachial artery were measured once again. Through electrical sympathetic activation, the vessel diameter and overall flow rate decreased. We recorded the baseline and post-stimulus data from the brachial artery. After 7 d, the baseline diameter and flow rate measurements were repeated on the right arm with the same intensity of electrical current supplied to the ulnar nerve (Figure 1C). On the fifth second, images from the brachial artery were recorded once more. The differences in the diameter and flow rate of the brachial artery in response to median and ulnar nerve activation were compared.
Frequency distributions for categorical variables and medians (min-max) for the numerical variables were presented. The Mann-Whitney U test was used to compare numerical variables between independent groups. For two of the dependent groups, numerical comparisons were made using the Wilcoxon test.
A P value of less than 0.05 was considered a statistically significant result. A statistical analysis was performed using the Statistical Package for Social Sciences for Windows software version 20.0. developed in Armonk, NY.
Females accounted for 46.7% of participants. All participants were between the ages of 20-years-old and 51-years-old, with a median age of 29-years-old.
The median flow rate of the brachial artery before the median nerve stimulus for the entire group was 79.80 (32.90-201.10) mL/min, whereas the post-stimulus flow rate was 44.00 (10.90-103.00) mL/min. This flow rate reduction was statistically significant (P = 0.001) (Table 1). The absolute decrease in the flow rate following the stimulus was 39.40 (19.90-124.50) mL/min on average. When the flow rate before the stimulus was compared to the flow rate after the stimulus, a median drop of 50.25% (28.97-71.84) was recorded.
| n | Median | Min | Max | P value | |
| Median pre-stimulation flow rate (mL/min) | 15 | 79, 80 | 32, 9 | 201, 1 | 0.001 |
| Median afer-stimulation flow rate (mL/min) | 15 | 44 | 10, 9 | 103 |
For the entire group, the median diameter of the brachial artery before medial nerve stimulus was 0.35 (0.26-0.50) cm, whereas the post-stimulus diameter was 0.31 (0.16-0.45) cm. The difference in diameter prior to and following the stimulus was determined to be statistically significant (P = 0.001) (Table 2). With stimulation, the absolute change in diameter was 0.05 (0.01-0.14) cm, and the percentage change in comparison to the original diameter was 14.63% (2.86-4.67).
| n | Median | Min | Max | P value | |
| Median pre-stimulation diameter of the brachial artery (cm) | 15 | 0.35 | 0.26 | 0.5 | 0.001 |
| Median after-stimulation diameter of the brachial artery (cm) | 15 | 0.31 | 0.16 | 0.45 |
For the entire group, the median flow rate of the brachial artery before ulnar nerve stimulus was 56.40 (28.10-214.00) mL/min, whereas the post-stimulus flow rate was 30.00 (15.00-78.00) mL/min. The difference between the two was statistically significant (P = 0.001) (Table 3). After ulnar nerve stimulation, the absolute decrease in flow rate was determined to be 25.10 (7.90-170.10) mL/min, whereas the percentage decrease was 48.08% (20.84-79.67).
| n | Median | Min | Max | P value | |
| Ulnar pre-stimulation flow rate (mL/min) | 15 | 56, 40 | 28, 10 | 214 | 0.001 |
| Ulnar after-stimulation flow rate (mL/min) | 15 | 30 | 15 | 78 |
The median diameter of the artery prior to ulnar nerve stimulation was 0.36 (0.23-0.50) cm, whereas the median diameter following the stimulus was 0.28 (0.19-0.48) cm. The difference in diameters prior to and following the stimulus was determined to be statistically significant (P = 0.001) (Table 4). With stimulation, the median absolute decrease in diameter was 0.04 (0.00-0.14) cm, and the median percentage decrease in diameter relative to the initial diameter was 17.14% (0.00-38.89) for the total cohort. Table 4 shows the diameter of the brachial artery (cm) before and after ulnar nerve stimulation for the entire group.
| n | Median | Min | Max | P value | |
| Ulnar pre-stimulation diameter of the brachial artery (cm) | 15 | 36 | 23 | 50 | 0.001 |
| Ulnar after-stimulation diameter of the brachial artery (cm) | 15 | 28 | 19 | 48 |
The difference in absolute decrease of flow rate between median nerve stimulation and ulnar nerve stimulation was not statistically significant for the entire group (P = 0.733). There were no statistically significant variations observed when the same assessment was stratified by female and male. Males and females were found to have P = 0.237 and 0.327, respectively (Table 5).
| Absolute change in flow rate with median stimulation (mL/min) | Absolute change in flow rate with ulnar stimulation (mL/min) | ||
| Entire group (n = 15) | Median | 3940 | 2510 |
| Min | 1990 | 790 | |
| Max | 12450 | 17010 | |
| Female (n = 7) | Median | 3090 | 2270 |
| Min | 2040 | 790 | |
| Max | 6010 | 5300 | |
| Male (n = 8) | Median | 4130 | 6865 |
| Min | 1990 | 1210 | |
| Max | 12450 | 17010 |
For the total group, no significant difference in diameter change was seen between medial and ulnar nerve stimulation (P = 0.648) for either sex (P = 0.610 for males and P = 0.833 for females) (Table 6).
| Change in diameter with a median stimulation (cm) | Change in diameter with an ulnar stimulation (cm) | P value | ||
| Entire group (n = 15) | Median | 5 | 4 | 0.648 |
| Minimum | 1 | 0 | ||
| Maximum | 14 | 14 | ||
| Female (n = 7) | Median | 8 | 6 | 0.610 |
| Minimum | 2 | 0 | ||
| Maximum | 14 | 14 | ||
| Male (n = 8) | Median | 4 | 4 | 0.833 |
| Minimum | 1 | 1 | ||
| Maximum | 11 | 8 |
It is well established that the sympathetic fibres in the median nerve exert control over the flow parameters of the arteries in the upper extremities. For example, Badal et al[7] discovered that medial nerve blockage increased radial artery peak velocity. According to Galea et al[8] triggering sympathetic activation induced by coughing, followed by deep inspiration, does not significantly increase the Pulsatile Index of the radialis indicis artery in patients with carpal tunnel syndrome compared to healthy controls. Similarly, Ghasemi-Esfe et al[9] showed that people with carpal tunnel syndrome have a Pulsatile Index value for the radialis indicis artery that is much lower than the healthy controls.
These observations imply that a peripheral nerve injury causes vasomotor paralysis and higher blood flow to the large and medium arteries of the extremities; conversely, physiological activation of a peripheral nerve causes lower blood flow. Our study depended on this pathophysiological background and aimed to investigate the anatomical innervation of the brachial artery by utilizing a neuro
Campero et al[10] reported a study based on invasive approaches that was scientifically similar to our work; according to their findings, the median and ulnar nerves blockage resulted in cutaneous vasodilatation at the respective innervation areas, but a similar result was not observed for radial nerve blockage. On the other hand, the approach we developed was non-invasive and generated local sympathetic discharge through peripheral nerve stimulation, allowing for rapid, practical, and reproducible measurements of vasomotor responses. This advantage has made comparative investigations in neurophysiology and functional anatomy easier. Our findings indicated that stimulating both the median and ulnar nerves produced identical changes in the diameter and flow rate of the brachial artery and corroborated the assumption that the median and ulnar nerves exert equal control and equally innervate the brachial artery. This result may have impact not just on the neurophysiological field but also on vascular surgery investigations in the future. On the other hand, the limitation of this study was the small sample size, and further studies in larger sample sizes are needed.
Our results also suggested that unlike muscles and sensorial skin areas the vasomotor control of some large arteries was supplied by several peripheral nerves. However, when small arteries are considered, target organs are separated once again. This assumption was supported by two reports that demonstrated that the medial nerve was the single supplier of the vasomotor control of the medial and radial hand areas, the ulnar nerve is the single supplier of the ulnar hand area, and the radial nerve does not have a meaningful impact on arterial sympathetic control of the hand[10,11]. However, current research in this field has been limited, and further neurophysiological or interventional studies are needed to determine the neural-vascular liaisons of the extremities in more detail.
As a target organ, the brachial artery receives an equal amount of sympathetic innervation from the median and ulnar nerves. Expanding similar neurophysiological research to other arteries in the upper and lower limbs will contribute to advancing the field of functional neuroanatomy.
The sympathetic nervous system is primarily responsible for controlling the peripheral arteries. It decreases the size of medium and large peripheral arteries to slow the blood flow rate.
There is insufficient information to determine which artery receives more postganglionic sympathetic innervation from which peripheral nerve.
To determine if the ulnar and median nerves contribute equally to sympathetic stimulation of the brachial artery.
We developed a neurophysiological autonomous test that measured the effects of peripheral sympathetic fibres on peripheral arteries. The brachial artery baseline diameter and flow rate were measured in the right arm of the patients. Afterwards, electrical stimulus was applied to the medial nerve for 5 s. Through electrical sympathetic activation, the vessel diameter and overall flow rate will decrease. After 7 d, a similar experiment was repeated using the ulnar nerve.
The differences in diameter and flow rate of the brachial artery in response to median and ulnar nerve activation were compared. In the total group, no significant difference in diameter was seen between medial and ulnar nerve stimulation (P = 0.648). The difference in absolute slowdown of flow rate between median nerve stimulation and ulnar nerve stimulation was not statistically significant for the entire group (P = 0.733).
The brachial artery serves as a target organ and is equally innervated by the median and ulnar nerves.
Expanding similar neurophysiological research to other arteries in the upper and lower limbs will contribute to advancing the field of functional neuroanatomy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ahmed Kamal M, Egypt; Alkhatib AJ, Jordan; Darbari A, India; Ghimire R, Nepal; Xu B, China S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Aalkjær C, Nilsson H, De Mey JGR. Sympathetic and Sensory-Motor Nerves in Peripheral Small Arteries. Physiol Rev. 2021;101:495-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Morgan RF, Reisman NR, Wilgis EF. Anatomic localization of sympathetic nerves in the hand. J Hand Surg Am. 1983;8:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Balogh B, Auterith A, Behrus R, Maier S, Vesely M, Piza-Katzer H. [The sympathetic axons of the nerves of the hand]. Handchir Mikrochir Plast Chir. 2002;34:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | LeBouef T, Yaker Z, Whited L. Physiology, Autonomic Nervous System. 2022 May 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] |
| 5. | Gutrecht JA. Sympathetic skin response. J Clin Neurophysiol. 1994;11:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Eiber CD, Delbeke J, Cardoso J, de Neeling M, John SE, Won Lee C, Skefos J, Sun A, Prodanov D, McKinney Z. Preliminary Minimum Reporting Requirements for In-Vivo Neural Interface Research: I. Implantable Neural Interfaces. IEEE Open J Eng Med Biol. 2021;2:74-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Badal JJ, Kiesau A, Boyle P. Effects of median nerve block on radial artery diameter and peak velocity. Local Reg Anesth. 2010;3:5-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Galea LA, Mercieca A, Sciberras C, Gatt R, Schembri M. Evaluation of sympathetic vasomotor fibres in carpal tunnel syndrome using continuous wave Doppler ultrasonography. J Hand Surg Br. 2006;31:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Ghasemi-Esfe AR, Morteza A, Khalilzadeh O, Mazloumi M, Ghasemi-Esfe M, Rahmani M. Color Doppler ultrasound for evaluation of vasomotor activity in patients with carpal tunnel syndrome. Skeletal Radiol. 2012;41:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Campero M, Verdugo RJ, Ochoa JL. Vasomotor innervation of the skin of the hand: a contribution to the study of human anatomy. J Anat. 1993;182 ( Pt 3):361-368. [PubMed] |
| 11. | Lange KH, Jansen T, Asghar S, Kristensen PL, Skjønnemand M, Nørgaard P. Skin temperature measured by infrared thermography after specific ultrasound-guided blocking of the musculocutaneous, radial, ulnar, and median nerves in the upper extremity. Br J Anaesth. 2011;106:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |